Cleft Lip and Palate in Four Full-Sib Puppies from a Single Litter of Staffordshire Bull Terrier Dogs: An Anatomical and Genetic Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anatomical Evaluation
2.3. Cytogenetic Analysis
2.4. Molecular Genetic Analysis
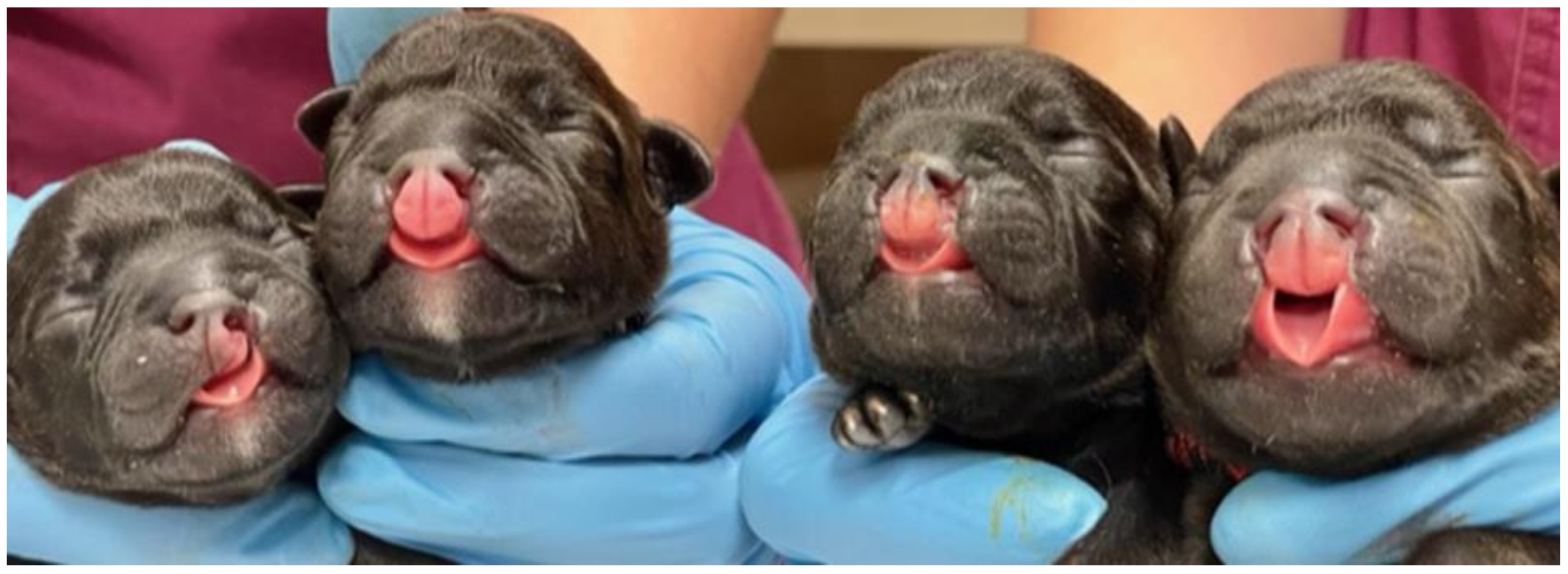
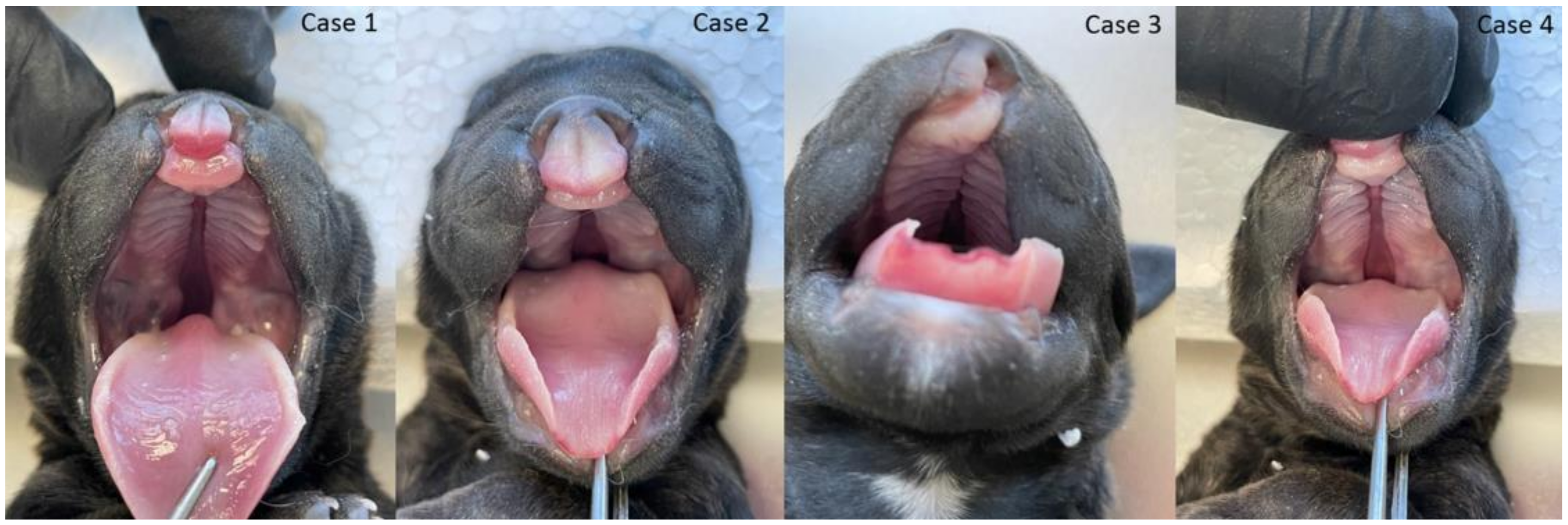
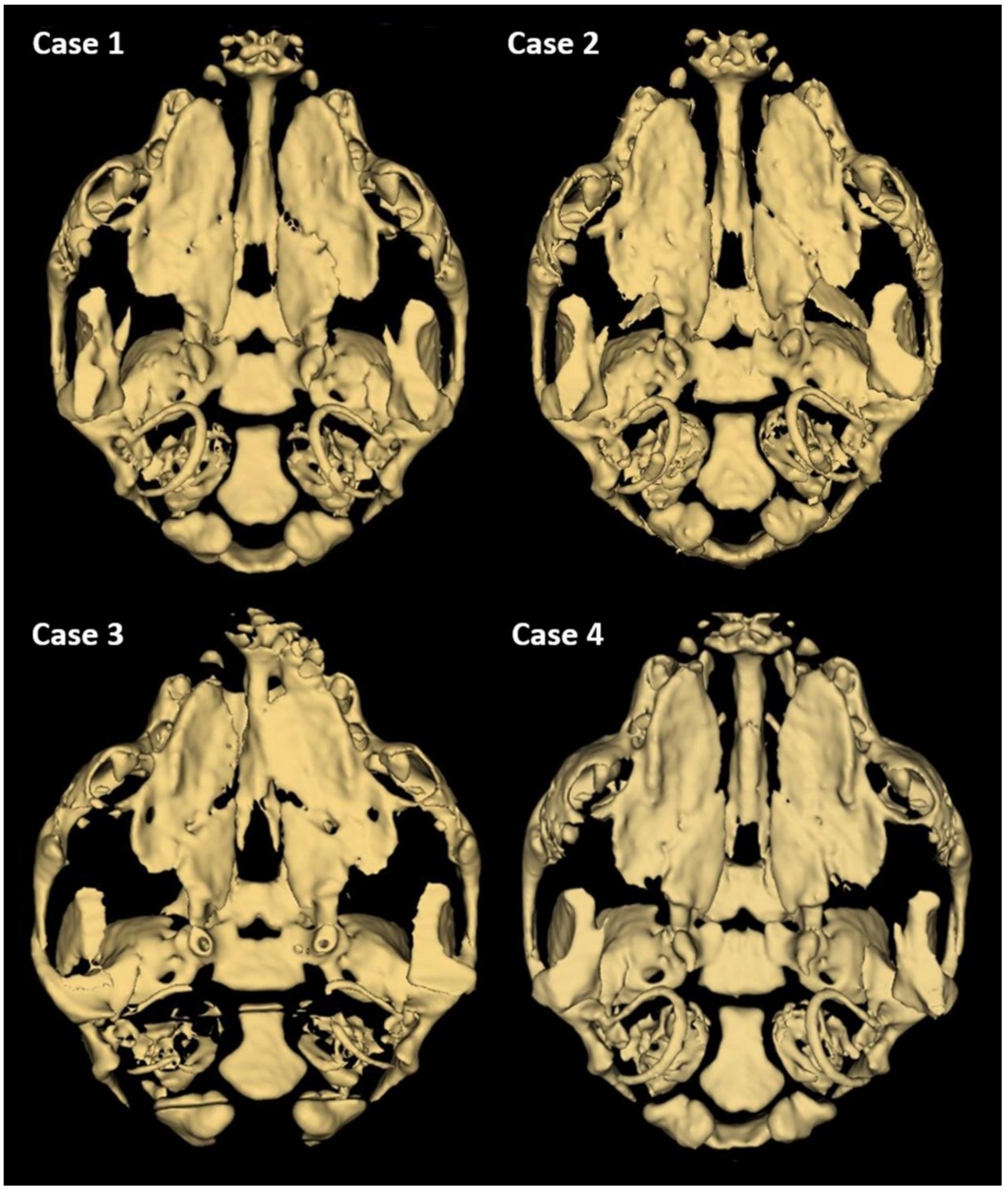
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roman, N.; Carney, P.C.; Fiani, N.; Peralta, S. Incidence Patterns of Orofacial Clefts in Purebred Dogs. PLoS ONE 2019, 14, e0224574. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Avila, J.R.; Daack-Hirsch, S.; Dragan, E.; Félix, T.M.; Rahimov, F.; Harrington, J.; Schultz, R.R.; Watanabe, Y.; Johnson, M.; et al. Medical Sequencing of Candidate Genes for Nonsyndromic Cleft Lip and Palate. PLoS Genet. 2005, 1, e64. [Google Scholar] [CrossRef]
- Wolf, Z.T.; Brand, H.A.; Shaffer, J.R.; Leslie, E.J.; Arzi, B.; Willet, C.E.; Cox, T.C.; McHenry, T.; Narayan, N.; Feingold, E.; et al. Genome-Wide Association Studies in Dogs and Humans Identify ADAMTS20 as a Risk Variant for Cleft Lip and Palate. PLoS Genet. 2015, 11, e1005059. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, J.J.; Mulvihill, C.G.; Priester, W.A. Cleft Palate in Domestic Animals: Epidemiologic Features. Teratology 1980, 21, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.H.; Ihle, S.L. Small Animal Internal Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Vaiman, A.; Fritz, S.; Beauvallet, C.; Boussaha, M.; Grohs, C.; Daniel-Carlier, N.; Relun, A.; Boichard, D.; Vilotte, J.-L.; Duchesne, A. Mutation of the MYH3 Gene Causes Recessive Cleft Palate in Limousine Cattle. Genet. Sel. Evol. 2022, 54, 71. [Google Scholar] [CrossRef]
- Shupe, J.L.; James, L.F.; Binns, W.; Keeler, R.F. Cleft Palate in Cattle. Cleft Palate J. 1968, 5, 346–355. [Google Scholar]
- Lobodzinska, A.; Gruszczynska, J.; Max, A.; Bartyzel, B.J.; Mikula, M.; Mikula, I., Jr.; Grzegrzolka, B. Cleft Palate in the Domestic Dog, Canis Lupus Familiaris–Etiology, Pathophysiology, Diagnosis, Prevention, and Treatment. Acta Sci. Polon. Zootech. 2014, 13, 5–28. [Google Scholar]
- Estevam, M.V.; Beretta, S.; Smargiassi, N.F.; Apparício, M.; Toniollo, G.H.; Pereira, G.T. Congenital Malformations in Brachycephalic Dogs: A Retrospective Study. Front. Vet. Sci. 2022, 9, 981923. [Google Scholar] [CrossRef]
- Moura, E.; Pimpão, C.T.; Almasri, M. Cleft Lip and Palate in the Dog: Medical and Genetic Aspects. In Designing Strategies for Cleft Lip and Palate Care; InTech: Rijeka, Croatia, 2017; p. 143. [Google Scholar]
- Kelly, K.M.; Bardach, J. Biologic Basis of Cleft Palate and Palatal Surgery. In Oral and Maxillofacial Surgery in Dogs and Cats; Elsevier: Amsterdam, The Netherlands, 2012; pp. 343–350. [Google Scholar]
- Martinelli, M.; Palmieri, A.; Carinci, F.; Scapoli, L. Non-Syndromic Cleft Palate: An Overview on Human Genetic and Environmental Risk Factors. Front. Cell Dev. Biol. 2020, 8, 592271. [Google Scholar] [CrossRef]
- Ludwig, K.; Böhmer, A.C.; Bowes, J.; Nikolić, M.; Ishorst, N.; Wyatt, N.; Hammond, N.; Gölz, L.; Thieme, F.; Barth, S.; et al. Imputation of orofacial clefting data identifies novel risk loci and sheds light on the genetic background of cleft lip ± cleft palate and cleft palate only. Hum. Mol. Genet. 2017, 26, 829–842. [Google Scholar] [CrossRef]
- Awotoye, W.; Mossey, P.A.; Hetmanski, J.B.; Gowans, L.J.J.; Eshete, M.A.; Adeyemo, W.L.; Alade, A.; Zeng, E.; Adamson, O.; Naicker, T.; et al. Whole-Genome Sequencing Reveals De-Novo Mutations Associated with Nonsyndromic Cleft Lip/Palate. Sci. Rep. 2022, 12, 11743. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Z.T.; Leslie, E.J.; Arzi, B.; Jayashankar, K.; Karmi, N.; Jia, Z.; Rowland, D.J.; Young, A.; Safra, N.; Sliskovic, S.; et al. A LINE-1 Insertion in DLX6 Is Responsible for Cleft Palate and Mandibular Abnormalities in a Canine Model of Pierre Robin Sequence. PLoS Genet. 2014, 10, e1004257. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria, 6th ed.; Editorial Committee: Hanover, Germany, 2017; pp. 73–147. [Google Scholar]
- Pankowski, F.; Paśko, S.; Max, A.; Szal, B.; Dzierzęcka, M.; Gruszczyńska, J.; Szaro, P.; Gołębiowski, M.; Bartyzel, B.J. Computed Tomographic Evaluation of Cleft Palate in One-Day-Old Puppies. BMC Vet. Res. 2018, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Jiao, S.; He, M.; Lin, D.; Zuo, H.; Han, J.; Sun, Y.; Cao, G.; Chen, Z.; Liu, H. Chromatin Conformation of Human Oral Epithelium Can Identify Orofacial Cleft Missing Functional Variants. Int. J. Oral Sci. 2022, 14, 43. [Google Scholar] [CrossRef]
- Lan, Y.; Jiang, R. Chapter Two—Mouse Models in Palate Development and Orofacial Cleft Research: Understanding the Crucial Role and Regulation of Epithelial Integrity in Facial and Palate Morphogenesis. Curr. Top. Dev. Biol. 2022, 148, 13–50. [Google Scholar] [PubMed]
- Conze, T.; Ritz, I.; Hospes, R.; Wehrend, A. Management of Cleft Palate in Puppies Using A Temporary Prosthesis: A Report of Three Cases. Vet. Sci. 2018, 5, 61. [Google Scholar] [CrossRef]
- Rossell-Perry, P.; Caceres Nano, E.; Gavino-Gutierrez, A.M. Association Between Palatal Index and Cleft Palate Repair Outcomes in Patients with Complete Unilateral Cleft Lip and Palate. JAMA Fac. Plast. Surg. 2014, 16, 206–210. [Google Scholar] [CrossRef]
- Fiani, N.; Verstraete, F.J.M.; Arzi, B. Reconstruction of Congenital Nose, Cleft Primary Palate, and Lip Disorders. Vet. Clin. Small Anim. Pract. 2016, 46, 663–675. [Google Scholar] [CrossRef]
- Nickel, R.; Schwarz, R. Vergleichende Betrachtung Der Kopfarterien Der Haus-Säugetiere (Katze, Hund, Schwein, Rind, Schaf, Ziege, Pferd). Zentralbl. Veterinärmed. Reihe A 1963, 10, 89–120. [Google Scholar] [CrossRef]
- Carroll, K.A.; Mathews, K.G. Ligation of the Maxillary Artery Prior to Caudal Maxillectomy in the Dog—A Description of the Technique, Retrospective Evaluation of Blood Loss, and Cadaveric Evaluation of Maxillary Artery Anatomy. Front. Vet. Sci. 2020, 7, 588945. [Google Scholar] [CrossRef]
- Nemec, A.; Daniaux, L.; Johnson, E.; Peralta, S.; Verstraete, F.J.M. Craniomaxillofacial Abnormalities in Dogs with Congenital Palatal Defects: Computed Tomographic Findings. Vet. Surg. 2015, 44, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Kimura-Kuroda, J.; Komuta, Y.; Yoshioka, N.; Li, H.P.; Kawamura, K.; Li, Y.; Raisman, G. Role of the Lesion Scar in the Response to Damage and Repair of the Central Nervous System. Cell Tissue Res. 2012, 349, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Askarian, S.; Gholami, M.; Khalili-Tanha, G.; Tehrani, N.C.; Joudi, M.; Khazaei, M.; Ferns, G.A.; Hassanian, S.M.; Avan, A.; Joodi, M. The genetic factors contributing to the risk of cleft lip-cleft palate and their clinical utility. Oral Maxillofac. Surg. 2023, 27, 177–186. [Google Scholar] [CrossRef]
- Beck, A.E.; McMillin, M.J.; Gildersleeve, H.I.S.; Shively, K.M.B.; Tang, A.; Bamshad, M.J. Genotype-Phenotype Relationships in Freeman–Sheldon Syndrome. Am. J. Med. Genet. Part A 2014, 164, 2808–2813. [Google Scholar] [CrossRef] [PubMed]
- Molina-Solana, R.; Yáñez-Vico, R.M.; Iglesias-Linares, A.; Mendoza-Mendoza, A.; Solano-Reina, E. Current concepts on the effect of environmental factors on cleft lip and palate. Int. J. Oral Maxillofac. Surg. 2013, 42, 177–184. [Google Scholar] [CrossRef]
- Leśków, A.; Nawrocka, M.; Łątkowska, M.; Tarnowska, M.; Galas, N.; Matejuk, A.; Całkosiński, I. Can contamination of the environment by dioxins cause craniofacial defects? Hum. Exp. Toxicol. 2019, 38, 1014–1023. [Google Scholar] [CrossRef]
- Butler, M.G. Imprinting disorders: Non-Mendelian mechanisms affecting growth. J. Pediatr. Endocrinol. Metab. 2002, 15 (Suppl. S5), 1279–1288. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruszkowski, J.J.; Nowacka-Woszuk, J.; Nowak, T.; Rozynek, J.; Serwanska-Leja, K.; Gogulski, M.; Kolodziejski, P.; Switonski, M.; Zdun, M.; Szczerbal, I. Cleft Lip and Palate in Four Full-Sib Puppies from a Single Litter of Staffordshire Bull Terrier Dogs: An Anatomical and Genetic Study. Animals 2023, 13, 2749. https://doi.org/10.3390/ani13172749
Ruszkowski JJ, Nowacka-Woszuk J, Nowak T, Rozynek J, Serwanska-Leja K, Gogulski M, Kolodziejski P, Switonski M, Zdun M, Szczerbal I. Cleft Lip and Palate in Four Full-Sib Puppies from a Single Litter of Staffordshire Bull Terrier Dogs: An Anatomical and Genetic Study. Animals. 2023; 13(17):2749. https://doi.org/10.3390/ani13172749
Chicago/Turabian StyleRuszkowski, Jakub J., Joanna Nowacka-Woszuk, Tomasz Nowak, Jedrzej Rozynek, Katarzyna Serwanska-Leja, Maciej Gogulski, Pawel Kolodziejski, Marek Switonski, Maciej Zdun, and Izabela Szczerbal. 2023. "Cleft Lip and Palate in Four Full-Sib Puppies from a Single Litter of Staffordshire Bull Terrier Dogs: An Anatomical and Genetic Study" Animals 13, no. 17: 2749. https://doi.org/10.3390/ani13172749
APA StyleRuszkowski, J. J., Nowacka-Woszuk, J., Nowak, T., Rozynek, J., Serwanska-Leja, K., Gogulski, M., Kolodziejski, P., Switonski, M., Zdun, M., & Szczerbal, I. (2023). Cleft Lip and Palate in Four Full-Sib Puppies from a Single Litter of Staffordshire Bull Terrier Dogs: An Anatomical and Genetic Study. Animals, 13(17), 2749. https://doi.org/10.3390/ani13172749





