Fine Particulate Matter Perturbs the Pulmonary Microbiota in Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. PM Preparation
2.2. Animal Experiment
2.3. PM Exposure Treatment
2.4. Sample Collection
2.5. Real-Time RT-PCR
2.6. Analysis of Histological
2.7. S rRNA Sequencing of Pulmonary Microbiota and Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
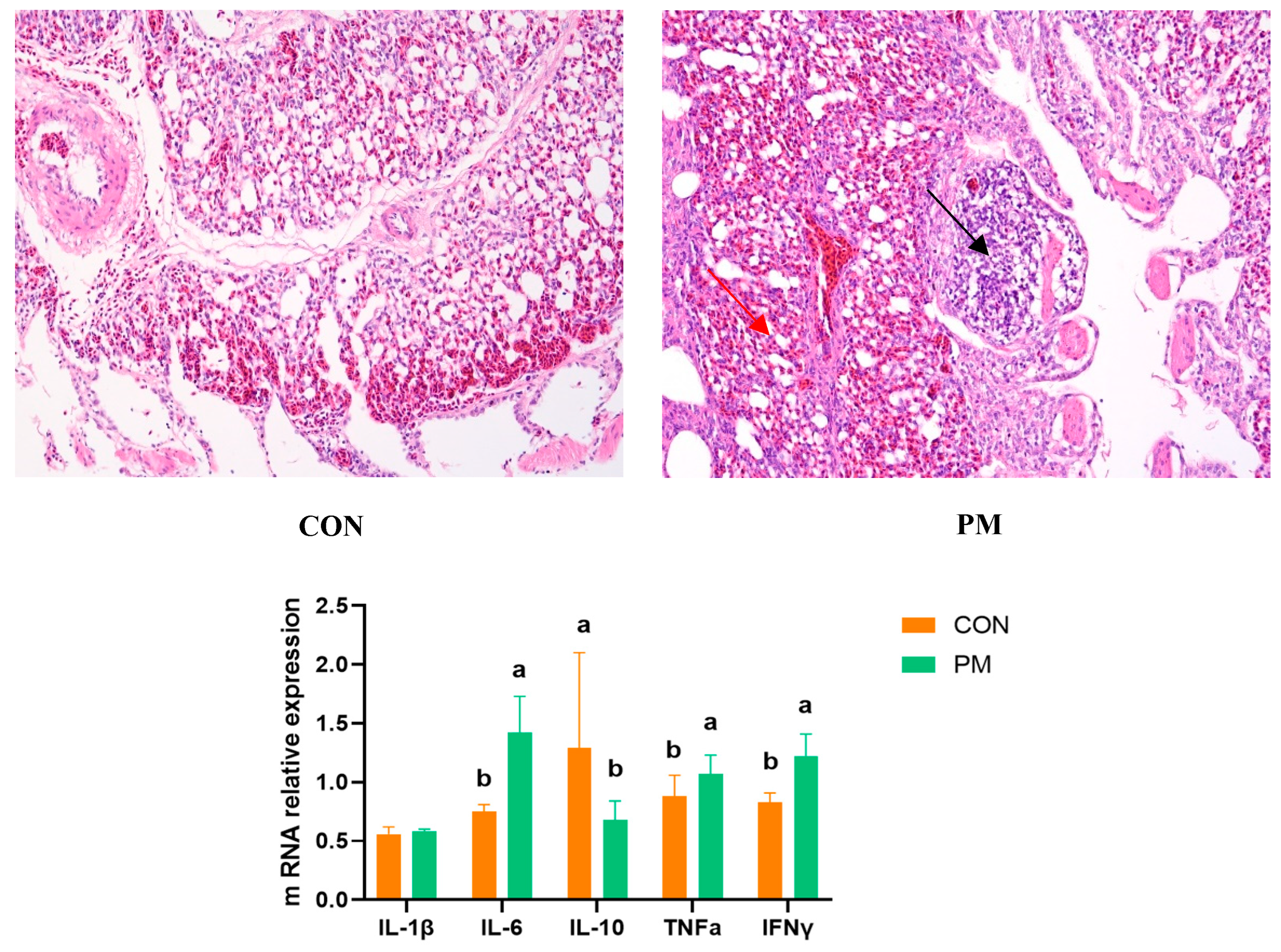
3.1. Pulmonary Injury
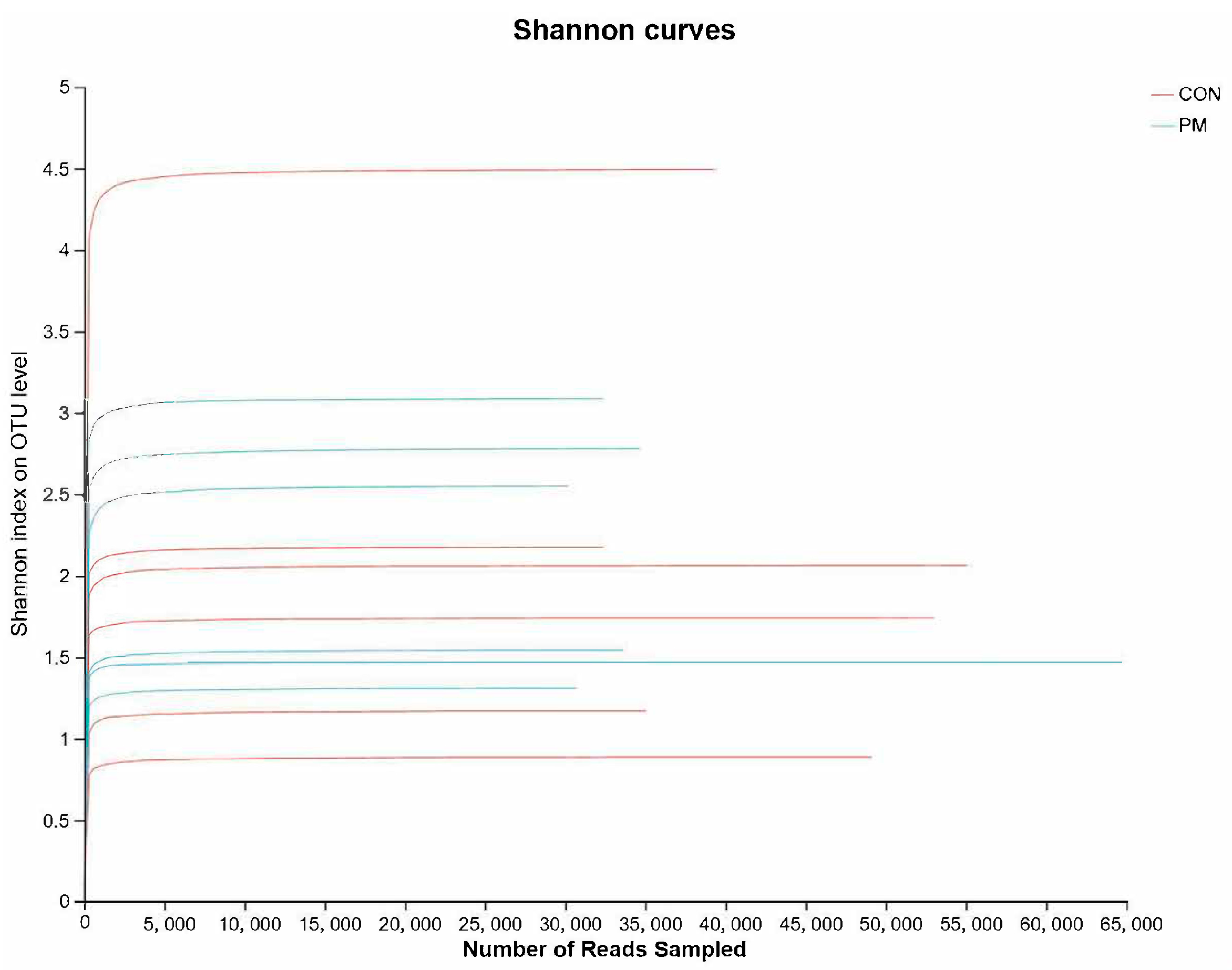
3.2. Rank–Abundance Curve
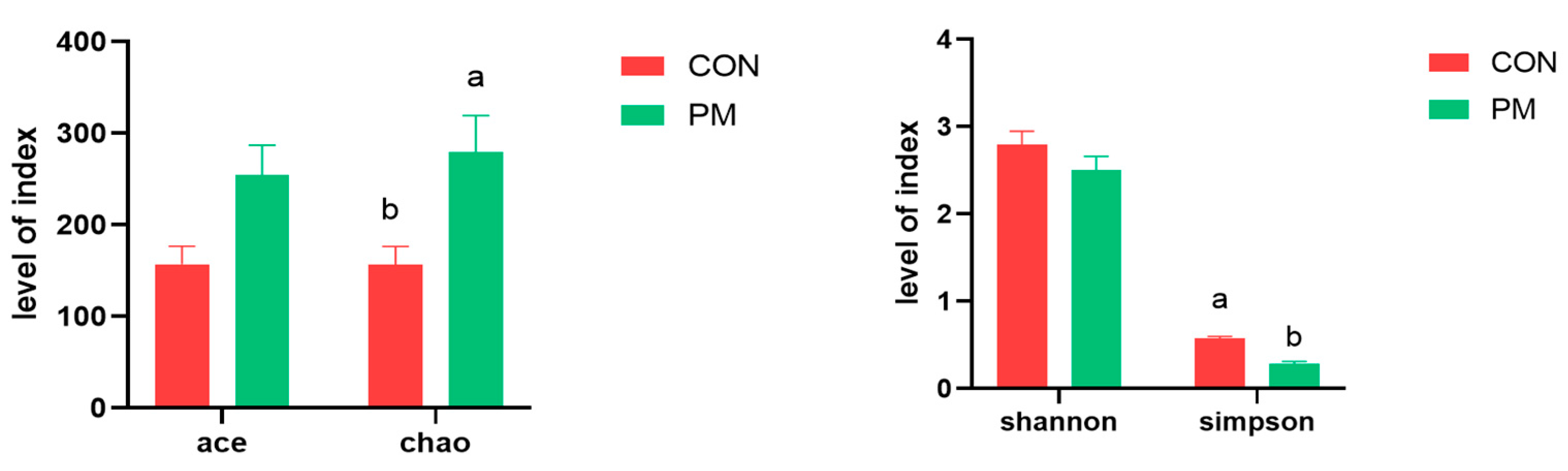
3.3. Alpha Diversity
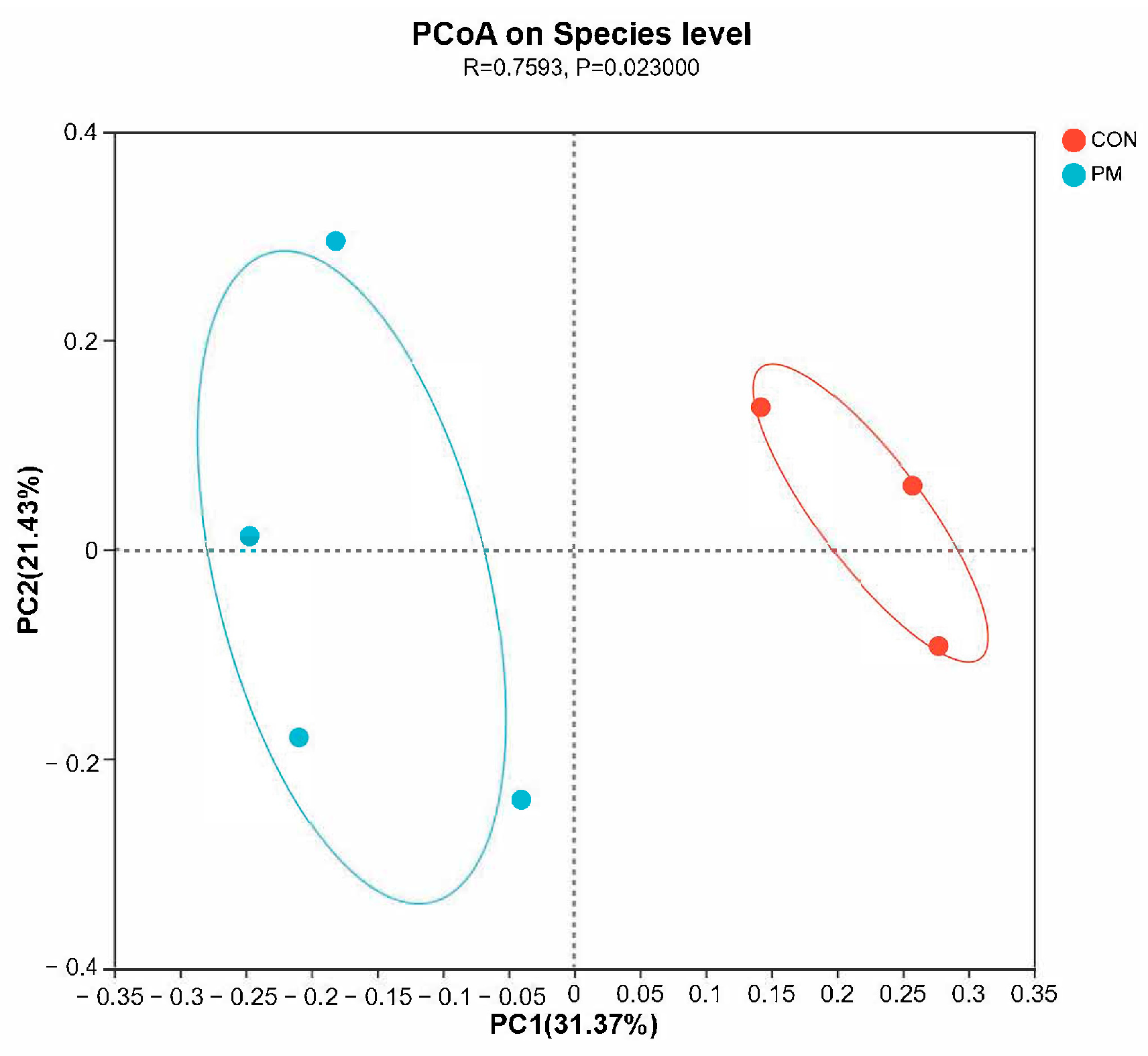
3.4. Beta Diversity
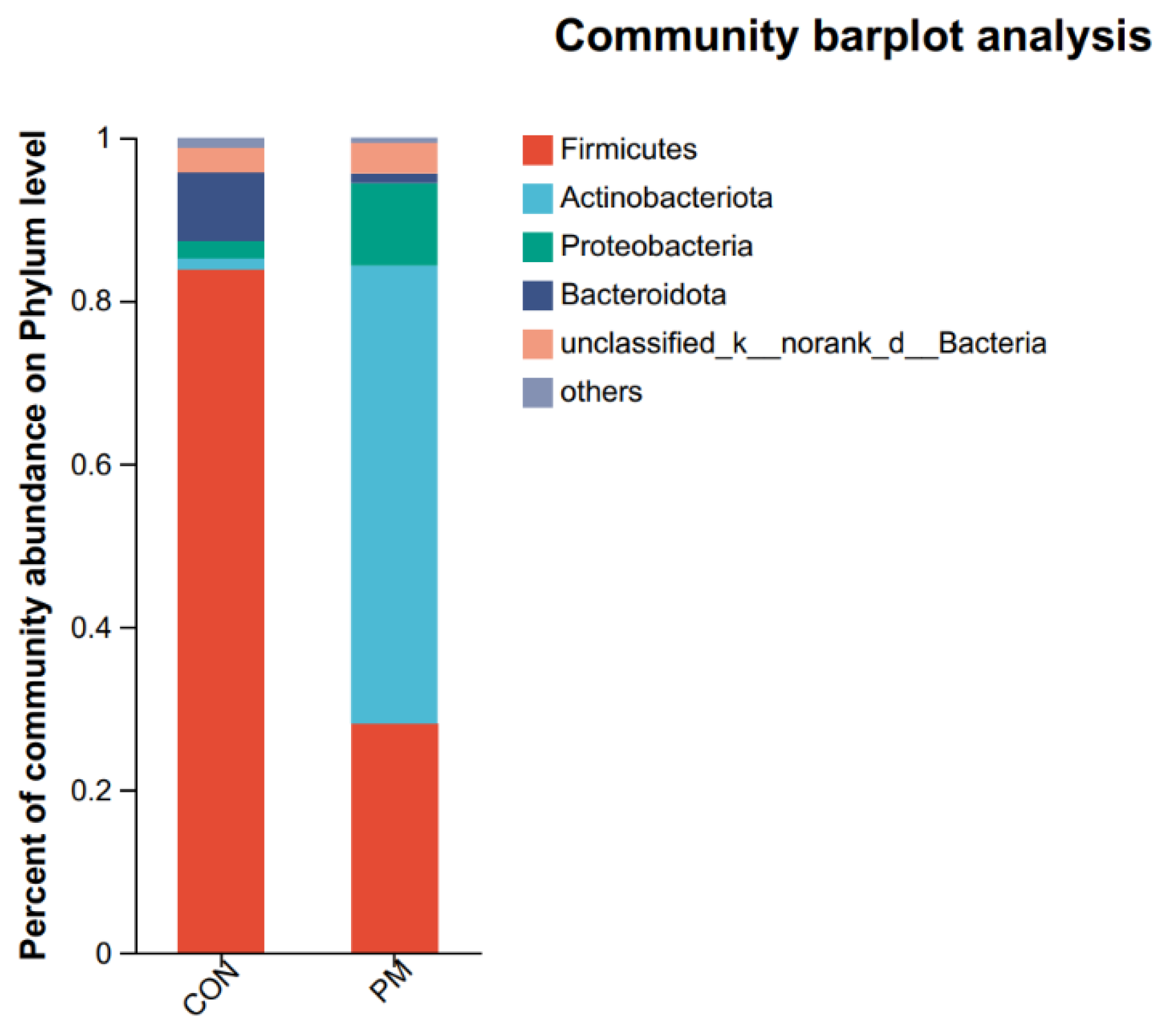
3.5. Pulmonary Microbiota Composition at the Phylum and Genus Levels
3.6. Test of Microbiota Composition between the CON and PM Groups at the Phylum and Genus Levels
3.7. Correlation between Pulmonary Microbiota and Inflammation under PM Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Li, H.; Li, H.; Guo, W.; An, Z.; Zeng, X.; Li, W.; Li, H.; Song, J.; Wu, W. Amelioration of PM2.5-induced lung toxicity in rats by nutritional supplementation with fish oil and Vitamin E. Respir. Res. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, M.; An, Z.; Jiang, J.; Li, J.; Wang, Y.; Du, S.; Zhang, X.; Zhou, H.; Cui, J.; et al. Associations between air pollution and outpatient visits for allergic rhinitis in Xinxiang, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 23565–23574. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Xiao, Q.; Gu, D.; Xu, M.; Tian, L.; Guo, Q.; Wu, Z.; Pan, X.; Liu, Y. Satellite-based short- and long-term exposure to PM2.5 and adult mortality in urban Beijing, China. Environ. Pollut. 2018, 242, 492–499. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Y.; Huang, L.; Wang, K.; Shen, H.; Li, Z. Pollution characteristics and toxic effects of PM1.0 and PM2.5 in Harbin, China. Environ. Sci. Pollut. Res. Int. 2021, 28, 13229–13242. [Google Scholar] [CrossRef]
- Cambra-López, M.; Aarnink, A.J.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Almuhanna, E.A. Characteristics of air contaminants in naturally and mechanically ventilated poultry houses in Al-Ahsa, Saudi Arabia. Trans. ASABE 2011, 54, 1433–1443. [Google Scholar] [CrossRef]
- Ni, J.Q.; Chai, L.; Chen, L.; Bogan, B.W.; Wang, K.; Cortus, E.L.; Diehl, C.A. Characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter concentrations in high-rise and manure-belt layer hen houses. Atmos. Environ. 2012, 57, 165–174. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, D.; Zhao, B.; Ma, S.; Liu, X.; Li, M.; Liu, X. Seasonal variations and spatial distribution of particulate matter emissions from a ventilated laying hen house in Northeast China. Int. J. Agric. Biol. Eng. 2020, 13, 57–63. [Google Scholar] [CrossRef]
- Hong, E.C.; Kang, H.K.; Jeon, J.J.; You, A.S.; Kim, H.S.; Son, J.S.; Kim, H.J.; Yun, Y.S.; Kang, B.S.; Kim, J.H. Studies on the concentrations of particulate matter and ammonia gas from three laying hen rearing systems during the summer season. J. Environ. Sci. Health B. 2021, 56, 753–760. [Google Scholar] [CrossRef]
- Kim, Y.H.; Suh, H.; Kim, J.; Jung, Y.H.; Moon, K.W. Evaluation of Environmental Circumstance Within Swine and Chicken Houses in South Korea for the Production of Safe and Hygienic Animal Food Products. Korean J. Food Sci. Anim. Resour. 2008, 28, 623–628. [Google Scholar] [CrossRef]
- Ali, M.Z. Common respiratory diseases of poultry in Bangladesh: A review. SAARC J. Agric. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Hamid, A.; Ahmad, A.S.; Khan, N. Respiratory and other health risks among poultry-farm workers and evaluation of management practices in poultry farms. Braz. J. Poult. Sci. 2018, 20, 111–118. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Szablewski, T.; Nowaczewski, S.; Gornowicz, E. Chemical and microbiological hazards related to poultry farming. Med. Sr. 2018, 21, 53–63. [Google Scholar]
- Wang, K.; Shen, D.; Dai, P.; Li, C. Particulate matter in poultry house on poultry respiratory disease: A systematic review. Poult. Sci. 2023, 102, 102556. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Falkowski, N.R.; Hunter, E.M.; Ashley, S.L.; Huffnagle, G.B. The Lung Microbiota of Healthy Mice Are Highly Variable, Cluster by Environment, and Reflect Variation in Baseline Lung Innate Immunity. Am. J. Respir. Crit. Care Med. 2018, 198, 497–508. [Google Scholar] [CrossRef]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef]
- Dickson, R.P.; Schultz, M.J.; van der Poll, T.; Schouten, L.R.; Falkowski, N.R.; Luth, J.E.; Sjoding, M.W.; Brown, C.A.; Chanderraj, R.; Huffnagle, G.B.; et al. Biomarker Analysis in Septic ICU Patients (BASIC) Consortium. Lung Microbiota Predict Clinical Outcomes in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2020, 201, 555–563. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, D.N.; Ashley, S.L.; Gurczynski, S.J.; Xia, M.; Wilke, C.; Falkowski, N.R.; Norman, K.C.; Arnold, K.B.; Huffnagle, G.B.; Salisbury, M.L.; et al. Lung Microbiota Contribute to Pulmonary Inflammation and Disease Progression in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1127–1138. [Google Scholar] [CrossRef]
- D’Alessandro-Gabazza, C.N.; Yasuma, T.; Kobayashi, T.; Toda, M.; Abdel-Hamid, A.M.; Fujimoto, H.; Hataji, O.; Nakahara, H.; Takeshita, A.; Nishihama, K.; et al. Inhibition of lung microbiota-derived proapoptotic peptides ameliorates acute exacerbation of pulmonary fibrosis. Nat. Commun. 2022, 13, 1558. [Google Scholar] [CrossRef]
- Russo, C.; Colaianni, V.; Ielo, G.; Valle, M.S.; Spicuzza, L.; Malaguarnera, L. Impact of Lung Microbiota on COPD. Biomedicines 2022, 10, 1337. [Google Scholar] [CrossRef]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 42, pp. 75–93. [Google Scholar]
- Thibeault, C.; Suttorp, N.; Opitz, B. The microbiota in pneumonia: From protection to predisposition. Sci. Transl. Med. 2021, 13, eaba0501. [Google Scholar] [CrossRef]
- Budden, K.F.; Shukla, S.D.; Rehman, S.F.; Bowerman, K.L.; Keely, S.; Hugenholtz, P.; Armstrong-James, D.P.H.; Adcock, I.M.; Chotirmall, S.H.; Chung, K.F.; et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir. Med. 2019, 7, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, H.; Wang, D.; Zhao, B.; Zhang, J.; Cheng, L.; Yao, P.; Di Narzo, A.; Shen, Y.; Yu, J.; et al. Airway microbiome is associated with respiratory functions and responses to ambient particulate matter exposure. Ecotoxicol. Environ. Saf. 2019, 167, 269–277. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Tian, Y.; Hu, X. The Lung Microbiota Affects Pulmonary Inflammation and Oxidative Stress Induced by PM2.5 Exposure. Environ. Sci. Technol. 2022, 56, 12368–12379. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, Y.; Si, H.; Li, J.; Zhao, Y.; Gao, T.; Pi, J.; Zhang, R.; Chen, R.; Chen, W.; et al. The effect of real-ambient PM2.5 exposure on the lung and gut microbiomes and the regulation of Nrf2. Ecotoxicol. Environ. Saf. 2023, 254, 114702. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, F.; Liao, B.; Zhou, Y.; Li, B.; Ran, P. Exposure to ambient particulate matter alters the microbial composition and induces immune changes in rat lung. Respir. Res. 2017, 18, 143. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Zhang, M.; Feng, J. Gut microbiota dysbiosis exaggerates ammonia-induced tracheal injury Via TLR4 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 246, 114206. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell 2019, 176, 998–1013. [Google Scholar] [CrossRef]
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The lung microbiome: Progress and promise. J. Clin. Investig. 2021, 131, e150473. [Google Scholar] [CrossRef]
- Yagi, K.; Huffnagle, G.B.; Lukacs, N.W.; Asai, N. The Lung Microbiome during Health and Disease. Int. J. Mol. Sci. 2021, 22, 10872. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ji, L.; Ma, Y.; Tian, G.; Lv, K.; Yang, J. Intratumoral Microbiota-Host Interactions Shape the Variability of Lung Adenocarcinoma and Lung Squamous Cell Carcinoma in Recurrence and Metastasis. Microbiol. Spectr. 2023, 11, e0373822. [Google Scholar] [CrossRef] [PubMed]
- Natalini, J.G.; Singh, S.; Segal, L.N. The dynamic lung microbiome in health and disease. Nat. Rev. Microbiol. 2023, 21, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Greathouse, K.L.; White, J.R.; Vargas, A.J.; Bliskovsky, V.V.; Beck, J.A.; von Muhlinen, N.; Polley, E.C.; Bowman, E.D.; Khan, M.A.; Robles, A.I.; et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhu, G.; Zhu, M.; Song, J.; Cai, H.; Song, Y.; Wang, J.; Jin, M. Edaravone Attenuated Particulate Matter-Induced Lung Inflammation by Inhibiting ROS-NF-κB Signaling Pathway. Oxid. Med. Cell Longev. 2022, 2022, 6908884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Zhu, J.; Li, C.; Zhang, T.; Liu, H.; Xu, Q.; Ye, X.; Zhou, L.; Ye, L. Effect of Atmospheric PM2.5 on Expression Levels of NF-κB Genes and Inflammatory Cytokines Regulated by NF-κB in Human Macrophage. Inflammation 2018, 41, 784–794. [Google Scholar] [CrossRef]
- Hayden, F.G.; Fritz, R.; Lobo, M.C.; Alvord, W.; Strober, W.; Straus, S.E. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J. Clin. Investig. 1998, 101, 643–649. [Google Scholar] [CrossRef]
- Li, J.; An, Z.; Song, J.; Du, J.; Zhang, L.; Jiang, J.; Ma, Y.; Wang, C.; Zhang, J.; Wu, W. Fine particulate matter-induced lung inflammation is mediated by pyroptosis in mice. Ecotoxicol. Environ. Saf. 2021, 219, 112351. [Google Scholar] [CrossRef]
- Jia, H.; Liu, Y.; Guo, D.; He, W.; Zhao, L.; Xia, S. PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway. Environ. Toxicol. 2021, 36, 298–307. [Google Scholar] [CrossRef]
- Yang, D.; Xing, Y.; Song, X.; Qian, Y. The impact of lung microbiota dysbiosis on inflammation. Immunology 2020, 159, 156–166. [Google Scholar] [CrossRef]
- Ramírez-Labrada, A.G.; Isla, D.; Artal, A.; Arias, M.; Rezusta, A.; Pardo, J.; Gálvez, E.M. The Influence of Lung Microbiota on Lung Carcinogenesis, Immunity, and Immunotherapy. Trends Cancer. 2020, 6, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Claassen, S.L.; Reese, J.M.; Mysliwiec, V.; Mahlen, S.D. Achromobacter xylosoxidans infection presenting as a pulmonary nodule mimicking cancer. J. Clin. Microbiol. 2011, 49, 2751–2754. [Google Scholar] [CrossRef]
- Gómez-Cerezo, J.; Suárez, I.; Ríos, J.J.; Peña, P.; García de Miguel, M.J.; de José, M.; Monteagudo, O.; Linares, P.; Barbado-Cano, A.; Vázquez, J.J. Achromobacter xylosoxidans bacteremia: A 10-year analysis of 54 cases. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 360–363. [Google Scholar] [CrossRef]
- Iyobe, S.; Kusadokoro, H.; Takahashi, A.; Yomoda, S.; Okubo, T.; Nakamura, A.; O’Hara, K. Detection of a variant metallo-beta-lactamase, IMP-10, from two unrelated strains of Pseudomonas aeruginosa and an alcaligenes xylosoxidans strain. Antimicrob. Agents Chemother. 2002, 46, 2014–2016. [Google Scholar] [CrossRef]
- Kostadinova, S.; Ivanov, A.; Kamberov, E. Purification and some properties of phospholipase C from Achromobacter xylosoxidans. J. Chromatogr. 1991, 568, 315–324. [Google Scholar] [CrossRef]
- Traglia, G.M.; Almuzara, M.; Merkier, A.K.; Adams, C.; Galanternik, L.; Vay, C.; Centrón, D.; Ramírez, M.S. Achromobacter xylosoxidans: An emerging pathogen carrying different elements involved in horizontal genetic transfer. Curr. Microbiol. 2012, 65, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Green, S.J.; Scheller, L.F.; Marletta, M.A.; Seguin, M.C.; Klotz, F.W.; Slayter, M.; Nelson, B.J.; Nacy, C.A. Nitric oxide: Cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol. Lett. 1994, 43, 87–94. [Google Scholar] [CrossRef]
- Weinstock, D.M.; Brown, A.E. Rhodococcus equi: An emerging pathogen. Clin. Infect. Dis. 2002, 34, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Swenson, C.E.; Sadikot, R.T. Achromobacter respiratory infections. Ann. Am. Thorac. Soc. 2015, 12, 252–258. [Google Scholar] [CrossRef]
- Veschetti, L.; Boaretti, M.; Saitta, G.M.; Passarelli Mantovani, R.; Lleò, M.M.; Sandri, A.; Malerba, G. Achromobacter spp. prevalence and adaptation in cystic fibrosis lung infection. Microbiol. Res. 2022, 263, 127140. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Hauser, A.R. Pseudomonas aeruginosa virulence and antimicrobial resistance: Two sides of the same coin? Crit. Care. Med. 2014, 42, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Haller, S.; Quinten, C.; Kärki, T.; Zacher, B.; Eckmanns, T.; Abu Sin, M.; Plachouras, D.; Kinross, P.; Suetens, C. ECDC PPS study group. Healthcare-associated pneumonia in acute care hospitals in European Union/European Economic Area countries: An analysis of data from a point prevalence survey, 2011 to 2012. Euro Surveill. 2018, 23, 1700843. [Google Scholar] [CrossRef] [PubMed]
- Berube, B.J.; Rangel, S.M.; Hauser, A.R. Pseudomonas aeruginosa: Breaking down barriers. Curr. Genet. 2016, 62, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, G.I.; Sethi, S. Pseudomonas infection in chronic obstructive pulmonary disease. Future Microbiol. 2012, 7, 1129–1132. [Google Scholar] [CrossRef]
- Vidaillac, C.; Chotirmall, S.H. Pseudomonas aeruginosa in bronchiectasis: Infection, inflammation, and therapies. Expert Rev. Respir. Med. 2021, 15, 649–662. [Google Scholar] [CrossRef]
- Agaronyan, K.; Sharma, L.; Vaidyanathan, B.; Glenn, K.; Yu, S.; Annicelli, C.; Wiggen, T.D.; Penningroth, M.R.; Hunter, R.C.; Dela Cruz, C.S.; et al. Tissue remodeling by an opportunistic pathogen triggers allergic inflammation. Immunity 2022, 55, 895–911. [Google Scholar] [CrossRef]
- Lyons, J.D.; Mandal, P.; Otani, S.; Chihade, D.B.; Easley, K.F.; Swift, D.A.; Burd, E.M.; Liang, Z.; Koval, M.; Mocarski, E.S.; et al. The RIPK3 Scaffold Regulates Lung Inflammation During Pseudomonas Aeruginosa Pneumonia. Am. J. Respir. Cell Mol. Biol. 2023, 68, 150–160. [Google Scholar] [CrossRef]
- Ryan, M.P.; Pembroke, J.T. The Genus Ochrobactrum as Major Opportunistic Pathogens. Microorganisms 2020, 8, 1797. [Google Scholar] [CrossRef]
- Ko, H.M.; Jo, J.H.; Baek, H.G. Effective Identification of Ochrobactrum anthropi Isolated from Clinical Specimens. Korean J. Clin. Lab. Sci. 2020, 52, 221–228. [Google Scholar] [CrossRef]
- Kettaneh, A.; Weill, F.X.; Poilane, I.; Fain, O.; Thomas, M.; Herrmann, J.L.; Hocqueloux, L. Septic shock caused by Ochrobactrum anthropi in an otherwise healthy host. J. Clin. Microbiol. 2003, 41, 1339–1341. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, D.; Soypacaci, Z.; Sahin, I.; Bicik, Z.; Sencan, I. Ochrobactrum anthropi endocarditis and septic shock in a patient with no prosthetic valve or rheumatic heart disease: Case report and review of the literature. Jpn. J. Infect Dis. 2006, 59, 264–265. [Google Scholar]
- Crost, E.H.; Tailford, L.E.; Le Gall, G.; Fons, M.; Henrissat, B.; Juge, N. Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain-dependent. PLoS ONE 2013, 8, e76341. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, L.; Sheng, Y.; Liu, J.; Xu, Z.; Kong, W.; Tang, L.; Chen, Z. Airway microbiota in children with bronchial mucus plugs caused by Mycoplasma pneumoniae pneumonia. Respir. Med. 2020, 170, 105902. [Google Scholar] [CrossRef] [PubMed]
- McCormack, U.M.; Curião, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F.; Magowan, E.; Metzler-Zebeli, B.U.; Berry, D.; et al. Exploring a Possible Link between the Intestinal Microbiota and Feed Efficiency in Pigs. Appl. Environ. Microbiol. 2017, 83, e00380-17. [Google Scholar] [CrossRef]




| Target Gene | Primer Sequence (5′ to 3′) | Length | Login ID |
|---|---|---|---|
| GAPDH | F:TGAAAGTCGGAGTCAACGGAT | 230 bp | NM_204305.1 |
| R:ACGCTCCTGGAAGATAGTGAT | |||
| IL-1β | F:AGAAGAAGCCTCGCCTGGAT | 131 bp | NM_204524.1 |
| R:CCTCCGCAGCAGTTTGGT | |||
| IFN-γ | F:AGTCAAAGCCGCACATCAAACAC | 133 bp | NM_205149.1 |
| R:CGCTGGATTCTCAAGTCGTTCATC | |||
| TNF-α | F:GGACAGCCTATGCCAACAAG | 168 bp | NM_204267.1 |
| R:ACACGACAGCCAAGTCAACG | |||
| IL-6 | F:CCTCCTCGCCAATCTGAAGTCA | 210 bp | NM_204628.1 |
| R:AACGGAACAACACTGCCATCTG | |||
| IL-10 | F:ATCCAACTGCTCAGCTCTGAACTG | 101 bp | NM_001004414.2 |
| R:GGCAGGACCTCATCTGTGTAGAAG |
| Steps | Kit or Instrument | Measuring Methods |
|---|---|---|
| 1. DNA extraction | E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA), | Referring to kit’s instructions |
| 2. DNA detection | determined by 1.0% agarose gel electrophoresis and a NanoDrop® ND-2000 spectrophotometer (Thermo Scientific Inc., Waltham, MA, USA) | According to instrument’s instructions |
| 3. PCR amplification | ABI GeneAmp® 9700 PCR thermocycler (ABI, Los Angeles, CA, USA) | V3-V4 of the bacterial 16S rRNA gene: primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′); the PCR reaction mixture and PCR amplification cycling conditions were based on our previous research (Zhou et al., 2022 [28]) |
| 4. Purification and quantification of PCR products | According to kit’s instructions | |
| 5. Illumina MiSeq sequence | Referring to the previous research (Zhou et al., 2022 [28]) | |
| 6. Bioinformatic analysis | Majorbio Cloud platform (https://cloud.majorbio.com, accessed on 24 November 2022) | Alpha diversity indices, principal coordinate analysis (PCoA), spearman’s correlation, etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Xu, B.; Wang, L.; Zhang, C.; Li, S. Fine Particulate Matter Perturbs the Pulmonary Microbiota in Broiler Chickens. Animals 2023, 13, 2862. https://doi.org/10.3390/ani13182862
Zhou Y, Xu B, Wang L, Zhang C, Li S. Fine Particulate Matter Perturbs the Pulmonary Microbiota in Broiler Chickens. Animals. 2023; 13(18):2862. https://doi.org/10.3390/ani13182862
Chicago/Turabian StyleZhou, Ying, Bin Xu, Linyi Wang, Chaoshuai Zhang, and Shaoyu Li. 2023. "Fine Particulate Matter Perturbs the Pulmonary Microbiota in Broiler Chickens" Animals 13, no. 18: 2862. https://doi.org/10.3390/ani13182862
APA StyleZhou, Y., Xu, B., Wang, L., Zhang, C., & Li, S. (2023). Fine Particulate Matter Perturbs the Pulmonary Microbiota in Broiler Chickens. Animals, 13(18), 2862. https://doi.org/10.3390/ani13182862





