Ultrasonography and Sonoelastography Characteristics of Benign vs. Malignant Mesenteric Lymph Nodes in Cats: An Update
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyman, H.T.; O’Brien, R.T. The sonographic evaluation of lymph nodes. Clin. Tech. Small Anim. Pract. 2007, 22, 128–137. [Google Scholar] [CrossRef]
- Llabrés-Díaz, F.J. Ultrasonography of the medial iliac lymph nodes in the dog. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2004, 45, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Arámbulo, R.; Wrigley, R.; Powers, B. Sonographic features of histiocytic neoplasms in the canine abdomen. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2004, 45, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Nyman, H.T.; Kristensen, A.T.; Skovgaard, I.M.; McEvoy, F.J. Characterization of normal and abnormal canine superficial lymph nodes using gray-scale B-mode, color flow mapping, power, and spectral Doppler ultrasonography: A multivariate study. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2005, 46, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Uscategui, R.A.R.; Maronezi, M.C.; Gasser, B.; Pavan, L.; Gatto, I.R.H.; de Almeida, V.T.; Vicente, W.R.R.; Feliciano, M.A.R. Ultrasonography for lymph nodes metastasis identification in bitches with mammary neoplasms. Sci. Rep. 2018, 8, 17708. [Google Scholar] [CrossRef]
- De Swarte, M.; Alexander, K.; Rannou, B.; D’Anjou, M.A.; Blond, L.; Beauchamp, G. Comparison of sonographic features of benign and neoplastic deep lymph nodes in dogs. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2011, 52, 451–456. [Google Scholar] [CrossRef]
- Stan, F.; Gudea, A.; Damian, A.; Gal, A.F.; Papuc, I.; Pop, A.R.; Martonos, C. Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs. Animals 2020, 10, 2366. [Google Scholar] [CrossRef]
- Prieto, S.; Gomez-Ochoa, P.; De Blas, I.; Gascón, M.; Aceña, C.; Corda, A.; Sosa, I.; Gregori, T.; Couto, G. Pathologic correlation of resistive and pulsatility indices in canine abdominal lymph nodes. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2009, 50, 525–529. [Google Scholar] [CrossRef]
- Agthe, P.; Caine, A.R.; Posch, B.; Herrtage, M.E. Ultrasonographic appearance of jejunal lymph nodes in dogs without clinical signs of gastrointestinal disease. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2009, 50, 195–200. [Google Scholar] [CrossRef]
- Davé, A.C.; Zekas, L.J.; Auld, D.M. Correlation of cytologic and histopathologic findings with perinodal echogenicity of abdominal lymph nodes in dogs and cats. Vet. Radiol. Ultrasound. 2017, 58, 463–470. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Ophir, J.; Garra, B.; Kallel, F.; Konofagou, E.; Krouskop, T.; Righetti, R.; Varghese, T. Elastographic imaging. Ultrasound Med. Biol. 2000, 26 (Suppl. S1), S23–S29. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Prado-Costa, R.; Rebelo, J.; Monteiro-Barroso, J.; Preto, A.S. Ultrasound elastography: Compression elastography and shear-wave elastography in the assessment of tendon injury. Insights Imaging 2018, 9, 791–814. [Google Scholar] [CrossRef]
- Chang, J.M.; Won, J.K.; Lee, K.B.; Park, I.A.; Yi, A.; Moon, W.K. Comparison of shear-wave and strain ultrasound elastography in the differentiation of benign and malignant breast lesions. AJR Am. J. Roentgenol. 2013, 201, W347–W356. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.S.S.; Cho, C.C.M.; Yuen, Y.H.; Rasalkar, D.D.; King, A.D.; Ahuja, A.T. Real-time qualitative ultrasound elastography of cervical lymph nodes in routine clinical practice: Interobserver agreement and correlation with malignancy. Ultrasound Med. Biol. 2010, 36, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Yoon, J.; Choi, M. Semi-quantitative strain elastography may facilitate pre-surgical prediction of mandibular lymph nodes malignancy in dogs. J. Vet. Sci. 2019, 20, e62. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Ambroziak, M.; Pietruski, P.; Noszczyk, B.; Paluch, Ł. Ultrasonographic elastography in the evaluation of normal and pathological skin—A review. Postepy Dermatol. Allergol. 2019, 36, 667–672. [Google Scholar] [CrossRef]
- Carlsen, J.; Ewertsen, C.; Sletting, S.; Vejborg, I.; Schäfer, F.K.W.; Cosgrove, D.; Bachmann Nielsen, M. Ultrasound Elastography in Breast Cancer Diagnosis. Ultraschall Med. 2015, 36, 550–562, quiz 563–5. [Google Scholar] [CrossRef]
- Tyloch, D.J.; Tyloch, J.F.; Adamowicz, J.; Juszczak, K.; Ostrowski, A.; Warsiński, P.; Wilamowski, J.; Ludwikowska, J.; Drewa, T. Elastography in prostate gland imaging and prostate cancer detection. Med. Ultrason. 2018, 20, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cai, J.; Wang, X. Real-time ultrasound elastography for differentiation of benign and malignant thyroid nodules: A meta-analysis. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2014, 33, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, Y.; Li, H.; Ji, H. Elastography for the differentiation of benign and malignant cervical lymph node: A meta-analysis. Int. J. Clin. Exp. Med. 2016, 9, 16094–16101. [Google Scholar]
- Carlsen, J.F.; Ewertsen, C.; Lönn, L.; Nielsen, M.B. Strain Elastography Ultrasound: An Overview with Emphasis on Breast Cancer Diagnosis. Diagnostics 2013, 3, 117–125. [Google Scholar] [CrossRef]
- Nyman, H.T.; Lee, M.H.; McEvoy, F.J.; Nielsen, O.L.; Martinussen, T.; Kristensen, A.T. Comparison of B-mode and Doppler ultrasonographic findings with histologic features of benign and malignant superficial lymph nodes in dogs. Am. J. Vet. Res. 2006, 67, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Gaschen, L.; Angelette, N.; Stout, R. Contrast-enhanced harmonic ultrasonography of medial iliac lymph nodes in healthy dogs. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2010, 51, 634–637. [Google Scholar] [CrossRef]
- Salwei, R.M.; O’Brien, R.T.; Matheson, J.S. Characterization of lymphomatous lymph nodes in dogs using contrast harmonic and Power Doppler ultrasound. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2005, 46, 411–416. [Google Scholar] [CrossRef]
- Nyman, H.T.; Kristensen, A.T.; Flagstad, A.; McEvoy, F.J. A review of the sonographic assessment of tumor metastases in liver and superficial lymph nodes. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2004, 45, 438–448. [Google Scholar] [CrossRef]
- Hinz, T.; Hoeller, T.; Wenzel, J.; Bieber, T.; Schmid-Wendtner, M.H. Real-time tissue elastography as promising diagnostic tool for diagnosis of lymph node metastases in patients with malignant melanoma: A prospective single-center experience. Dermatology 2013, 226, 81–90. [Google Scholar] [CrossRef]
- Ishibashi, N.; Yamagata, K.; Sasaki, H.; Seto, K.; Shinya, Y.; Ito, H.; Shinozuka, K.; Yanagawa, T.; Onizawa, K.; Bukawa, H. Real-time tissue elastography for the diagnosis of lymph node metastasis in oral squamous cell carcinoma. Ultrasound Med. Biol. 2012, 38, 389–395. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.H.; Lim, H.K.; Kim, S.Y.; Han, M.W.; Cho, K.J.; Baek, J.H. Quantitative shear wave elastography in the evaluation of metastatic cervical lymph nodes. Ultrasound Med. Biol. 2013, 39, 935–940. [Google Scholar] [CrossRef]
- Lo, W.C.; Cheng, P.W.; Wang, C.T.; Liao, L.J. Real-time ultrasound elastography: An assessment of enlarged cervical lymph nodes. Eur. Radiol. 2013, 23, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Naito, K.; Horiguchi, J.; Fukuda, H.; Tachikake, T.; Ito, K. Accuracy of Sonographic Elastography in the Differential Diagnosis of Enlarged Cervical Lymph Nodes: Comparison with Conventional B-Mode Sonography. Am. J. Roentgenol. 2008, 191, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Brizzi, G.; Crepaldi, P.; Roccabianca, P.; Morabito, S.; Zini, E.; Auriemma, E.; Zanna, G. Strain elastography for the assessment of skin nodules in dogs. Vet. Dermatol. 2021, 32, 272.e75. [Google Scholar] [CrossRef] [PubMed]
- Thanaboonnipat, C.; Sutayatram, S.; Buranakarl, C.; Choisunirachon, N. Renal ultrasonographic strain elastography and symmetric dimethylarginine (SDMA) in canine and feline chronic kidney disease. J. Vet. Med. Sci. 2020, 82, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Lee, G.; Lee, S.K.; Kim, H.; Yu, D.; Choi, J. Ultrasonographic elastography of the liver, spleen, kidneys, and prostate in clinically nor-mal beagle dogs [corrected]. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2015, 56, 425–431. [Google Scholar]
- Piccionello, A.; Serrani, D.; Busoni, V.; Salvaggio, A.; Bonazzi, M.; Bergamino, C.; Volta, A.; Serrani, D. Sonoelastographic Features of the Patellar Ligament in Clinically Normal Dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, 279–284. [Google Scholar] [CrossRef]
- Seiler, G.S.; Griffith, E. Comparisons between elastographic stiffness scores for benign versus malignant lymph nodes in dogs and cats. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2018, 59, 79–88. [Google Scholar] [CrossRef]
- Belotta, A.F.; Gomes, M.C.; Rocha, N.S.; Melchert, A.; Giuffrida, R.; Silva, J.P.; Mamprim, M.J. Sonography and sonoelastography in the detection of malignancy in superficial lymph nodes of dogs. J. Vet. Intern. Med. 2019, 33, 1403–1413. [Google Scholar] [CrossRef]
- Marsilio, S.; Freiche, V.; Johnson, E.; Leo, C.; Langerak, A.W.; Peters, I.; Ackermann, M.R. ACVIM consensus statement guidelines on diagnosing and distinguishing low-grade neoplastic from inflammatory lymphocytic chronic enteropathies in cats. J. Vet. Intern. Med. 2023, 37, 794–816. [Google Scholar] [CrossRef]
- Daniaux, L.A.; Laurenson, M.P.; Marks, S.L.; Moore, P.F.; Taylor, S.L.; Chen, R.X.; Zwingenberger, A.L. Ultrasonographic thickening of the muscularis propria in feline small intestinal small cell T-cell lymphoma and inflammatory bowel disease. J. Feline Med. Surg. 2014, 16, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zwingenberger, A.L.; Marks, S.L.; Baker, T.W.; Moore, P.F. Ultrasonographic Evaluation of the Muscularis Propria in Cats with Diffuse Small Intestinal Lymphoma or Inflammatory Bowel Disease. J. Vet. Intern. Med. 2010, 24, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraju, V.; Rakshith, A.V.B.; Devanand, B.; Rajakumar, R. Utility of Ultrasound Elastography to Differentiate Benign from Malignant Cervical Lymph Nodes. J. Med. Ultrasound. 2019, 28, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Randall, E. Lymph Node Evaluation with Diagnostic Imaging. Adv. Small Anim. Care 2020, 1, 75–90. [Google Scholar] [CrossRef]
- Kinns, J.; Mai, W. Association between malignancy and sonographic heterogeneity in canine and feline abdominal lymph nodes. Vet. Radiol. Ultrasound. Assoc. 2007, 48, 565–569. [Google Scholar] [CrossRef]
- Ruppel, M.; Pollard, R.; Willcox, J. Ultrasonographic characterization of cervical lymph nodes in healthy dogs. Vet. Radiol. Ultrasound 2019, 60, 560–566. [Google Scholar] [CrossRef]
- Kanagaraju, V.; Ashlyin, P.V.K.; Elango, N.; Devanand, B. Role of Transrectal Ultrasound Elastography in the Diagnosis of Prostate Carcinoma. J. Med. Ultrasound. 2020, 28, 173–178. [Google Scholar] [CrossRef]
- Zampieri, B.; Church, M.E.; Walsh, K.; Lennon, E.M. Feline eosinophilic sclerosing fibroplasia—A characteristic inflammatory response in sites beyond the gastrointestinal tract: Case report and proposed nomenclature. J. Feline Med. Surg. Open Rep. 2022, 8, 20551169221117516. [Google Scholar] [CrossRef]
- Linton, M.; Nimmo, J.S.; Norris, J.M.; Churcher, R.; Haynes, S.; Zoltowska, A.; Hughes, S.; Lessels, N.S.; Wright, M.; Malik, R. Feline gastrointestinal eosinophilic sclerosing fibroplasia: 13 cases and review of an emerging clinical entity. J. Feline Med. Surg. 2015, 17, 392–404. [Google Scholar] [CrossRef]
- Munday, J.S.; Martinez, A.W.; Soo, M. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia mimicking metastatic neoplasia. N. Z. Vet. J. 2014, 62, 356–360. [Google Scholar] [CrossRef]
- Dudea, S.M.; Botar-Jid, C.; Dumitriu, D.; Vasilescu, D.; Manole, S.; Lenghel, M.L. Differentiating benign from malignant superficial lymph nodes with sonoelastography. Med. Ultrason. 2013, 15, 132–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dietrich, C.F.; Barr, R.G.; Farrokh, A.; Dighe, M.; Hocke, M.; Jenssen, C.; Dong, Y.; Saftoiu, A.; Havre, R.F. Strain Elastography—How To Do It? Ultrasound Int. Open 2017, 3, E137–E149. [Google Scholar] [CrossRef] [PubMed]
- Finotello, R.; Vasconi, M.E.; Sabattini, S.; Agnoli, C.; Giacoboni, C.; Annoni, M.; Dentini, A.; Bettini, G.; Guazzi, P.; Stefanello, D.; et al. Feline large granular lymphocyte lymphoma: An Italian Society of Veterinary Oncology (SIONCOV) retrospective study. Vet. Comp. Oncol. 2018, 16, 159–166. [Google Scholar] [CrossRef]
- Ku, C.K.; Kass, P.H.; Christopher, M.M. Cytologic–histologic concordance in the diagnosis of neoplasia in canine and feline lymph nodes: A retrospective study of 367 cases. Vet. Comp. Oncol. 2017, 15, 1206–1217. [Google Scholar] [CrossRef] [PubMed]

| Cytology | Histology | Cytology/Histology | |
|---|---|---|---|
| Reactive hyperplasia | n = 31 (77.5%) | n = 4 (10%) | n = 5 (12.5%) |
| Eosinophilic sclerosing lymphadenitis | n = 6 (100%) | ||
| Lymphoma | n = 26 (62%) | n = 16 (38%) |
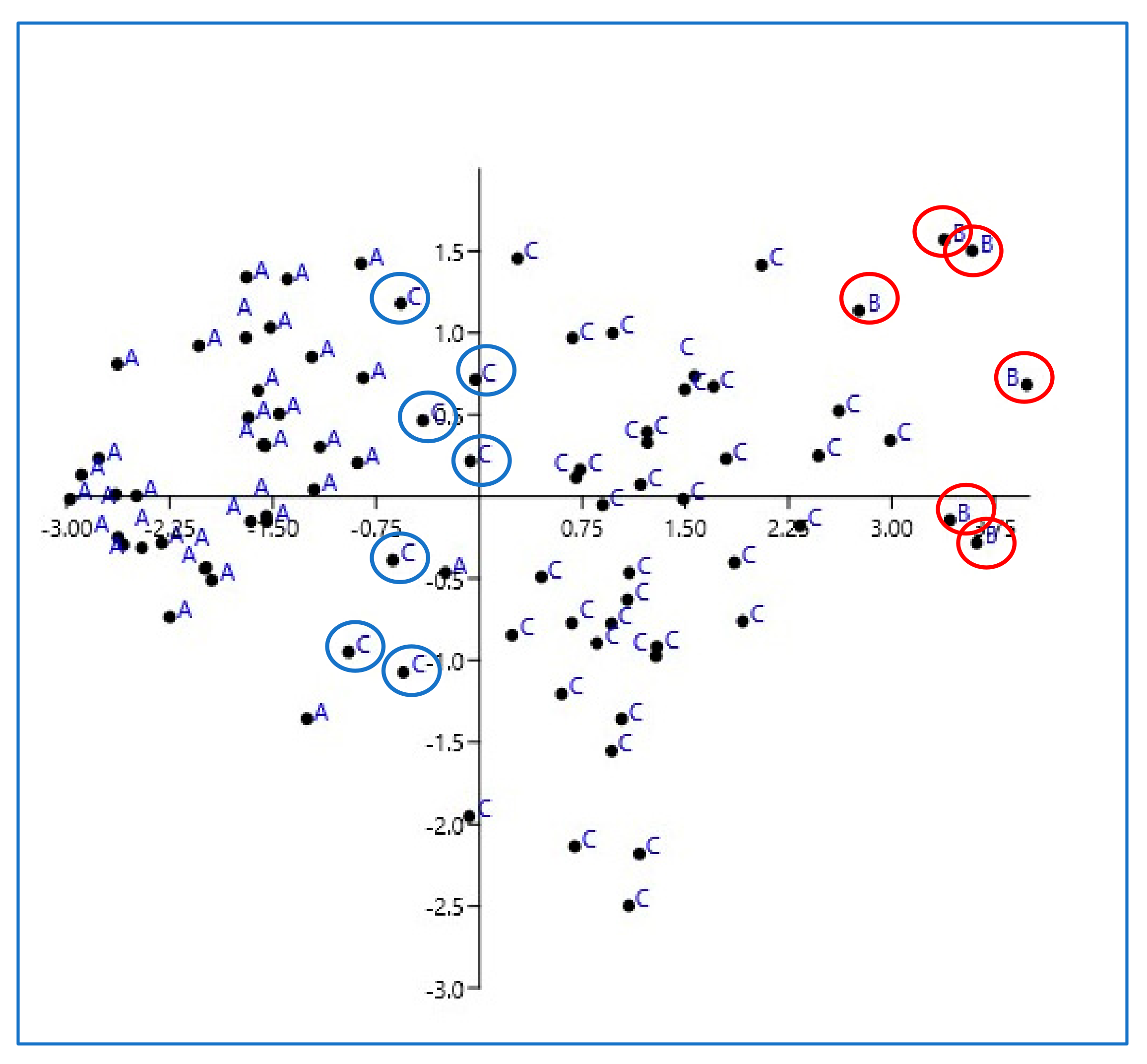
| Reactive Lymph Nodes Group A | Eosinophilic Sclerosing Lymphadenitis Group B | Lymphoma Group C | p-Value | |
|---|---|---|---|---|
| B-mode and color Doppler | ||||
| Borders | ||||
| regular | n = 29 (77%) | n = 0 (0%) | n = 21 (50%) | |
| irregular | n = 11 (23%) | n = 6 (100%) | n = 21 (50%) | |
| Echogenicity | ||||
| homogeneous | n = 12 (28.5%) | n = 0 (0%) | n = 4 (11%) | |
| heterogeneous | n = 26 (71.5%) | n = 6 (100%) | n = 38 (89%) | |
| Hilum | ||||
| present | n = 37 (98%) | n = 0 (0%) | n = 0 (0%) | |
| absent | n = 3 (2%) | n = 6 (100%) | n = 42 (100%) | |
| Vascularization | ||||
| hilar/absent | n = 31 (82.85%) | n = 0 (0%) | n = 2 (4%) | |
| peripherical/mixed | n = 9 (17.15%) | n = 6 (100%) | n = 40 (96%) | |
| S/L axis | ||||
| <0.7 | n = 40 (100%) | n = 3 (50%) | n = 22 (54.5%) | |
| >0.7 | n = 0 (0%) | n = 3 (50%) | n = 20 (45.5%) | |
| US score, Median (IQR) | 5.6 (5–8) | 9.5 (9–10) | 8.4 (7–9) | 0.01 |
| Sonoelastography | ||||
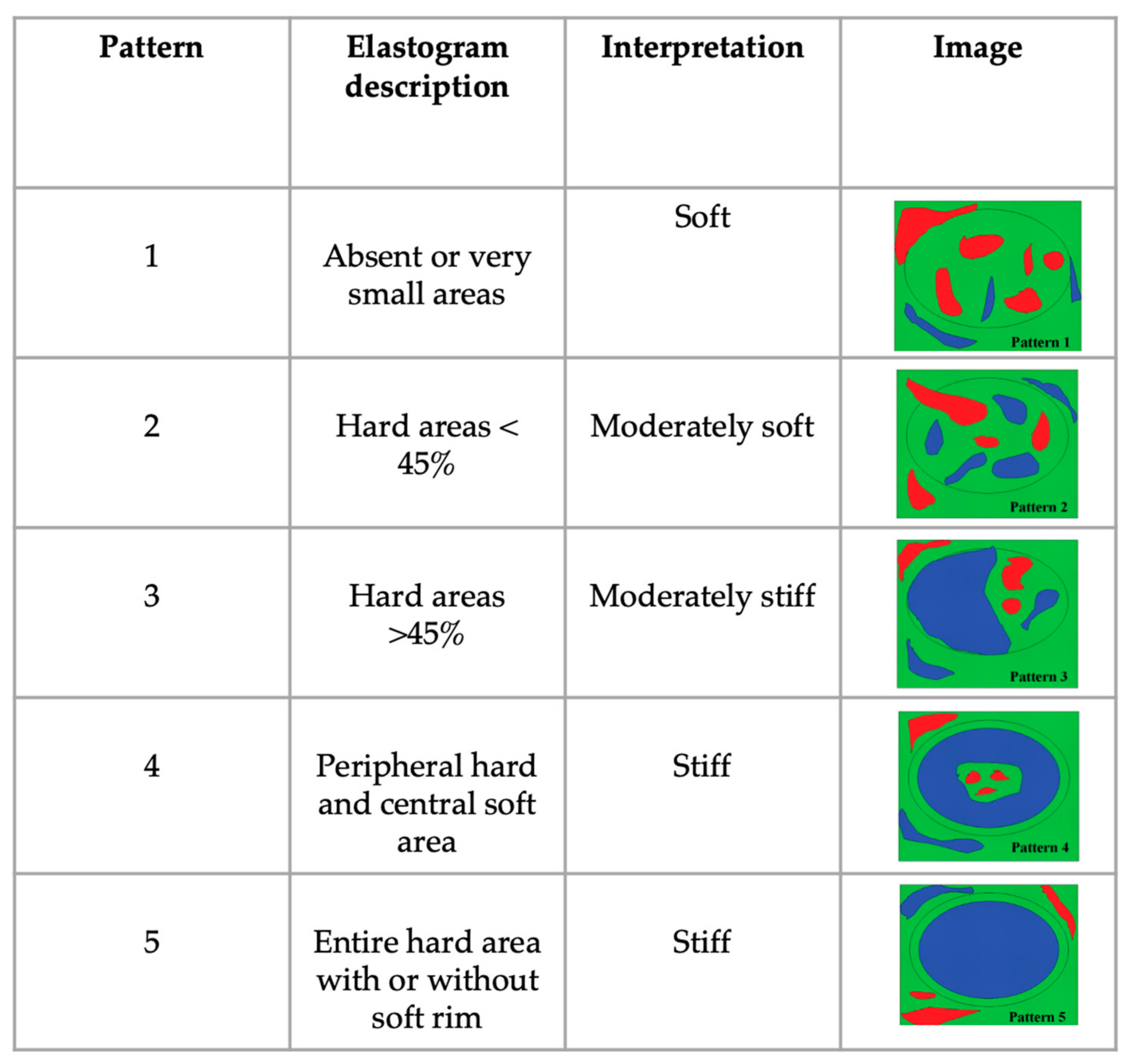
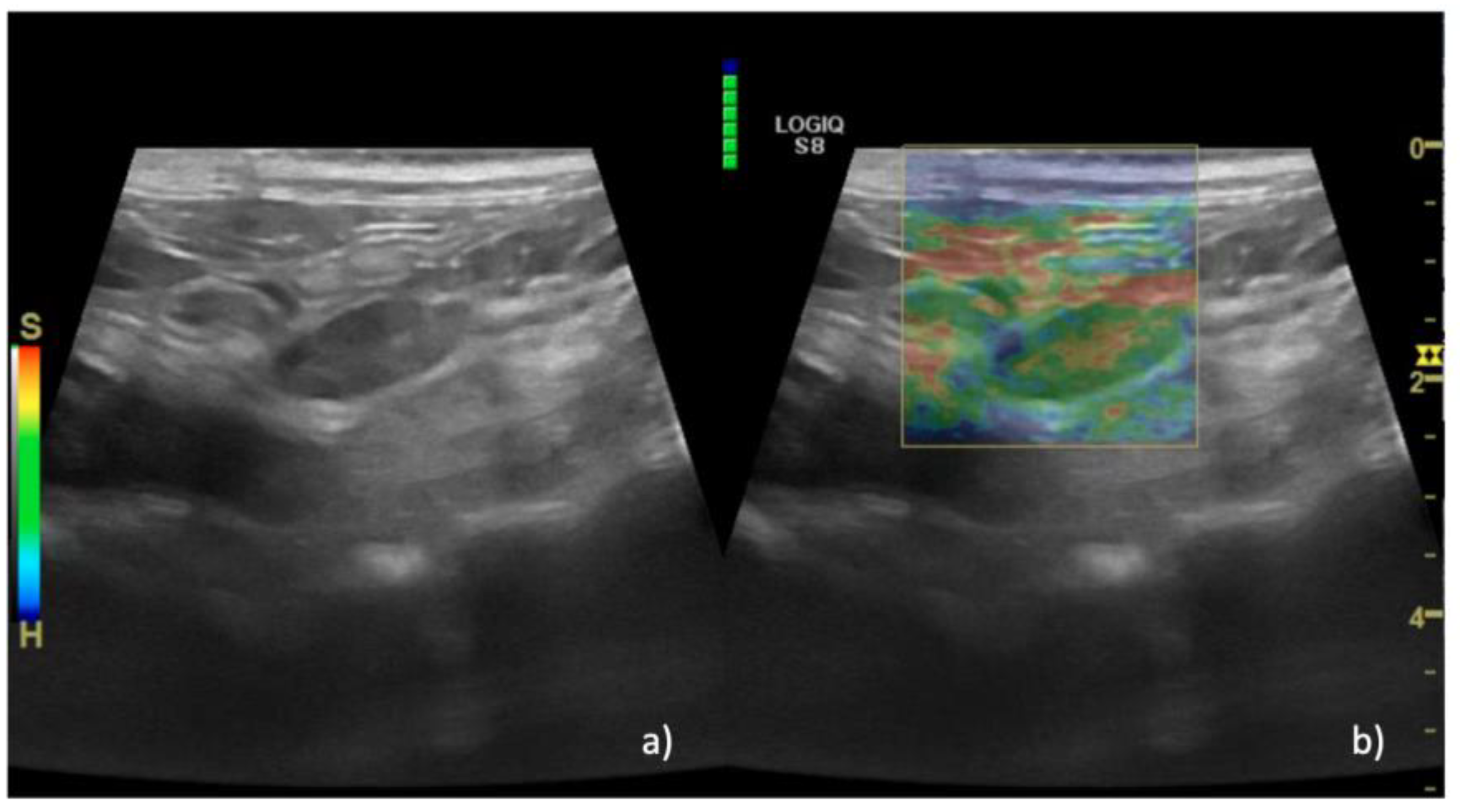
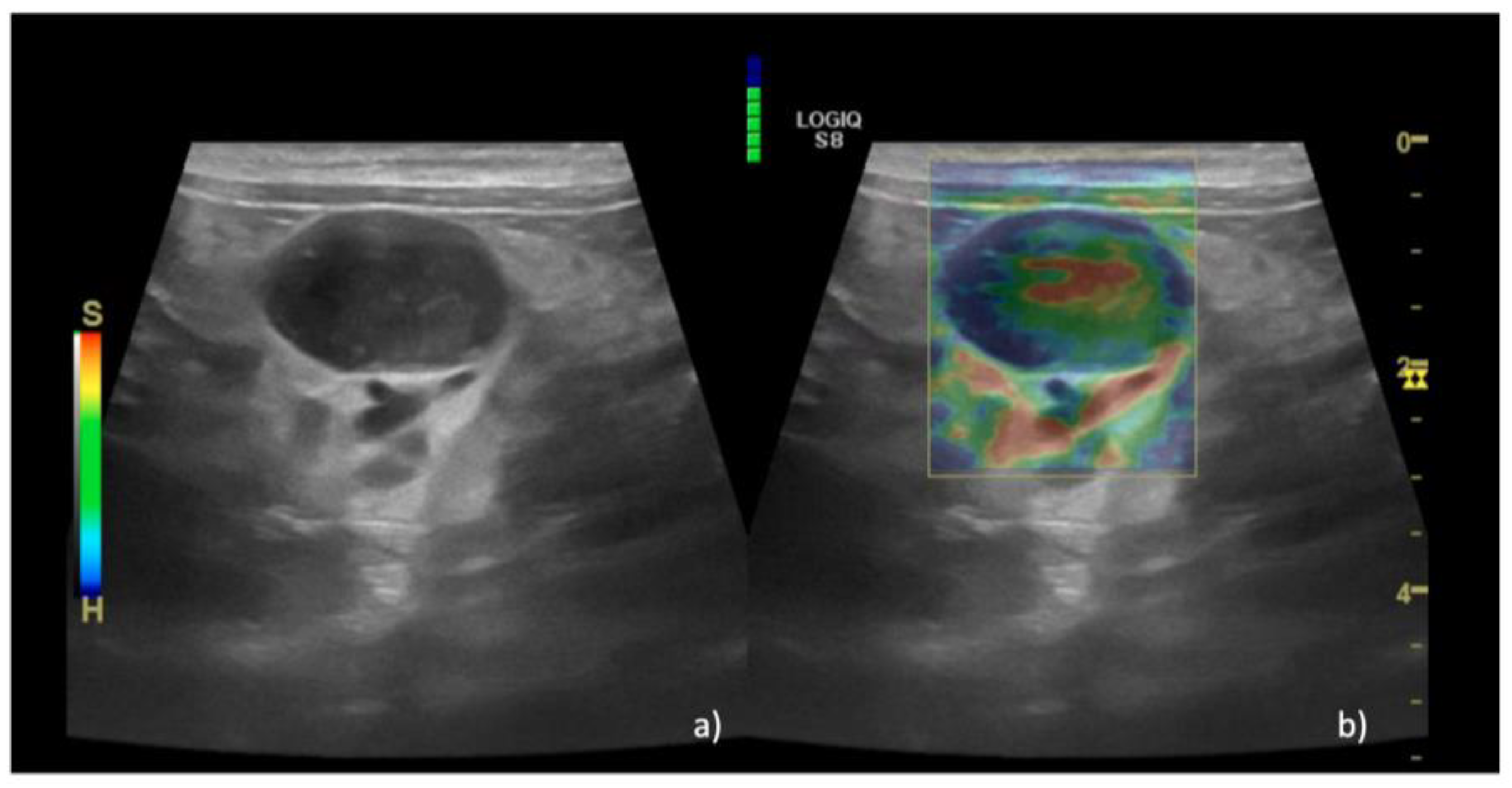
| EP | 1 n = 15 (36%) | 1 n = 0 | 1 n = 0 | |
| 2 n = 14 (35%) | 2 n = 0 | 2 n = 2 (4%) | ||
| 3 n = 11 (29%) | 3 n = 0 | 3 n = 15 (36%) | ||
| 4 n = 0 | 4 n = 0 | 4 n = 24 (58%) | ||
| 5 n = 0 | 5 n = 6 (100%) | 5 n = 1 (2%) | ||
| Median (IQR) | 2 (1–3) | 5 (5–5) | 4 (2–5) | 0.01 |
| SR, Median (IQR) | 0.5 (0.1–0.9) | 1.8 (1.5-2.2) | 0.7 (0.1–1.6) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Febo, E.; Del Signore, F.; Bernabò, N.; Paolini, A.; Simeoni, F.; De Bonis, A.; Rosto, M.; Canal, S.; Vignoli, M. Ultrasonography and Sonoelastography Characteristics of Benign vs. Malignant Mesenteric Lymph Nodes in Cats: An Update. Animals 2023, 13, 2664. https://doi.org/10.3390/ani13162664
Febo E, Del Signore F, Bernabò N, Paolini A, Simeoni F, De Bonis A, Rosto M, Canal S, Vignoli M. Ultrasonography and Sonoelastography Characteristics of Benign vs. Malignant Mesenteric Lymph Nodes in Cats: An Update. Animals. 2023; 13(16):2664. https://doi.org/10.3390/ani13162664
Chicago/Turabian StyleFebo, Elettra, Francesca Del Signore, Nicola Bernabò, Andrea Paolini, Francesco Simeoni, Andrea De Bonis, Martina Rosto, Sara Canal, and Massimo Vignoli. 2023. "Ultrasonography and Sonoelastography Characteristics of Benign vs. Malignant Mesenteric Lymph Nodes in Cats: An Update" Animals 13, no. 16: 2664. https://doi.org/10.3390/ani13162664
APA StyleFebo, E., Del Signore, F., Bernabò, N., Paolini, A., Simeoni, F., De Bonis, A., Rosto, M., Canal, S., & Vignoli, M. (2023). Ultrasonography and Sonoelastography Characteristics of Benign vs. Malignant Mesenteric Lymph Nodes in Cats: An Update. Animals, 13(16), 2664. https://doi.org/10.3390/ani13162664







