Molecular Characterization of Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and Escherichia coli in Dairy Goat Kids with Diarrhea in Partial Regions of Shaanxi Province, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
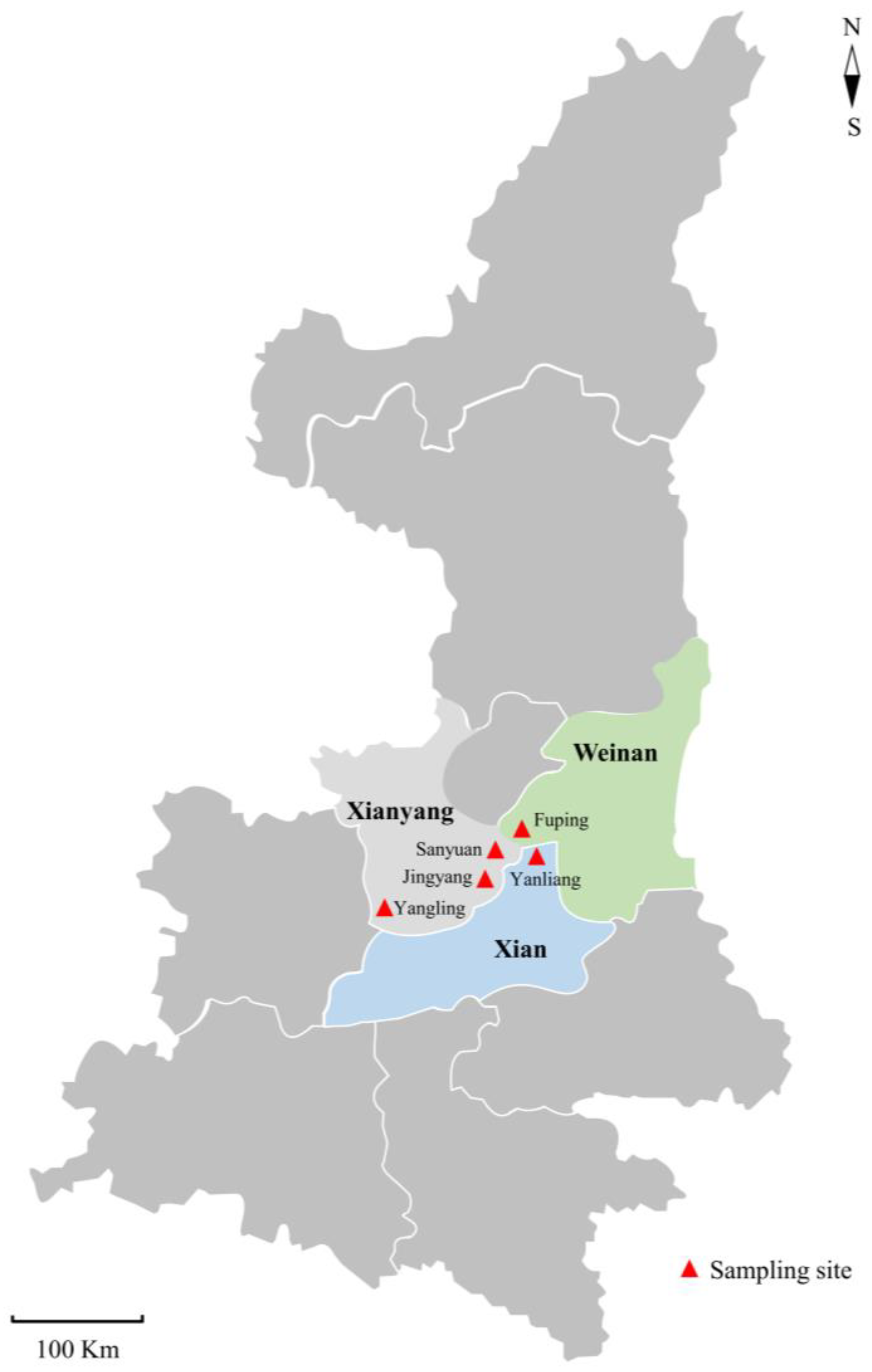
2.1. Samples
2.2. Genomic DNA Extraction
2.3. Detection, Genotyping and Subtyping of Cryptosporidium spp., G. duodenalis and E. bieneusi
2.4. Sequence Analysis
2.5. Bacterial Isolation and Identification
2.6. Virulence Factor Determination of E. coli Strains
2.7. Statistical Analysis
2.8. Nucleotide Sequence Accession Numbers
3. Results
3.1. Occurrence of Cryptosporidium Species and Subtypes in Dairy Goat Kids with Diarrhea
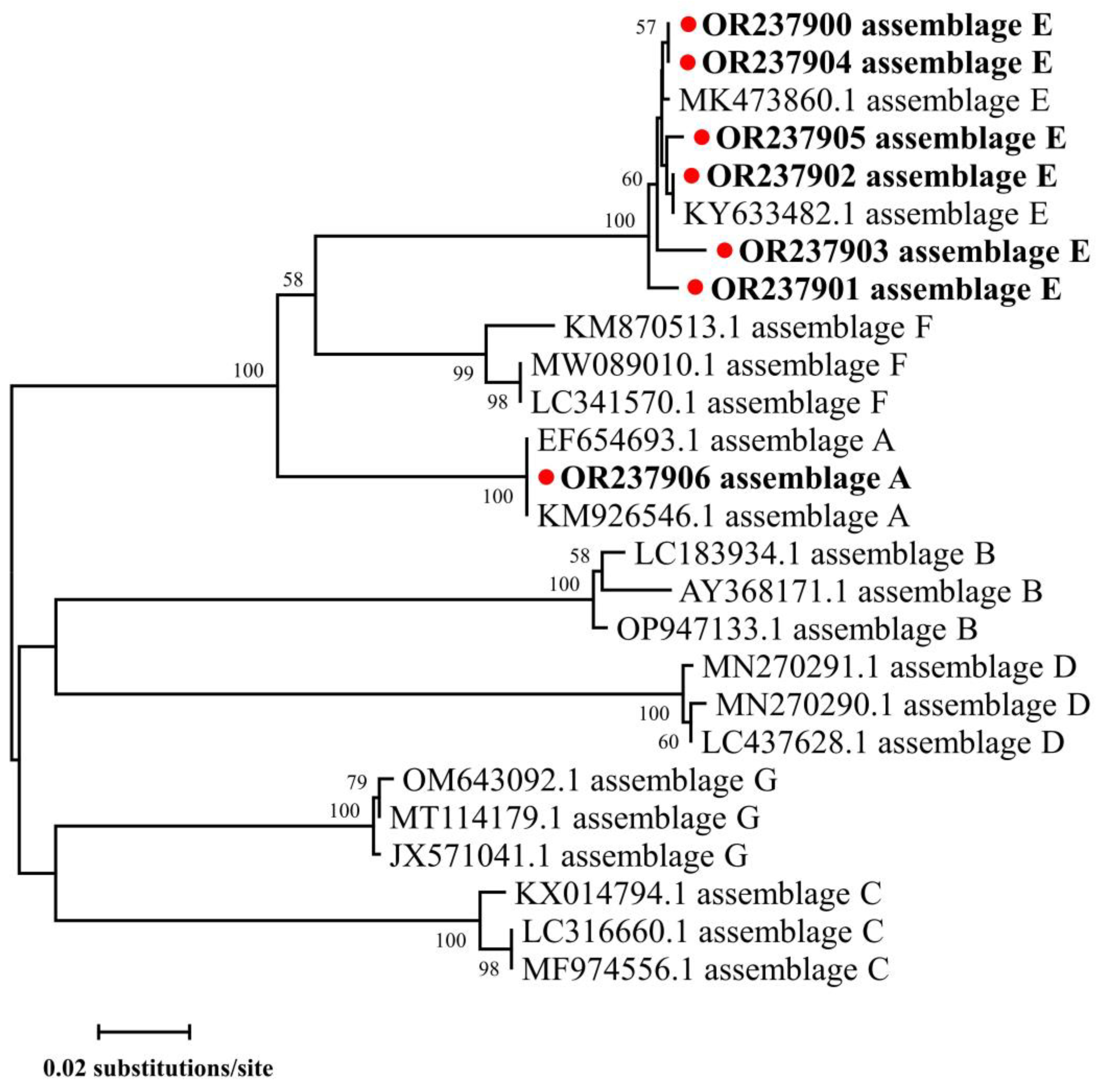
3.2. Occurrence of G. duodenalis Genotypes in Dairy Goat Kids with Diarrhea
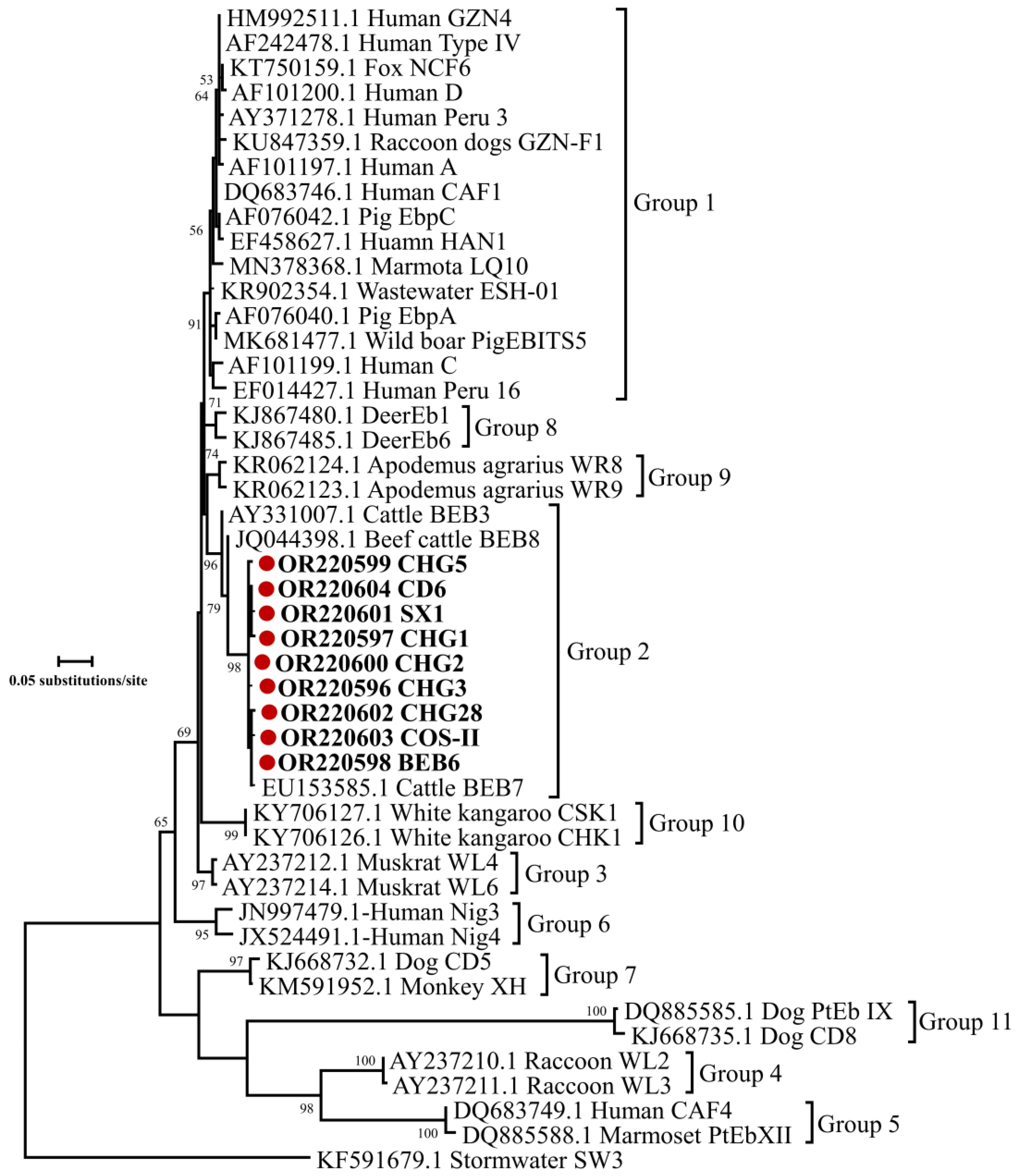
3.3. Occurrence of E. bieneusi Genotypes in Dairy Goat Kids with Diarrhea
3.4. Occurrence of E. coli Pathotypes in Dairy Goat Kids with Diarrhea
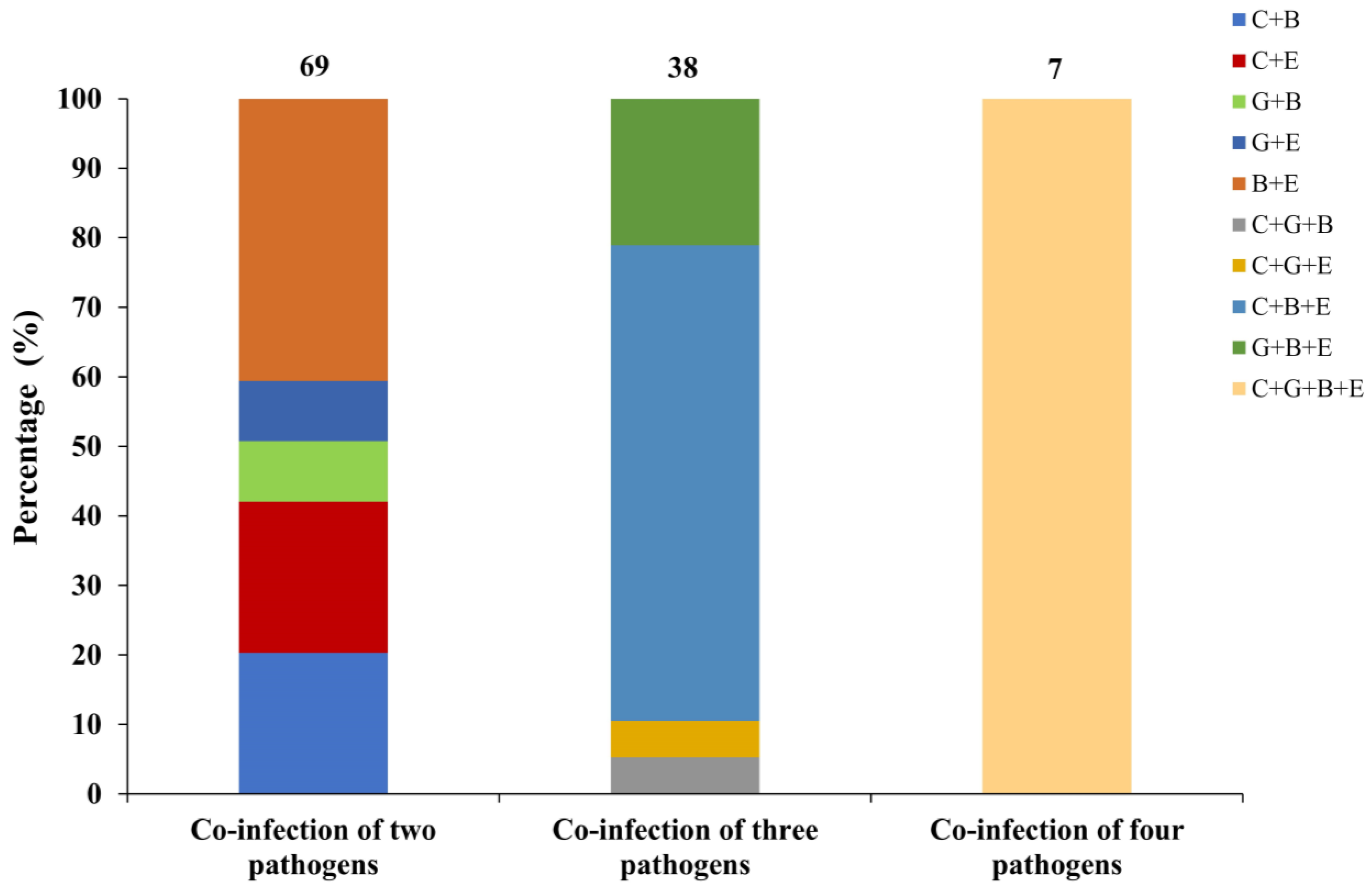
3.5. Co-Infection of Pathogens for Cryptosporidium spp., G. duodenalis, E. bieneusi and E. coli in Dairy Goat Kids with Diarrhea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Guo, Y.; Xiao, L.; Feng, Y. Molecular epidemiology of human cryptosporidiosis in low- and middle-income countries. Clin. Microbiol. Rev. 2021, 34, e00087-19. [Google Scholar] [CrossRef]
- Li, W.; Feng, Y.; Xiao, L. Enterocytozoon bieneusi. Trends Parasitol. 2022, 38, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef] [PubMed]
- Ferens, W.A.; Hovde, C.J. Escherichia coli O157:H7: Animal reservoir and sources of human infection. Foodborne Pathog. Dis. 2011, 8, 465–487. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, J.; Cuny, G.D. Current status and challenges in drug discovery against the globally important zoonotic cryptosporidiosis. Anim. Dis. 2021, 1, 3. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Gu, Y.; Liu, J.; Luo, J. Prevalence of Cryptosporidium, Giardia, Blastocystis, and trichomonads in domestic cats in East China. J. Vet. Med. Sci. 2019, 81, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Rojas-López, L.; Marques, R.C.; Svärd, S.G. Giardia duodenalis. Trends Parasitol. 2022, 38, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Y.R.; Cama, V.A. Foodborne transmission. In Cryptosporidium and Cryptosporidiosis, 2nd ed.; Fayer, R., Xiao, L., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 289–304. [Google Scholar]
- Hwang, S.B.; Chelliah, R.; Kang, J.E.; Rubab, M.; Banan-MwineDaliri, E.; Elahi, F.; Oh, D.H. Role of recent therapeutic applications and the infection strategies of Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2021, 11, 614963. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol. 2017, 8–9, 14–32. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, R.; Huang, J.; Wang, H.; Zhao, J.; Luo, N.; Li, J.; Zhang, Z.; Zhang, L. Cryptosporidiosis caused by Cryptosporidium parvum subtype IIdA15G1 at a dairy farm in Northwestern China. Parasit. Vectors 2014, 7, 529. [Google Scholar] [CrossRef]
- Li, N.; Wang, R.; Cai, M.; Jiang, W.; Feng, Y.; Xiao, L. Outbreak of cryptosporidiosis due to Cryptosporidium parvum subtype IIdA19G1 in neonatal calves on a dairy farm in China. Int. J. Parasitol. 2019, 49, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Matos, O.; Lobo, M.L.; Xiao, L. Epidemiology of Enterocytozoon bieneusi infection in Humans. J. Parasitol. Res. 2012, 2012, 981424. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Fischer, M.A.; Neumann, B.; Kiesewetter, K.; Hoffmann, I.; Werner, G.; Pfeifer, Y.; Lübbert, C. Carbapenemase-producing Gram-negative bacteria in hospital wastewater, wastewater treatment plants and surface waters in a metropolitan area in Germany, 2020. Sci. Total Environ. 2023, 890, 164179. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.; Panchalingham, S.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob. Health 2019, 7, e568–e584. [Google Scholar]
- Agholi, M.; Hatam, G.R.; Motazedian, M.H. HIV/AIDS-associated opportunistic protozoal diarrhea. AIDS Res. Hum. Retroviruses 2013, 29, 35–41. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Zhao, X.; Zhang, L.; Zhang, G.; Guo, M.; Liu, L.; Feng, Y.; Xiao, L. Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. J. Clin. Microbiol. 2013, 51, 557–563. [Google Scholar] [CrossRef]
- Rogawski, E.T.; Liu, J.; Platts-Mills, J.A.; Kabir, F.; Lertsethtakarn, P.; Siguas, M.; Khan, S.S.; Praharaj, I.; Murei, A.; Nshama, R.; et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: Longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob. Health 2018, 6, e1319–e1328. [Google Scholar] [CrossRef]
- Ryan, U.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Hira, P.R.; Zhou, L.; Al-Ali, F.M.; Al-Shelahi, F.A.; Shweiki, H.M.; Iqbal, J.; Khalid, N.; Xiao, L. Unique endemicity of cryptosporidiosis in children in Kuwait. J. Clin. Microbiol. 2005, 43, 2805–2809. [Google Scholar] [CrossRef]
- Jiang, W.; Roellig, D.M.; Guo, Y.; Li, N.; Feng, Y.; Xiao, L. Development of a subtyping tool for zoonotic pathogen Cryptosporidium canis. J. Clin. Microbiol. 2021, 59, e02474-20. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Beser, J.; Axén, C.; Lebbad, M. High applicability of a novel method for gp60-based subtyping of Cryptosporidium meleagridis. J. Clin. Microbiol. 2014, 52, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ryan, U.; Xiao, L.; Feng, Y. Zoonotic giardiasis: An update. Parasitol. Res. 2021, 120, 4199–4218. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, Y.; Zhang, L.; Xiao, L. Potential impacts of host specificity on zoonotic or interspecies transmission of Enterocytozoon bieneusi. Infect. Genet. Evol. 2019, 75, 104033. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, L.; Duan, L.; Ye, J.; Guo, Y.; Guo, M.; Liu, L.; Feng, Y. Concurrent infections of Giardia duodenalis, Enterocytozoon bieneusi, and Clostridium difficile in children during a cryptosporidiosis outbreak in a pediatric hospital in China. PLoS Negl. Trop. Dis. 2013, 7, e2437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Su, Y.; Liang, X.; Sun, X.; Peng, S.; Lu, H.; Jiang, N.; Yin, J.; Xiang, M.; et al. Identification and genotyping of Enterocytozoon bieneusi in China. J. Clin. Microbiol. 2011, 49, 2006–2008. [Google Scholar] [CrossRef]
- Habouria, H.; Pokharel, P.; Maris, S.; Garénaux, A.; Bessaiah, H.; Houle, S.; Veyrier, F.J.; Guyomard-Rabenirina, S.; Talarmin, A.; Dozois, C.M. Three new serine-protease autotransporters of Enterobacteriaceae (SPATEs) from extra-intestinal pathogenic Escherichia coli and combined role of SPATEs for cytotoxicity and colonization of the mouse kidney. Virulence 2019, 10, 568–587. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef]
- Mi, R.; Wang, X.; Huang, Y.; Zhou, P.; Liu, Y.; Chen, Y.; Chen, J.; Zhu, W.; Chen, Z. Prevalence and molecular characterization of Cryptosporidium in goats across four provincial level areas in China. PLoS ONE 2014, 9, e111164. [Google Scholar] [CrossRef]
- Peng, X.Q.; Tian, G.R.; Ren, G.J.; Yu, Z.Q.; Lok, J.B.; Zhang, L.X.; Wang, X.T.; Song, J.K.; Zhao, G.H. Infection rate of Giardia duodenalis, Cryptosporidium spp. and Enterocytozoon bieneusi in cashmere, dairy and meat goats in China. Infect. Genet. Evol. 2016, 41, 26–31. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Cui, B.; Huang, J.; Cui, Z.; Zhang, S.; Dong, H.; Yue, D.; Zhang, L.; Ning, C.; et al. Prevalence, molecular characterization and zoonotic potential of Cryptosporidium spp. in goats in Henan and Chongqing, China. Exp. Parasitol. 2014, 142, 11–16. [Google Scholar] [CrossRef]
- Zhong, Z.; Tu, R.; Ou, H.; Yan, G.; Dan, J.; Xiao, Q.; Wang, Y.; Cao, S.; Shen, L.; Deng, J.; et al. Occurrence and genetic characterization of Giardia duodenalis and Cryptosporidium spp. from adult goats in Sichuan Province, China. PLoS ONE 2018, 13, e0199325. [Google Scholar] [CrossRef]
- Xiao, L.; Escalante, L.; Yang, C.; Sulaiman, I.; Escalante, A.A.; Montali, R.J.; Fayer, R.; Lal, A.A. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 1999, 65, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, X.; Guo, S.; Yang, F.; Yang, X.; Guo, Y.; Feng, Y.; Xiao, L.; Li, N. Subtyping Cryptosporidium xiaoi, a common pathogen in sheep and goats. Pathogens 2021, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.M.; Fayer, R.; Bern, C.; Gilman, R.H.; Trout, J.M.; Schantz, P.M.; Das, P.; Lal, A.A.; Xiao, L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003, 9, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Lalle, M.; Pozio, E.; Capelli, G.; Bruschi, F.; Crotti, D.; Cacciò, S.M. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 2005, 35, 207–213. [Google Scholar] [CrossRef]
- Cacciò, S.M.; Beck, R.; Lalle, M.; Marinculic, A.; Pozio, E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int. J. Parasitol. 2008, 38, 1523–1531. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Fayer, R.; Lal, A.A.; Trout, J.M.; Schaefer, F.W.; Xiao, L. Molecular characterization of microsporidia indicates that wild mammals Harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl. Environ. Microbiol. 2003, 69, 4495–4501. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, Y.; Adam, F.E.A.; Xie, Q.; Wang, B.; Bai, X.; Wang, X.; Shan, H.; Wang, X.; Liu, H.; et al. Comparison of antimicrobial resistance, virulence genes, phylogroups, and biofilm formation of Escherichia coli isolated from intensive farming and free-range sheep. Front. Microbiol. 2021, 12, 699927. [Google Scholar] [CrossRef]
- Greisen, K.; Loeffelholz, M.; Purohit, A.; Leong, D. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J. Clin. Microbiol. 1994, 32, 335–351. [Google Scholar] [CrossRef]
- López-Saucedo, C.; Cerna, J.F.; Villegas-Sepulveda, N.; Thompson, R.; Velazquez, F.R.; Torres, J.; Tarr, P.I.; Estrada-García, T. Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli. Emerg. Infect. Dis. 2003, 9, 127–131. [Google Scholar] [CrossRef]
- Chapman, T.A.; Wu, X.Y.; Barchia, I.; Bettelheim, K.A.; Driesen, S.; Trott, D.; Wilson, M.; Chin, J.J. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 2006, 72, 4782–4795. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Lu, Y.; Higa, N.; Nakasone, N.; Chinen, I.; Baschkier, A.; Rivas, M.; Iwanaga, M. Multiplex PCR assay for identification of human diarrheagenic Escherichia coli. J. Clin. Microbiol. 2003, 41, 2669–2671. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. Molecular Epidemiological Investigation of Cryptosporidium spp. in Sheep and Goats in Midwest China and Development of a Subtyping Tool for Cryptosporidium xiaoi. Master’s Thesis, South China Agricultural University, Guangzhou, China, 27 August 2020. (In Chinese). [Google Scholar]
- Mi, R.; Wang, X.; Huang, Y.; Mu, G.; Zhang, Y.; Jia, H.; Zhang, X.; Yang, H.; Wang, X.; Han, X.; et al. Sheep as a potential source of zoonotic cryptosporidiosis in China. Appl. Environ. Microbiol. 2018, 84, e00868-18. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Gong, Q.L.; Zhao, B.; Cai, Y.N.; Zhao, Q. Prevalence of Cryptosporidium infection in sheep and goat flocks in China during 2010–2019: A systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021, 21, 692–706. [Google Scholar] [CrossRef]
- Chen, J.; Shen, K.; Ren, H.; Gao, L.; Ning, C. Investigation of goats Cryptosporidium infection in some breeding farms of Chongqing. Chin. J. Vet. Med. 2012, 48, 15–17. (In Chinese) [Google Scholar]
- Jiang, Y. Genotyping of Three Enteric Protozoans in Sheep from Heilongjiang Province and Assessment of Their Zoonotic Potential. Master’s Thesis, Northeast Agricultural University, Harbin, China, 14 June 2016. (In Chinese). [Google Scholar]
- Wu, Y.; Chang, Y.; Chen, Y.; Zhang, X.; Li, D.; Zheng, S.; Wang, L.; Li, J.; Ning, C.; Zhang, L. Occurrence and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi from Tibetan sheep in Gansu, China. Infect. Genet. Evol. 2018, 64, 46–51. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, Z.; Zhao, A.; Jing, B.; Guan, G.; Luo, J.; Zhang, L. Distribution and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi amongst grazing adult sheep in Xinjiang, China. Parasitol. Int. 2019, 71, 80–86. [Google Scholar] [CrossRef]
- Li, P.; Cai, J.; Cai, M.; Wu, W.; Li, C.; Lei, M.; Xu, H.; Feng, L.; Ma, J.; Feng, Y.; et al. Distribution of Cryptosporidium species in Tibetan sheep and yaks in Qinghai, China. Vet. Parasitol. 2016, 215, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Xiao, L.; Wang, Y.; Wang, L.; Amer, S.; Roellig, D.M.; Guo, Y.; Feng, Y. Periparturient transmission of Cryptosporidium xiaoi from ewes to lambs. Vet. Parasitol. 2013, 197, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Quílez, J.; Torres, E.; Chalmers, R.M.; Hadfield, S.J.; Del Cacho, E.; Sánchez-Acedo, C. Cryptosporidium genotypes and subtypes in lambs and goat kids in Spain. Appl. Environ. Microbiol. 2008, 74, 6026–6031. [Google Scholar] [CrossRef]
- Yu, F.; Li, D.; Chang, Y.; Wu, Y.; Guo, Z.; Jia, L.; Xu, J.; Li, J.; Qi, M.; Wang, R.; et al. Molecular characterization of three intestinal protozoans in hospitalized children with different disease backgrounds in Zhengzhou, central China. Parasit. Vectors 2019, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Z.; Zhang, Y.; Yang, Y.; Zhao, J.; Wang, R.; Jian, F.; Ning, C.; Zhang, W.; Zhang, L. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in deer in Henan and Jilin, China. Parasit. Vectors 2018, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, B.; Li, J.; Yu, S.; Zhang, N.; Liu, S.; Zhang, Y.; Li, J.; Ma, N.; Cai, Y.; et al. Development of a quantitative real-time PCR assay for detection of Cryptosporidium spp. infection and threatening caused by Cryptosporidium parvum subtype IIdA19G1 in diarrhea calves from Northeastern China. Vector Borne Zoonotic Dis. 2021, 21, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Jian, F.; Liu, A.; Wang, R.; Zhang, S.; Qi, M.; Zhao, W.; Shi, Y.; Wang, J.; Wei, J.; Zhang, L.; et al. Common occurrence of Cryptosporidium hominis in horses and donkeys. Infect. Genet. Evol. 2016, 43, 261–266. [Google Scholar] [CrossRef]
- Wang, H.; Qi, M.; Zhang, K.; Li, J.; Huang, J.; Ning, C.; Zhang, L. Prevalence and genotyping of Giardia duodenalis isolated from sheep in Henan Province, central China. Infect. Genet. Evol. 2016, 39, 330–335. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Wang, R.; Liu, A.; Shen, Y.; Ling, H.; Cao, J.; Yang, F.; Zhang, X.; Zhang, L. Genetic characterizations of Giardia duodenalis in sheep and goats in Heilongjiang Province, China and possibility of zoonotic transmission. PLoS Negl. Trop. Dis. 2012, 6, e1826. [Google Scholar] [CrossRef]
- Yang, F.; Ma, L.; Gou, J.M.; Yao, H.Z.; Ren, M.; Yang, B.K.; Lin, Q. Seasonal distribution of Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi in Tibetan sheep in Qinghai, China. Parasit. Vectors 2022, 15, 394. [Google Scholar] [CrossRef]
- Chen, D.; Zou, Y.; Li, Z.; Wang, S.S.; Xie, S.C.; Shi, L.Q.; Zou, F.C.; Yang, J.F.; Zhao, G.H.; Zhu, X.Q. Occurrence and multilocus genotyping of Giardia duodenalis in black-boned sheep and goats in southwestern China. Parasit. Vectors 2019, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.L.; Zhang, H.J.; Yuan, Y.J.; Tang, H.; Chen, D.; Jing, S.; Wu, H.X.; Wang, S.S.; Zhao, G.H. Prevalence and multi-locus genotyping of Giardia duodenalis from goats in Shaanxi province, northwestern China. Acta Trop. 2018, 182, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Cacciò, S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Field, D.; Ryan, U. Molecular typing of Giardia duodenalis in humans in Queensland—First report of assemblage E. Parasitology 2017, 144, 1154–1161. [Google Scholar] [CrossRef]
- Ye, J.; Xiao, L.; Wang, Y.; Guo, Y.; Roellig, D.M.; Feng, Y. Dominance of Giardia duodenalis assemblage A and Enterocytozoon bieneusi genotype BEB6 in sheep in Inner Mongolia, China. Vet. Parasitol. 2015, 210, 235–239. [Google Scholar] [CrossRef]
- Berrilli, F.; D’Alfonso, R.; Giangaspero, A.; Marangi, M.; Brandonisio, O.; Kaboré, Y.; Glé, C.; Cianfanelli, C.; Lauro, R.; Di Cave, D. Giardia duodenalis genotypes and Cryptosporidium species in humans and domestic animals in Côte d’Ivoire: Occurrence and evidence for environmental contamination. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 191–195. [Google Scholar] [CrossRef]
- Li, W.C.; Wang, K.; Gu, Y.F. Detection and genotyping study of Enterocytozoon bieneusi in sheep and goats in East-central China. Acta Parasitol. 2019, 64, 44–50. [Google Scholar] [CrossRef]
- Shi, K.; Li, M.; Wang, X.; Li, J.; Karim, M.R.; Wang, R.; Zhang, L.; Jian, F.; Ning, C. Molecular survey of Enterocytozoon bieneusi in sheep and goats in China. Parasit. Vectors 2016, 9, 23. [Google Scholar] [CrossRef]
- Xie, S.C.; Zou, Y.; Li, Z.; Yang, J.F.; Zhu, X.Q.; Zou, F.C. Molecular detection and genotyping of Enterocytozoon bieneusi in black goats (Capra hircus) in Yunnan Province, Southwestern China. Animals 2021, 11, 3387. [Google Scholar] [CrossRef]
- Karim, M.R.; Wang, R.; Dong, H.; Zhang, L.; Li, J.; Zhang, S.; Rume, F.I.; Qi, M.; Jian, F.; Sun, M.; et al. Genetic polymorphism and zoonotic potential of Enterocytozoon bieneusi from nonhuman primates in China. Appl. Environ. Microbiol. 2014, 80, 1893–1898. [Google Scholar] [CrossRef]
- Fayer, R.; Santín, M.; Trout, J.M. Enterocytozoon bieneusi in mature dairy cattle on farms in the eastern United States. Parasitol. Res. 2007, 102, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, W.; Wang, R.; Liu, W.; Liu, A.; Yang, D.; Yang, F.; Karim, M.R.; Zhang, L. Enterocytozoon bieneusi in sika deer (Cervus nippon) and red deer (Cervus elaphus): Deer specificity and zoonotic potential of ITS genotypes. Parasitol. Res. 2014, 113, 4243–4250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.H.; Du, S.Z.; Wang, H.B.; Hu, X.F.; Deng, M.J.; Yu, S.K.; Zhang, L.X.; Zhu, X.Q. First report of zoonotic Cryptosporidium spp., Giardia intestinalis and Enterocytozoon bieneusi in golden takins (Budorcas taxicolor bedfordi). Infect. Genet. Evol. 2015, 34, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, L.; Yu, X.; Zhong, Z.; Wang, Q.; Liu, X.; Niu, L.; Xie, N.; Deng, J.; Lei, S.; et al. Multilocus genotypes and broad host-range of Enterocytozoon bieneusi in captive wildlife at zoological gardens in China. Parasit. Vectors 2016, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Dong, H.; Yu, F.; Jian, F.; Zhang, L.; Wang, R.; Zhang, S.; Rume, F.I.; Ning, C.; Xiao, L. Genetic diversity in Enterocytozoon bieneusi isolates from dogs and cats in China: Host specificity and public health implications. J. Clin. Microbiol. 2014, 52, 3297–3302. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, S.; Yang, Z.; Zhang, Y.; Zhang, L.; Wang, R.; Zhang, W.; Yang, F.; Liu, A. Genotyping of Enterocytozoon bieneusi (Microsporidia) isolated from various birds in China. Infect. Genet. Evol. 2016, 40, 151–154. [Google Scholar] [CrossRef]
- Li, W.; Feng, Y.; Santin, M. Host specificity of Enterocytozoon bieneusi and public health implications. Trends Parasitol. 2019, 35, 436–451. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Bai, X.; Hu, B.; Jiang, D.; Jiao, H.; Lu, L.; Fan, R.; Hou, P.; Matussek, A.; et al. High prevalence and persistence of Escherichia coli strains producing Shiga toxin subtype 2k in goat herds. Microbiol. Spectr. 2022, 10, e0157122. [Google Scholar] [CrossRef]

| Factor | No. Examined | No. Positive (%) | Species (No.) | Subtype (No.) | |
|---|---|---|---|---|---|
| Location | |||||
| Fuping | Farm 1 | 54 | 23 (42.6) | C. parvum (18) C. xiaoi (5) | IIdA19G1 (16) XXIIIg (4), XXIIIa (1) |
| Farm 2 | 46 | 21 (45.7) | C. parvum (14) C. xiaoi (7) | IIdA19G1 (13) XXIIIg (3), XXIIIa (3) | |
| Farm 3 | 30 | 21 (70.0) | C. xiaoi (21) | XXIIIg (1), XXIIIa (18), XXIIIa + XXIIIg (2) | |
| Sub-total | 130 | 65 (50.0) | C. parvum (32) C. xiaoi (33) | IIdA19G1 (29) XXIIIa (22), XXIIIg (8), XXIIIa + XXIIIg (2) | |
| Yanliang | Farm 4 | 19 | 2 (10.5) | C. parvum (2) | IIdA19G1 (2) |
| Yangling | Farm 5 | 20 | 8 (40.0) | C. parvum (7) C. xiaoi (1) | IIdA19G1 (6), IIdA19G2 (1) NA |
| Jingyang | Farm 6 | 10 | 1 (10) | C. xiaoi (1) | XXIIIg (1) |
| Sanyuan | Farm 7 | 23 | 0 (0) | – | – |
| Age (weeks) | |||||
| <2 | 47 | 19 (40.4) | C. parvum (14) C. xiaoi (5) | IIdA19G1 (12), IIdA19G2 (1) XXIIIg (5) | |
| 2–4 | 102 | 53 (52.0) | C. parvum (24) C. xiaoi (29) | IIdA19G1 (22) XXIIIa (22), XXIIIg (4), XXIIIa + XXIIIg (2) | |
| 4–12 | 53 | 4 (7.5) | C. parvum (3) C. xiaoi (1) | IIdA19G1 (3) NA | |
| Total | 202 | 76 (37.6) | C. parvum (41) C. xiaoi (35) | IIdA19G1 (37), IIdA19G2 (1) XXIIIa (22), XXIIIg (9), XXIIIa + XXIIIg (2) |
| Factor | No. Examined | No. Positive (%) | Assemblage (No.) | |||||
|---|---|---|---|---|---|---|---|---|
| gdh | tpi | bg | gdh | tpi | bg | |||
| Location | ||||||||
| Fuping | Farm 1 | 54 | 5 (9.3) | 3 (5.6) | 4 (7.4) | A (2), E (3) | A (2), E (1) | A (1), E (3) |
| Farm 2 | 46 | 1 (2.2) | 0 (0) | 1 (2.2) | A (1) | – | A (1) | |
| Farm 3 | 30 | 8 (26.7) | 8 (26.7) | 8 (26.7) | A (2), E (6) | A (2), E (6) | A (2), E (6) | |
| Sub-total | 130 | 14 (10.8) | 11 (8.5) | 13 (10.0) | E (9), A (5) | E (7), A (4) | E (9), A (4) | |
| Yanliang | Farm 4 | 19 | 2 (10.5) | 0 (0) | 2 (10.5) | E (2) | – | E (2) |
| Yangling | Farm 5 | 20 | 5 (25.0) | 5 (25.0) | 5 (25.0) | E (5) | E (5) | E (5) |
| Jingyang | Farm 6 | 10 | 6 (60.0) | 0 (0) | 5 (50.0) | E (6) | – | E (5) |
| Sanyuan | Farm 7 | 23 | 6 (26.1) | 0 (0) | 5 (21.7) | E (6) | – | E (5) |
| Age (weeks) | ||||||||
| <2 | 47 | 5 (10.6) | 3 (6.4) | 4 (8.5) | E (3), A (2) | E (1), A (2) | E (3), A (1) | |
| 2–4 | 102 | 9 (8.8) | 8 (7.8) | 9 (8.8) | E (6), A (3) | E (6), A (2) | E (6), A (3) | |
| 4–12 | 53 | 19 (35.8) | 5 (9.4) | 17 (32.1) | E (19) | E (5) | E (17) | |
| Total | 202 | 33 (16.3) | 16 (7.9) | 30 (14.9) | E (28), A (5) | E (12), A (4) | E (26), A (4) | |
| Factor | No. Examined | No. Positive (%) | Genotype (No.) | |
|---|---|---|---|---|
| Location | ||||
| Fuping | Farm 1 | 54 | 27 (50.0) | CHG1 (16), CHG2 (1), CHG3 (4), CHG28 (1), BEB6 (3), COS-II (1), SX1 (1) |
| Farm 2 | 46 | 23 (50.0) | CHG1 (5), CHG2 (1), CHG3 (13), CHG5 (2), BEB6 (1), CD6 (1) | |
| Farm 3 | 30 | 24 (80.0) | CHG2 (1), CHG3 (20), BEB6 (1), SX1 (2) | |
| Sub-total | 130 | 74 (56.9) | CHG3 (37), CHG1(21), BEB6 (5), CHG2 (3), CHG5 (2), SX1 (3), CHG28 (1), COS-II (1), CD6 (1) | |
| Yanliang | Farm 4 | 19 | 13 (68.4) | CHG3 (7), CHG5 (4), SX1 (1), CHG2 (1) |
| Yangling | Farm 5 | 20 | 10 (50.0) | CHG1 (7), BEB6(2), CHG3(1) |
| Jingyang | Farm 6 | 10 | 2 (20.0) | CHG3 (1), CHG5 (1) |
| Sanyuan | Farm 7 | 23 | 13 (56.5) | CHG3 (12), BEB6 (1) |
| Age (weeks) | ||||
| <2 | 47 | 21 (44.7) | CHG1 (13), CHG3 (3), CHG5 (1), CHG28 (1), COS-II (1), SX1 (1), BEB6 (1) | |
| 2–4 | 102 | 62 (60.8) | CHG3 (38), CHG1 (10), BEB6 (4), CHG2 (4), SX1 (3), CHG5 (2), CD6 (1) | |
| 4–12 | 53 | 29 (54.7) | CHG3 (17), CHG1 (5), CHG5 (4), BEB6 (3) | |
| Total | 202 | 112 (55.4) | CHG3 (58), CHG1 (28), BEB6 (8), CHG5 (7), CHG2 (4), SX1 (4), CHG28 (1), COS-II (1), CD6 (1) |
| Factor | No. Examined | No. Positive (%) | Pathotype and Virulence Gene (No.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EPEC | EHEC | ETEC | EAEC | EIEC | |||||||
| ehxA | eae | stx2 | stx1 | lt | st | aggR | ipaH | ||||
| Location | |||||||||||
| Fuping | |||||||||||
| Farm 1 | 54 | 38 (70.4) | 31 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Farm 2 | 46 | 35 (76.1) | 5 | 16 | 2 | 6 | 0 | 0 | 0 | 0 | |
| Farm 3 | 30 | 27 (90.0) | 2 | 21 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Sub-total | 130 | 100 (76.9) | 38 | 70 | 2 | 7 | 0 | 0 | 0 | 0 | |
| Yanliang | Farm 4 | 20 | 13 (65.0) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yangling | Farm 5 | 19 | 17 (89.5) | 9 | 10 | 6 | 2 | 0 | 0 | 0 | 0 |
| Jingyang | Farm 6 | 10 | 9 (90.0) | 4 | 9 | 0 | 2 | 0 | 0 | 0 | 0 |
| Sanyuan | Farm 7 | 23 | 20 (87.0) | 7 | 16 | 11 | 4 | 0 | 0 | 0 | 0 |
| Age (weeks) | |||||||||||
| <2 | 47 | 36 (76.6) | 26 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2–4 | 102 | 81 (79.4) | 19 | 48 | 8 | 9 | 0 | 0 | 0 | 0 | |
| 4–12 | 53 | 42 (79.2) | 13 | 30 | 11 | 6 | 0 | 0 | 0 | 0 | |
| Total | 202 | 159 (78.7) | 58 | 106 | 19 | 15 | 0 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, J.; Huang, S.; Song, J.; Fan, Y.; Zhao, G. Molecular Characterization of Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and Escherichia coli in Dairy Goat Kids with Diarrhea in Partial Regions of Shaanxi Province, China. Animals 2023, 13, 2922. https://doi.org/10.3390/ani13182922
Yang X, Wang J, Huang S, Song J, Fan Y, Zhao G. Molecular Characterization of Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and Escherichia coli in Dairy Goat Kids with Diarrhea in Partial Regions of Shaanxi Province, China. Animals. 2023; 13(18):2922. https://doi.org/10.3390/ani13182922
Chicago/Turabian StyleYang, Xin, Junwei Wang, Shuang Huang, Junke Song, Yingying Fan, and Guanghui Zhao. 2023. "Molecular Characterization of Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and Escherichia coli in Dairy Goat Kids with Diarrhea in Partial Regions of Shaanxi Province, China" Animals 13, no. 18: 2922. https://doi.org/10.3390/ani13182922
APA StyleYang, X., Wang, J., Huang, S., Song, J., Fan, Y., & Zhao, G. (2023). Molecular Characterization of Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and Escherichia coli in Dairy Goat Kids with Diarrhea in Partial Regions of Shaanxi Province, China. Animals, 13(18), 2922. https://doi.org/10.3390/ani13182922






