Relationship between Microflora Changes and Mammary Lipid Metabolism in Dairy Cows with Mastitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Determination of Milk Quality
2.4. DNA Extraction and 16S rDNA Sequences
2.5. Bioinformatics Analysis
2.6. Non-Targeted Lipidomics Analysis
2.7. Statistical Analysis
3. Results
3.1. The Effects on Milk Performance
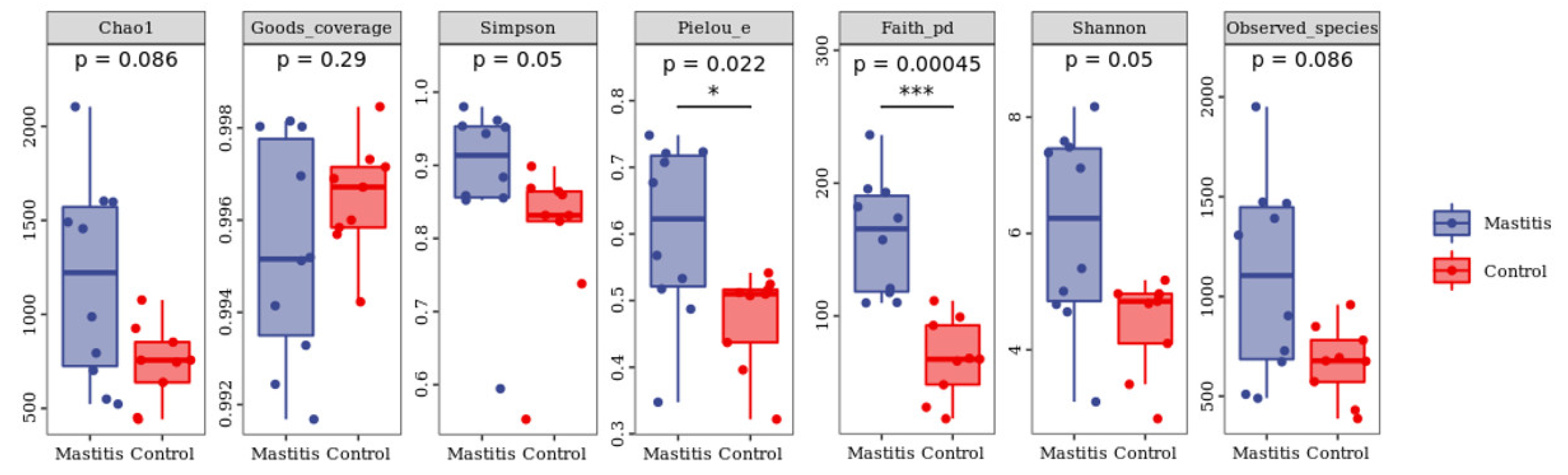
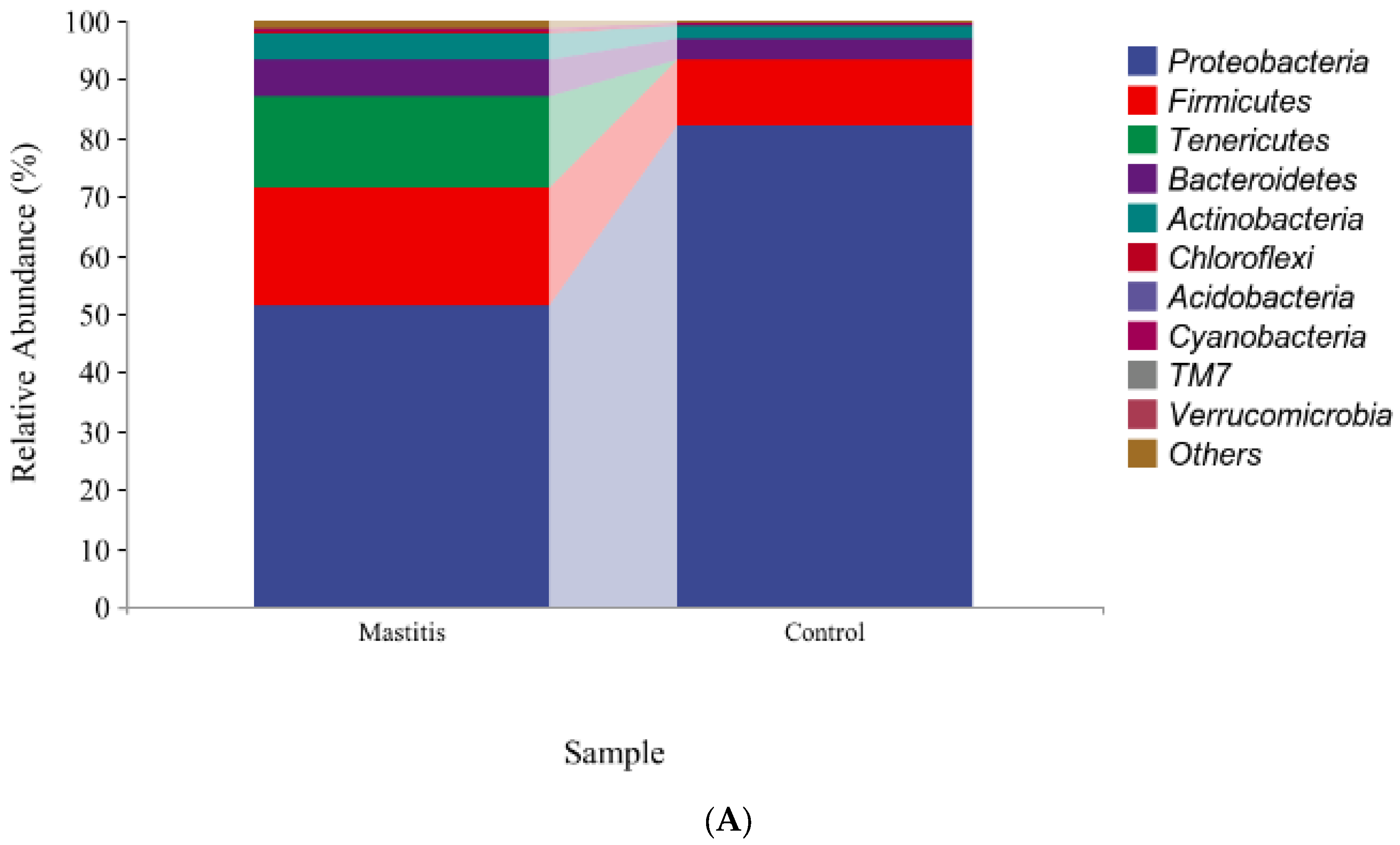
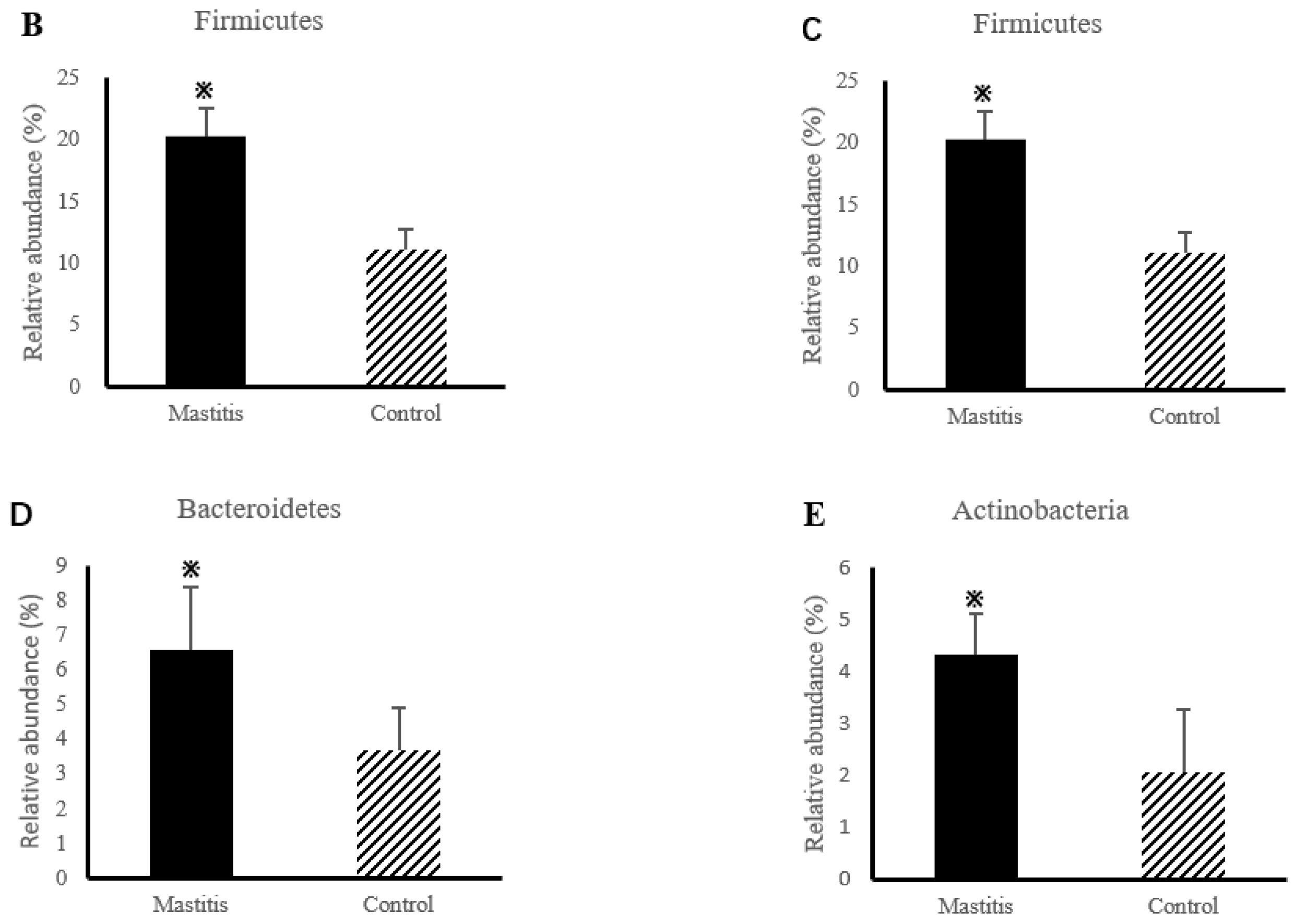
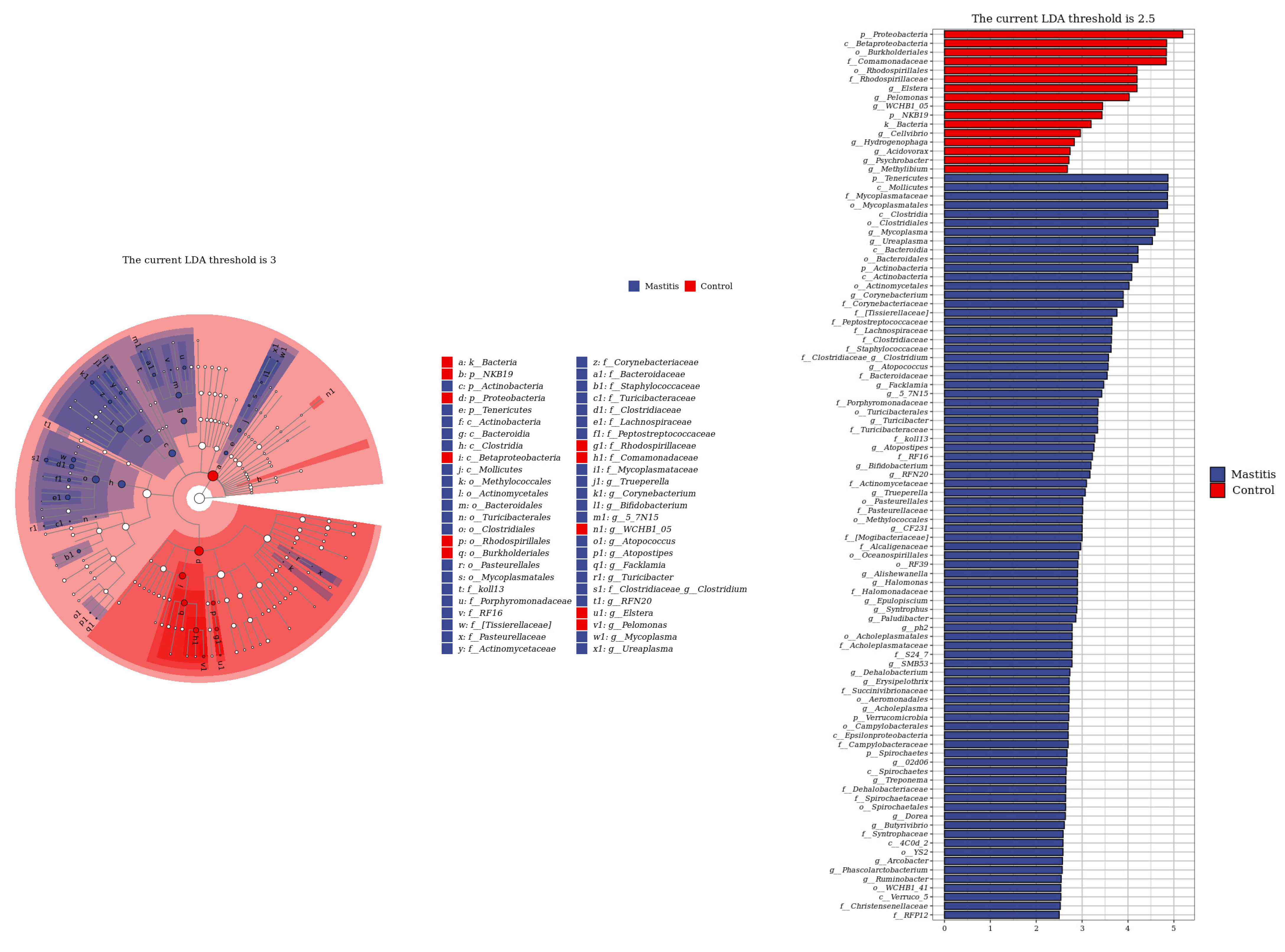
3.2. The Results of Milk-Associated Microbiome
3.3. Non-Targeted Metabolome Profiles of the Milk
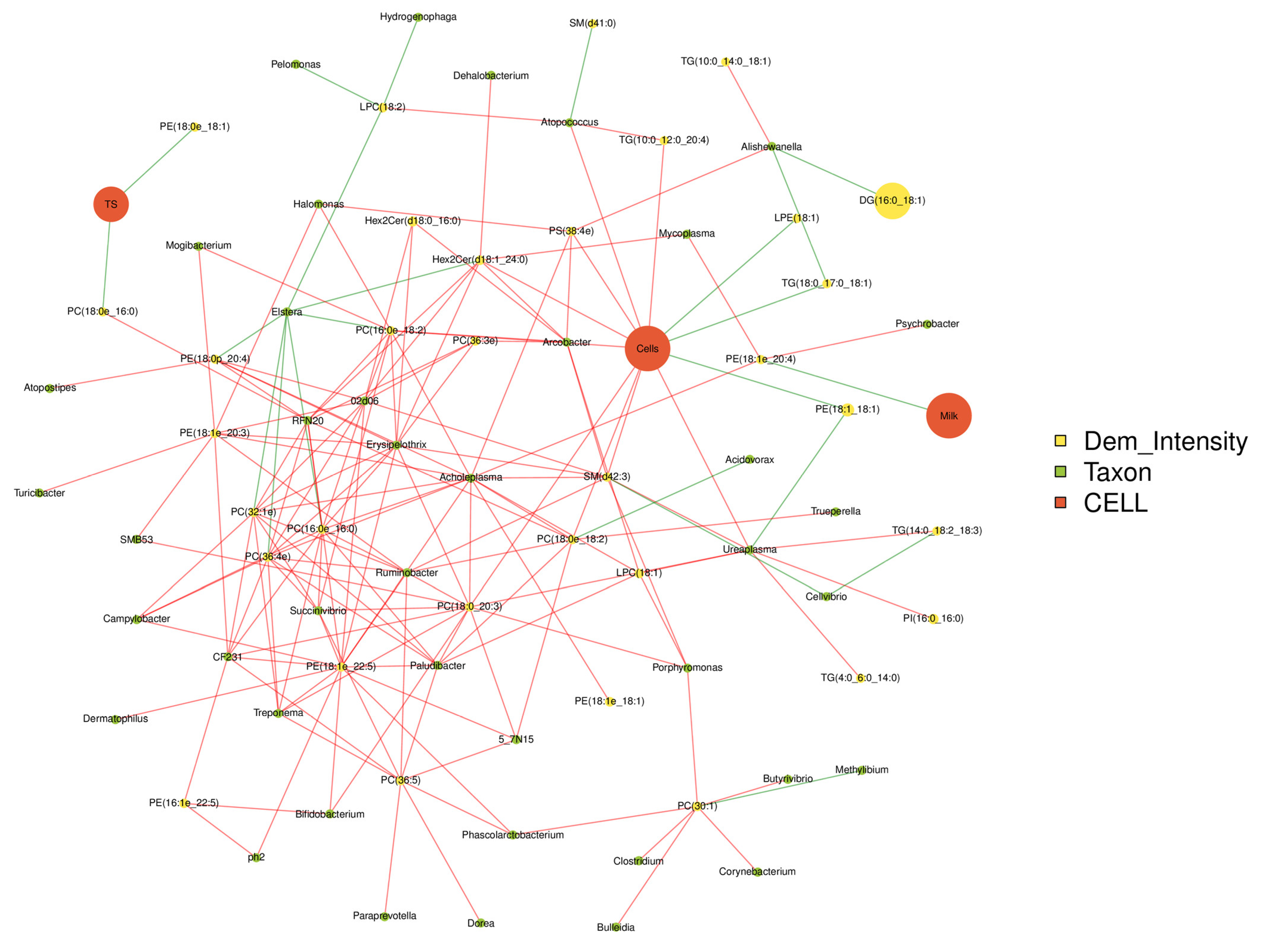
3.4. Correlation Analysis among the Milk Qualities, Microbiome and Lipidomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Chen, X.; Chen, Z. Identification of Key Candidate Genes in Dairy Cow in Response to Escherichia coli Mastitis by Bioinformatical Analysis. Front. Genet 2019, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Braem, G.; De Vliegher, S.; Verbist, B.; Heyndrickx, M.; Leroy, F.; De Vuyst, L. Culture-independent exploration of the teat apex microbiota of dairy cows reveals a wide bacterial species diversity. Vet. Microbiol. 2012, 157, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Perez-Casal, J.; Prysliak, T.; Potter, A.A. A GapC chimera retains the properties of the Streptococcus uberis wild-type GapC protein. Protein Expr. Purif. 2004, 33, 288–296. [Google Scholar] [CrossRef]
- de Boyer des Roches, A.; Faure, M.; Lussert, A.; Herry, V.; Rainard, P.; Durand, D.; Foucras, G. Behavioral and patho-physiological response as possible signs of pain in dairy cows during Escherichia coli mastitis: A pilot study. J. Dairy Sci. 2017, 100, 8385–8397. [Google Scholar] [CrossRef] [PubMed]
- Snel, G.G.; Monecke, S.; Ehricht, R.; Piccinini, R. Molecular characteristics of bap-positive Staphylococcus aureus strains from dairy cow mastitis. J. Dairy Res. 2015, 82, 312–316. [Google Scholar] [CrossRef]
- Bolton, A.; Song, X.M.; Willson, P.; Fontaine, M.C.; Potter, A.A.; Perez-Casal, J. Use of the surface proteins GapC and Mig of Streptococcus dysgalactiae as potential protective antigens against bovine mastitis. Can. J. Microb. 2004, 50, 423–432. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Li, B.Q.; Zhu, J.G. Construction of scFv that bind both fibronectin-binding protein A and clumping factor A of Stapylococcus aureus. Res. Vet. Sci. 2015, 100, 109–114. [Google Scholar] [CrossRef]
- Jamali, H.; Krylova, K.; Aider, M. Identification and frequency of the associated genes with virulence and antibiotic resistance of Escherichia coli isolated from cow’s milk presenting mastitis pathology. Anim. Sci. J. 2018, 89, 1701–1706. [Google Scholar] [CrossRef]
- Mu, T.; Hu, H.H.; Ma, Y.F.; Feng, X.F.; Zhang, J.; Gu, Y.L. Regulation of key genes for milk fat synthesis in ruminants. Front. Nutr. 2021, 8, 765147. [Google Scholar] [CrossRef]
- Liu, W.L.; Hou, Y.Y.; Jin, Y.Y.; Wang, Y.P.; Xu, X.K.; Han, J.Z. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends Food Sci. Tech. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Zhang, X.H.; Yu, J.H.; Yuan, T.L.; Zhao, P.; Tao, G.J.; Guo, Q.Z.; Du, Z.X. Comprehensive lipidomic analysis of milk polar lipids using ultraperformance supercritical fluid chromatography-mass spectrometry. Food Chem. 2022, 393, 133336. [Google Scholar] [CrossRef]
- Shi, Y.D.; Sun, G.Q.; Zhang, Z.G.; Deng, X.; Kang, X.H.; Liu, Z.D.; Ma, Y.; Sheng, Q.H. The chemical composition of human milk from Inner Mongolia of China. Food Chem. 2011, 127, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Zhao, T.Q.; Ma, Y.Y.; Liang, Y.; Lu, X.B.; Yang, Z.P. Effects of Different pasture sizes and different seasons on the Quality of Holstein Milk. Chinese J. Anim. Sci. 2022, 58, 252–257. [Google Scholar] [CrossRef]
- Thomas, V.; de Jong, A.; Moyaert, H.; Simjee, S.; El Garch, F.; Morrissey, I.; Mariona, H.; Vallé, M. Antimicrobial susceptibility monitoring of mastitis pathogens isolated from acute cases of clinical mastitis in dairy cows across Europe: VetPath results. Int. J Antimicrob. Agents. 2015, 46, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Audano, M.; Addis, M.F.; Lecchi, C.; Ghaffari, M.H.; Albertini, M.; Tangorra, F.; Piccinini, R.; Caruso, D.; Mitro, N.; et al. The untargeted lipidomic profile of quarter milk from dairy cows with subclinical intramammary infection by non-aureus staphylococci. J. Dairy Sci. 2021, 104, 10268–10281. [Google Scholar] [CrossRef]
- Toquet, M.; Gómez-Martín, Á.; Bataller, E. Review of the bacterial composition of healthy milk, mastitis milk and colostrum in small ruminants. Res. Vet. Sci. 2021, 140, 1–5. [Google Scholar] [CrossRef]
- Kong, Z.; Li, B.; Zhou, C.; He, Q.; Zheng, Y.; Tan, Z. Multi-Omics Analysis of Mammary Metabolic Changes in Dairy Cows Exposed to Hypoxia. Front Vet. Sci. 2021, 8, 764135. [Google Scholar] [CrossRef]
- NY/T 34–2004. Ministry of Agriculture. Feeding Standard of Dairy Cattle. Ministry of Agriculture of China: Beijing, China, 2004.
- Bailén, M.; Bressa, C.; Larrosa, M.; González-Soltero, R. Bioinformatic strategies to address limitations of 16rRNA short-read amplicons from different sequencing platforms. J. Microbiol. Methods 2020, 169, 105811. [Google Scholar] [CrossRef]
- Qin, S.J.; Zeng, H.X.; Wu, Q.Z.; Li, Q.Q.; Zeeshan, M.; Ye, L.Z.; Jiang, Y.; Zhang, R.; Jiang, X.H.; Li, M.; et al. An integrative analysis of lipidomics and transcriptomics in various mouse brain regions in response to real-ambient PM2.5 exposure. Sci. Total Environ. 2023, 895, 165112. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Han, L.; Li, T.; Du, M.; Chang, R.; Zhan, B.; Mao, X. Beneficial Effects of Potentilla discolor Bunge Water Extract on Inflammatory Cytokines Release and Gut Microbiota in High-Fat Diet and Streptozotocin-Induced Type 2 Diabetic Mice. Nutrients 2019, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y. The Harm of Cow Mastitis and the Preventive Measures of Integrated Traditional Chinese and Western Medicine. Anim. Husb. Feed Sci. 2013, 4, 121–122. [Google Scholar]
- Fernandes, L.; Guimaraes, I.; Noyes, N.R.; Caixeta, L.S.; Machado, V.S. Effect of subclinical mastitis detected in the first month of lactation on somatic cell count linear scores, milk yield, fertility, and culling of dairy cows in certified organic herds. J. Dairy Sci. 2020, 104, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Rošić, N.; Kule, J.; Horvati´, A.; Gelemanovic, A.; Galen, A.; Ljubić, B.B.; Benić, M.; Stevanović, V.; Mrljak, V.; et al. Milk and serum proteomes in subclinical and clinical mastitis in Simmental cows. J. Proteomics 2021, 244, 104277. [Google Scholar] [CrossRef] [PubMed]
- Martí-De Olives, A.; Peris, C.; Molina, M.P. Effect of subclinical mastitis on the yield and cheese-making properties of ewe’s milk. Small Rumin. Res. 2020, 184, 106044. [Google Scholar] [CrossRef]
- Villalobos, J.C.; Sigler, A.I.G.; Oliete, B.; Sánchez, R.A.; Jiménez, L.; Sánchez, N.N.; Marín, A.L.M. Relationship of somatic cell count and composition and coagulation properties of ewe’s milk. Mljekarstvo/Dairy 2015, 65, 138–143. [Google Scholar] [CrossRef]
- Vasquez, A.K.; Ganda, E.K.; Capel, M.B.; Eicker, S.; Virkler, P.D.; Bicalho, R.C.; Nydam, D.V. The microbiome of Escherichia coli and culture-negative nonsevere clinical mastitis: Characterization and associations with linear score and milk production. J. Dairy Sci. 2019, 102, 578–594. [Google Scholar] [CrossRef]
- Adkins, P.R.F.; Middleton, J.R. Methods for diagnosing mastitis. Vet. Clin. North Am. Food Anim. Pract. 2018, 34, 479–491. [Google Scholar] [CrossRef]
- Pazzola, M.; Cipolat-Gotet, C.; Bittante, G.; Cecchinato, A.; Dettori, M.L.; Vacca, G.M. Phenotypic and genetic relationships between indicators of the mammary gland health status and milk composition, coagulation, and curd firming in dairy sheep. J. Dairy Sci. 2018, 101, 3164–3175. [Google Scholar] [CrossRef]
- Goncalves, J.L.; Cue, R.I.; Botaro, B.G.; Horst, J.A.; Valloto, A.A.; Santos, M.V. Milk losses associated with somatic cell counts by parity and stage of lactation. J. Dairy Sci. 2018, 101, 4357–4366. [Google Scholar] [CrossRef]
- Deng, Z.; Lam, T.J.G.M.; Hogeveen, H.; Spaninks, M.; Heij, N.; Postema, M.; Werven, T.V.; Koop, G. Antimicrobial use and farmers’ attitude toward mastitis treatment on dairy farms with automatic or conventional milking systems. J. Dairy Sci. 2020, 103, 7302–7314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wu, K.; Bao, L.; Chen, L.; Feng, L.; Liu, Z.; Wang, Y.; Fu, Y.; Zhang, N.; Hu, X. Kynurenic acid protects against mastitis in mice by ameliorating inflammatory responses and enhancing blood-milk barrier integrity. Mol. Immunol. 2021, 137, 134–144. [Google Scholar] [CrossRef]
- Lima, S.F.; Teixeira, A.G.V.; Lima, F.S.; Ganda, E.K.; Higgins, C.H.; Oikonomou, G.; Bicalho, R.C. The bovine colostrum microbiome and its association with clinical mastitis. J. Dairy Sci. 2017, 100, 3031–3042. [Google Scholar] [CrossRef]
- Mateus-Barros, E.; Meneghine, A.K.; Bagatini, I.L.; Fernandes, C.C.; Kishi, L.T.; Vieira, A.A.H.; Sarmento, H. Comparison of two DNA extraction methods widely used in aquatic microbial ecology. J. Microbiol. Methods 2019, 159, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kunjadia, P.; Koringa, P.; Joshi, C.; Kunjadiya, A. Microbiological profiles in clinical and subclinical cases of mastitis in milking Jafarabadi buffalo. Res. Vet. Sci. 2019, 125, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Bisinotto, R.S.; Lima, S.F.; Kronauer, K.; Decter, D.H.; Oikonomou, G.; Schukken, Y.H.; Bicalho, R.C. Longitudinal metagenomic profiling of bovine milk to assess the impact of intramammary treatment using a third-generation cephalosporin. Sci. Rep. 2016, 6, 37565. [Google Scholar] [CrossRef]
- Rainard, P. Mammary microbiota of dairy ruminants: Fact or fiction? Vet. Res. 2017, 48, 25. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Ahir, V.B.; Koringa, P.G.; Jakhesara, S.J.; Rank, D.N.; Nauriyal, D.S.; Kunjadia, A.P.; Joshi, C.G. Milk microbiome signatures of subclinical mastitis-affected cattle analysed by shotgun sequencing. J. Appl. Microbiol. 2012, 112, 639–650. [Google Scholar] [CrossRef]
- Brouwer de Koning, I.M.; Lemson, A.; Renders, N.H.M.; Bessems, M.; Nooijen, P.T.G.A.; Draaisma, W.A.; Bosscha, K. Inflammatory granulomatous mastitis caused by Corynebacterium kroppenstedtii: A clinical challenge: Challenge of C. kroppenstedtii induced mastitis. Clin. Infect. Pract. 2022, 15, 100147. [Google Scholar] [CrossRef]
- Rodrogues, M.X.; Lima, S.F.; Canniatti-Brazaca, S.G.; Bicalho, R.C. The microbiome of bulk tank milk: Characterization and associations with somatic cell count and bacterial count. J. Dairy Sci. 2017, 100, 2536–2552. [Google Scholar] [CrossRef]
- Oliveira, L.; Hulland, C.; Ruegg, P.L. Characterization of clinical mastitis occurring in cows on 50 large dairy herds in Wisconsin. J. Dairy Sci. 2013, 96, 7538–7549. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Gaeta, N.; Sipka, A.; Pomeroy, B.; Oikonomou, G.; Schukken, Y.H.; Bicalho, R.C. Normal milk microbiome is reestablished following experimental infection with Escherichia coli independent of intramammary antibiotic treatment with a third-generation cephalosporin in bovines. Microbiome 2017, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Alba, C.; Aparicio, M.; Arroyo, R.; Jiménez, L.; Fernández, L.; Arias, R.; Rodríguez, J.M. Metataxonomic and immunological analysis of milk from ewes with or without a history of mastitis. J. Dairy Sci. 2019, 102, 9298–9311. [Google Scholar] [CrossRef] [PubMed]
- Dalanezi, F.M.; Joaquim, S.F.; Guimaraes, F.F.; Guerra, S.T.; Lopes, B.C.; Schmidt, E.M.S.; Cerri, R.L.A.; Langoni, H. Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy Sci. 2020, 103, 3648–3655. [Google Scholar] [CrossRef]
- Fox, L.K. Mycoplasma mastitis: Causes, transmission, and control. Vet. Clin. North. Am. Food Anim. Pract. 2012, 28, 225–237. [Google Scholar] [CrossRef]
- Horwood, P.F.; Schibrowski, M.I.; Fowler, E.V.; Gibson, J.S.; Barnes, T.S.; Mahony, T.J. Is Mycoplasma bovis a missing component of the bovine respiratory disease complex in Australia. Aust. Vet. J. 2014, 92, 185–191. [Google Scholar] [CrossRef]
- Qu, K.C. 1H Nuclear Magnetic Resonance-based Metabonomics on Early-stage Diagnosis of Dairy Cow Mastitis. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2020. [Google Scholar]
- Wang, X.M.; Li, T.; Fan, Q.Y.; Li, X.M.; Liu, W.W.; Dai, X.F. Research progress of Chinese herbal medicine for controlling recessive mastitis in dairy cows. China Dairy Cattle 2022, 3, 26–33. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Poulsen, N.A.; Larsen, L.B.; Bertram, H.C. Nuclear magnetic resonance metabonomics reveals strong association between milk metabolites and somatic cell count in bovine milk. J. Dairy Sci. 2013, 96, 290–299. [Google Scholar] [CrossRef]
- Huang, Y.X. Nontargeted metabolomics of bovine mammary epithelial cells induced by LPS. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2018. [Google Scholar]
- Zhao, L.; Zhang, J.; Ge, W.; Wang, J. Comparative Lipidomics Analysis of Human and Ruminant Milk Reveals Variation in Composition and Structural Characteristics. J. Agric. Food Chem. 2022, 70, 8994–9006. [Google Scholar] [CrossRef]
- Moradi, M.; Khalid Omer, A.; Razavi, R.; Valipour, S.; Guimaraes, J.T. The relationship between milk somatic cell count and cheese production, quality and safety: A review. Int. Dairy J. 2021, 113, 104884–104893. [Google Scholar] [CrossRef]
- Gross, J.J.; Grossen-Rösti, L.; Wall, S.K.; Wellnitz, O.; Bruckmaier, R.M. Metabolic status is associated with the recovery of milk somatic cell count and milk secretion after lipopolysaccharide-induced mastitis in dairy cows. J. Dairy Sci. 2019, 103, 5604–5615. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; El, R.R. Arachidonic acid: Physiological roles and potential health benefits-A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F. Leukocyte Lipid Bodies—Structure and Function as “Eicosasomes”. Trans. Am. Clin. Climatol. Assoc. 2016, 127, 328–340. [Google Scholar]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N.J. The essentiality of arachidonic acid in infant development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. BBA-Mol. Cell Biol. 2015, 1851, 414–421. [Google Scholar] [CrossRef]
- Chen, I.J.; Hee, S.W.; Liao, C.H.; Lin, S.Y.; Su, L.; Shun, C.T.; Chuang, L.M. Targeting the 15-keto-PGE2-PTGR2 axis modulates systemic inflammation and survival in experimental sepsis. Free Radic. Biol. Med. 2017, 115, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Tan, K.S.; Beng, H.N.; Liu, F.; Huang, J.; Kuai, Y.; Zhang, R.; Tan, W. Protective effect of isosteviol sodium against LPS-induced multiple organ injury by regulating of glycerophospholipid metabolism and reducing macrophage-driven inflammation. Pharmacol. Res. 2021, 172, 105781–105794. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Kinnun, J.J.; Cavazos, A.T.; Canner, S.W.; Shaikh, S.R.; Feller, S.E.; Wassalla, S.R. All n-3 PUFA are not the same: MD simulations reveal differences in membrane organization for EPA, DHA and DPA. BBA-Biomembr. 2018, 1860, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.J.; Hoeferlin, L.A.; Chalfant, C.E. Lipidomics in translational research and the clinical significance of lipid-based biomarkers. Transl. Res. 2017, 189, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef]
- Mendes-Frias, A.; Santos-Lima, B.; Furtado, D.Z.S.; Ruperez, F.J.; Assunção, N.A.; Matias, M.J.; Gomes, V.; Gaifem, J.; Barbas, C.; Castro, A.G.; et al. Dysregulation of glycerophospholipid metabolism during Behçet’s disease contributes to a pro-inflammatory phenotype of circulating monocytes. J. Transl. Autoimmun. 2020, 3, 100056–100065. [Google Scholar] [CrossRef]



| Items | Content (%) | Reference |
|---|---|---|
| Diet composition | [17] | |
| Chinese leymus | 37.5 | |
| Corn silage | 22.5 | |
| Corn | 15.2 | |
| Wheat bran | 5.3 | |
| Soybean meal | 9.2 | |
| DDGS | 8.4 | |
| Calcium hydrophosphate | 1.4 | |
| Premix1 | 0.5 | |
| Nutrient composition | ||
| CP | 13.1 | |
| NDF | 39.6 | |
| Ca | 0.6 | |
| P | 0.4 | |
| NEL2, MJ/kg DM | 5.4 |
| Item | Con | Mas | P |
|---|---|---|---|
| Milk production (kg) | 18.54 ± 1.290 | 15.46 ± 1.629 | 0.058 |
| Milk fat (%) | 3.74 ± 0.293 | 3.35 ± 0.351 | 0.217 |
| Milk protein (%) | 3.57 ± 0.146 | 3.57 ± 0.509 | 0.986 |
| Lactose (%) | 5.00 ± 0.136 | 4.71 ± 0.519 | 0.118 |
| Total solids (%) | 12.88 ± 0.733 | 12.23 ± 1.658 | 0.271 |
| SCC (1000/mL) | 21.25 ± 8.74 | 8380.0 ± 1885.96 | 0.001 |
| Urea nitrogen (mg/dL) | 25.11 ± 6.45 | 26.98 ± 9.39 | 0.610 |
| DIM(d) | 308.30 ± 3.529 | 308.80 ± 2.781 | 0.729 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Kong, Z.; Yang, B.; He, F.; Huan, C.; Li, J.; Yi, K. Relationship between Microflora Changes and Mammary Lipid Metabolism in Dairy Cows with Mastitis. Animals 2023, 13, 2773. https://doi.org/10.3390/ani13172773
Luo Y, Kong Z, Yang B, He F, Huan C, Li J, Yi K. Relationship between Microflora Changes and Mammary Lipid Metabolism in Dairy Cows with Mastitis. Animals. 2023; 13(17):2773. https://doi.org/10.3390/ani13172773
Chicago/Turabian StyleLuo, Yang, Zhiwei Kong, Bin Yang, Fang He, Cheng Huan, Jianbo Li, and Kangle Yi. 2023. "Relationship between Microflora Changes and Mammary Lipid Metabolism in Dairy Cows with Mastitis" Animals 13, no. 17: 2773. https://doi.org/10.3390/ani13172773
APA StyleLuo, Y., Kong, Z., Yang, B., He, F., Huan, C., Li, J., & Yi, K. (2023). Relationship between Microflora Changes and Mammary Lipid Metabolism in Dairy Cows with Mastitis. Animals, 13(17), 2773. https://doi.org/10.3390/ani13172773






