Commensal Escherichia coli Strains of Bovine Origin Competitively Mitigated Escherichia coli O157:H7 in a Gnotobiotic Murine Intestinal Colonization Model with or without Physiological Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Escherichia coli Strains
2.3. Design and Validation of Primers to Detect and Quantify EHEC O157:H7 Strains
2.4. Design and Validation of Primers to Detect and Quantify Commensal Escherichia coli Strains
2.5. Whole Genome Phylogenetic Analysis of Commensal Escherichia coli Strains
2.6. Commensal Escherichia coli Strain In Silico Phenomic Predictions
2.7. Colonization and Virulence of EHEC O157:H7 Strains in Mice
2.8. Competitive Colonization by EHEC O157:H7 in Mice under Physiological Stress
2.9. Propagation of EHEC O157:H7 and Commensal Escherichia coli Strains
2.10. Inoculation of Mice with EHEC O157:H7 and Commensal Escherichia coli Strains
2.11. Corticosterone Administration
2.12. Health Assessments
2.13. Behavioral Assessments
2.14. Sample Collection
2.15. Histopathologic Scoring
2.16. Detection and Quantification of EHEC O157:H7
2.17. Detection and Quantification of Commensal Escherichia coli
2.18. Quantification of Total Escherichia coli
2.19. Quantification of Inflammation Gene mRNA
2.20. Quantification of Corticosterone
2.21. Metabolomics
2.22. Statistical Analyses
3. Results
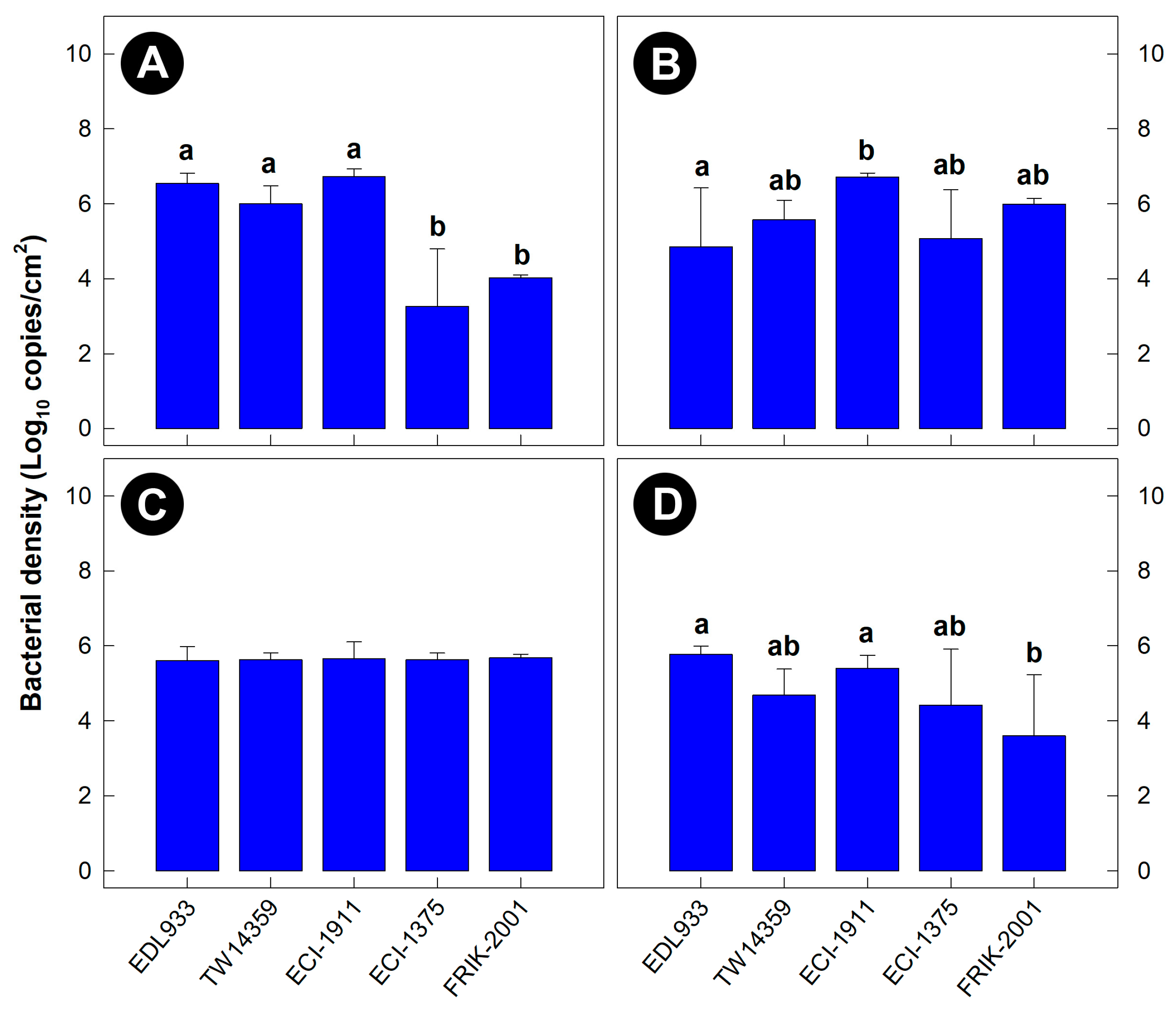
3.1. Intestinal Colonization and Virulence Differed among EHEC O157:H7 Strains
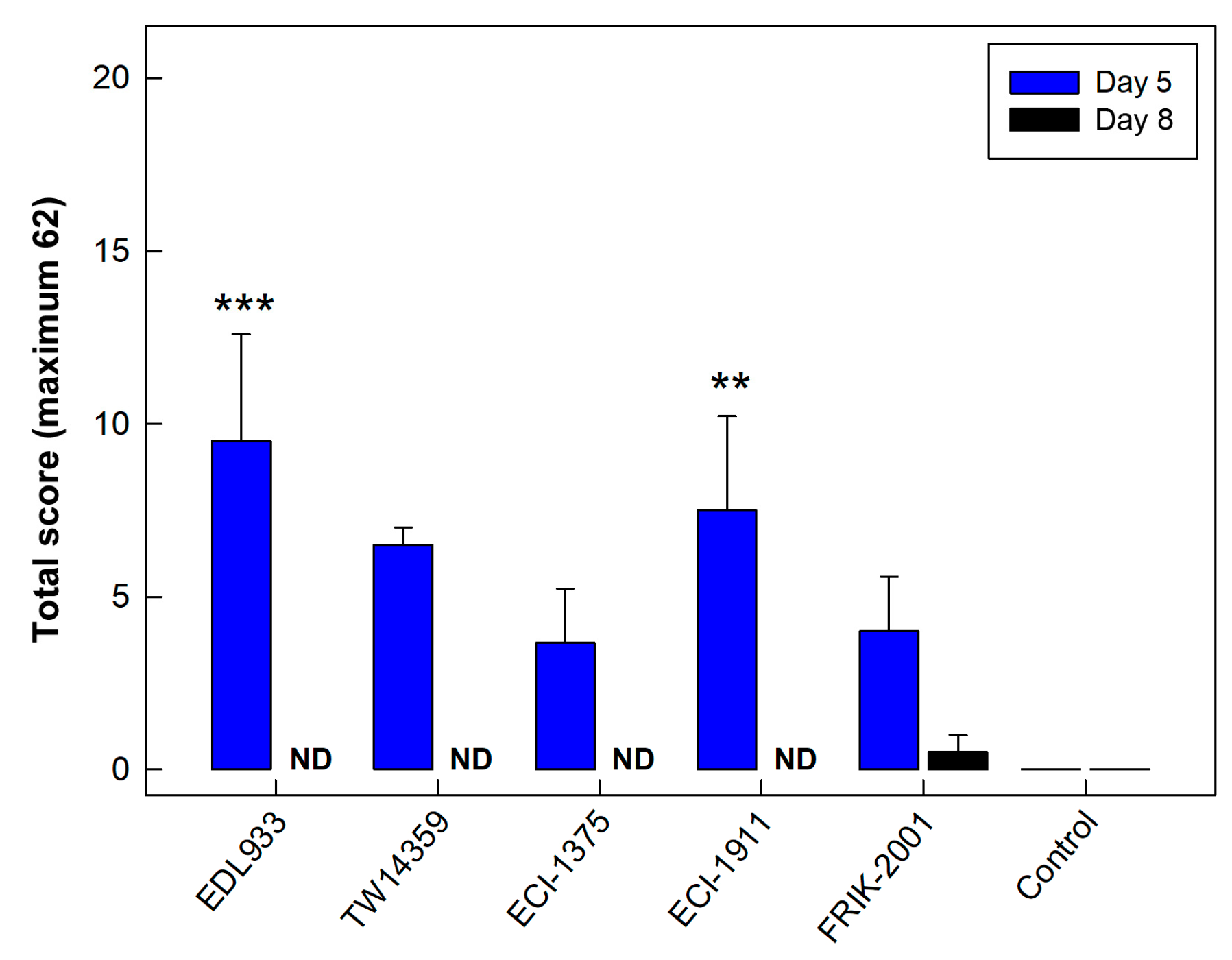
3.2. All EHEC O157:H7 Strains Generated Histopathological Changes in the Intestine
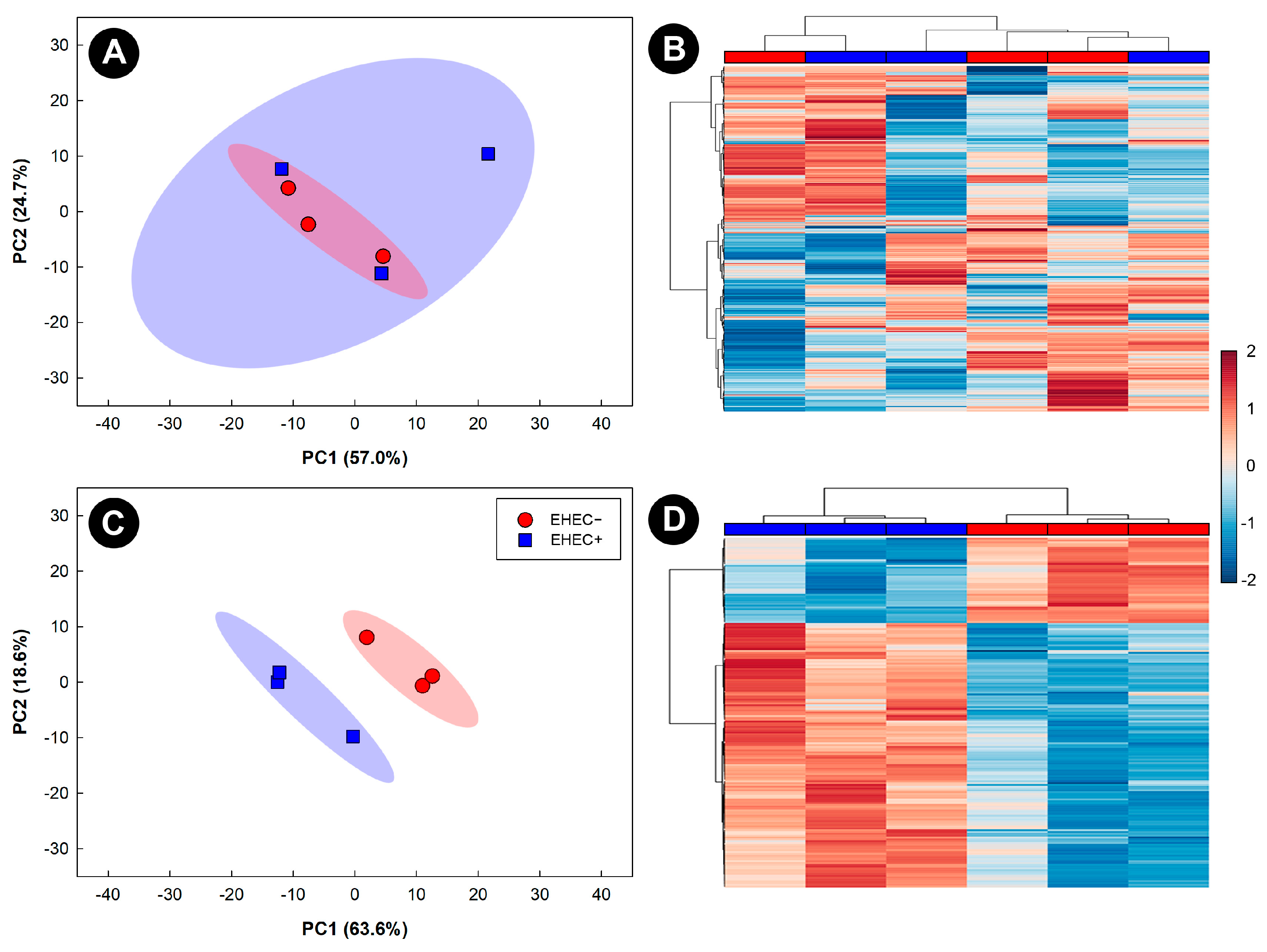
3.3. Metabolite Profiles Were Altered in Kidneys of Mice Inoculated with EHEC O157:H7 Strains
3.4. Physiological Stress Affected the Behavior of Gnotobiotic Mice
3.5. Corticosterone Concentrations Were Elevated in Mice Administered the Glucocorticoid
3.6. The Eighteen Commensal Escherichia coli Isolates from Cattle Were Genetically Distinct
3.7. DNA of Commensal Escherichia coli Strains Propagated Separately or Communally Were Detected in the Intestines of Mice
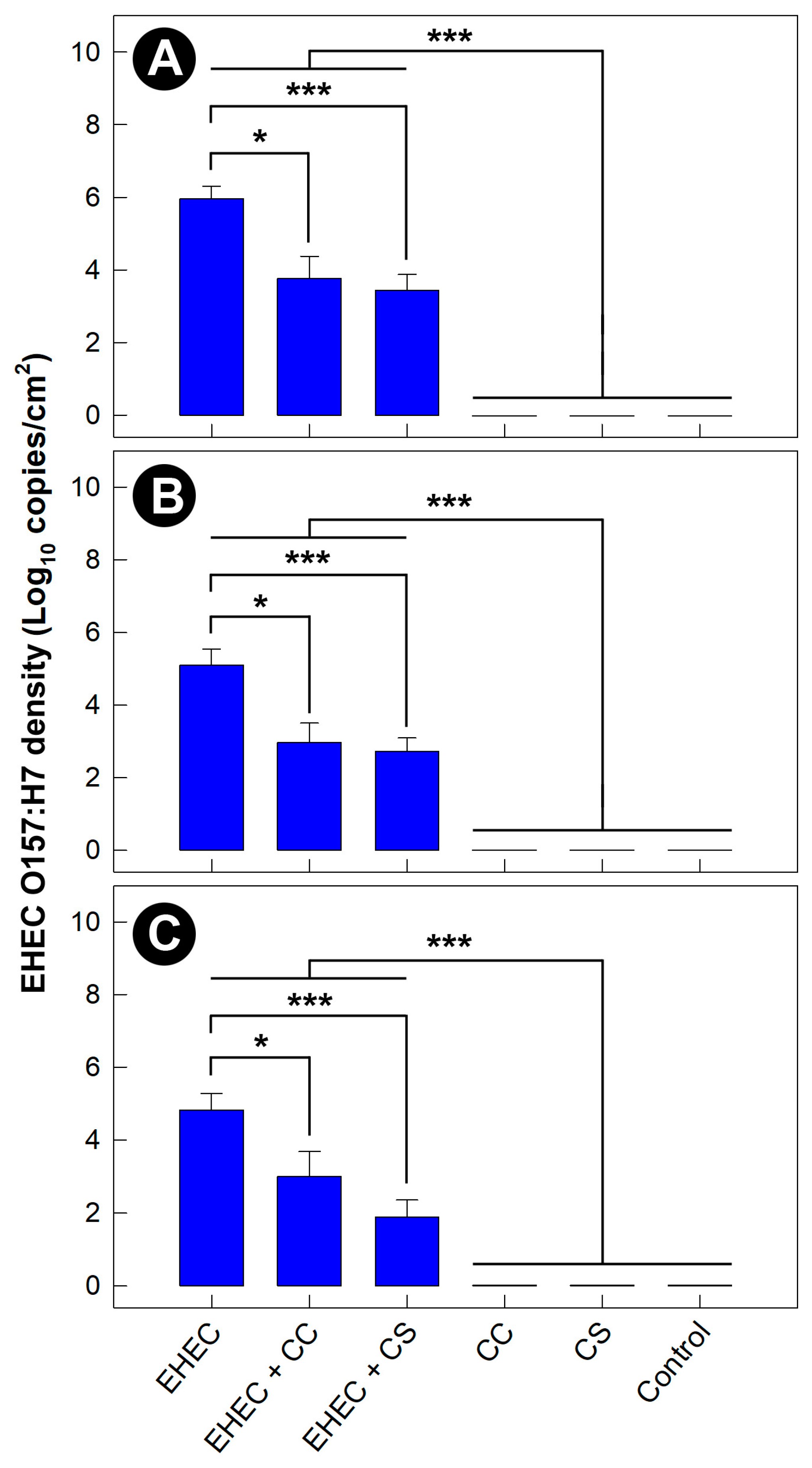
3.8. Density of EHEC O157:H7 Was Reduced in Mice Administered Commensal Escherichia coli
3.9. Histopathologic Changes Incited by EHEC O157:H7 Were Most Severe in the Distal Colon
3.10. Histopathologic Changes Incited by EHEC O157:H7 in the Distal Colon Were Reduced in Mice Administered Commensal Escherichia coli
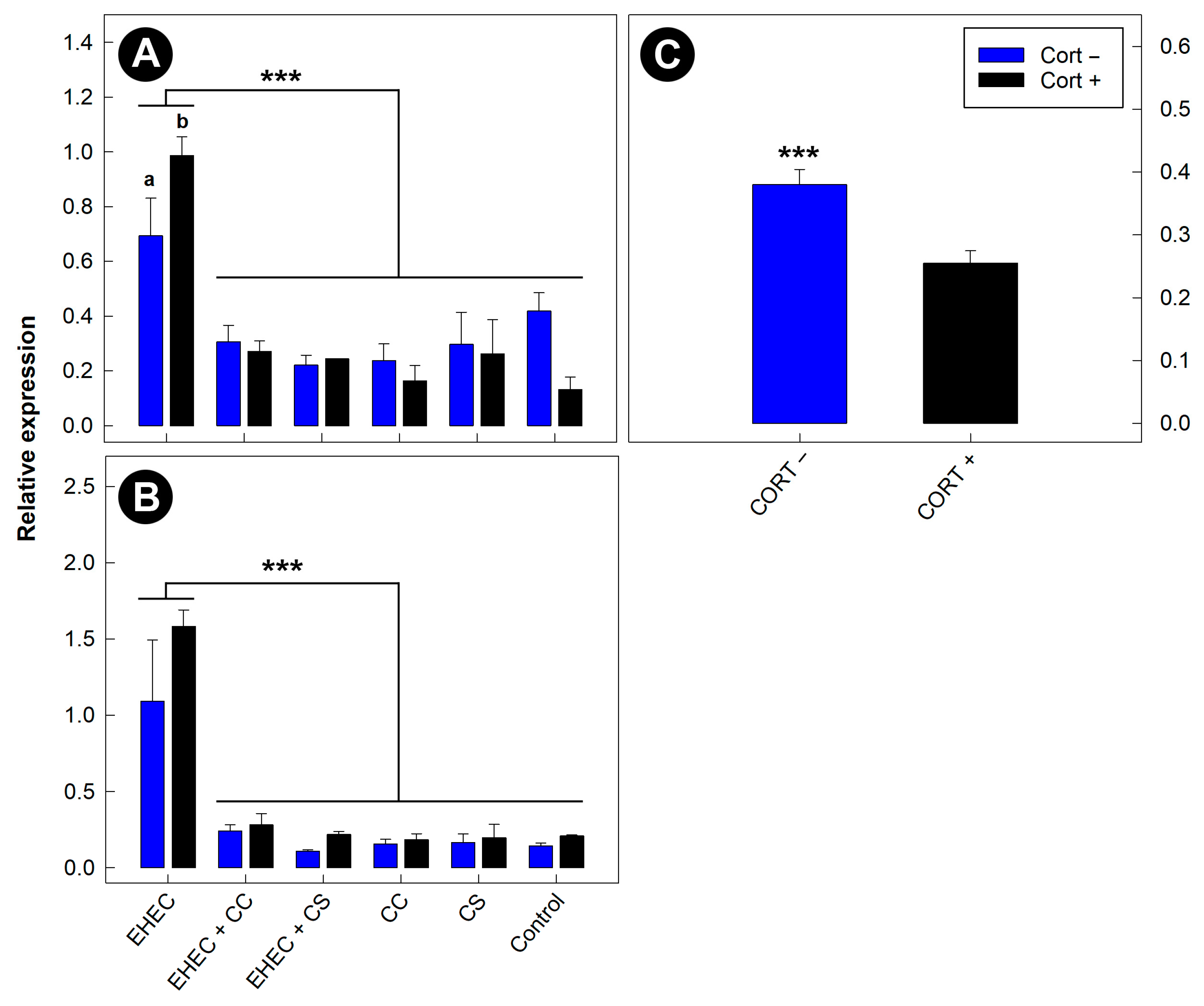
3.11. Expression of Inflammatory Markers in the Distal Colon Was Reduced in Mice Administered Commensal Escherichia coli
4. Discussion
4.1. Development of a Bovine EHEC O157:H7 Intestinal Colonization Model
4.2. Induction of Physiological Stress in Mice Had a Minimal Impact on EHEC O157:H7 Intestinal Colonization and Pathology
4.3. Commensal Escherichia coli Strains of Bovine Origin Administered to Mice Reduced EHEC O157:H7 Colonization and Pathology
4.4. Growing Commensal Escherichia coli Strains Communally Did Not Increase Efficacy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Yazdanparast, M.; Behzadi, E.; Salmanian, A.H.; Mousavi, S.L.; Nazarian, S.; Amani, J. A review on strategies for decreasing E. coli O157:H7 risk in animals. Microb. Pathog. 2017, 103, 186–195. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human shiga toxin-producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, K.D.; Selinger, L.B.; Stanford, K.; Guan, L.; Callaway, T.R.; McAllister, T.A. Perspectives on super-shedding of Escherichia coli O157:H7 by cattle. Foodborne Pathog. Dis. 2015, 12, 89–103. [Google Scholar] [CrossRef]
- Williams, K.J.; Ward, M.P.; Dhungyel, O.P.; Hall, E.J.; Van Breda, L. A longitudinal study of the prevalence and super-shedding of Escherichia coli O157 in dairy heifers. Vet. Microbiol. 2014, 173, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, A.; Sartorelli, P.; Agnes, F.; Bondiolotti, G.P.; Picotti, G.B. Adrenal response in the calf to repeated simulated transport. Br. Vet. J. 1989, 145, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Medica, P.; Alberghina, D.; Cavaleri, S.; Ferlazzo, A. Effect of long-distance road transport on thyroid and adrenal function and haematocrit values in Limousin cattle: Influence of body weight decrease. Vet. Res. Commun. 2005, 29, 713–719. [Google Scholar] [CrossRef]
- Agnes, F.; Sartorelli, P.; Abdi, B.H.; Locatelli, A. Effect of transport loading or noise on blood biochemical variables in calves. Am. J. Vet. Res. 1990, 51, 1679–1681. [Google Scholar]
- Bach, S.J.; McAllister, T.A.; Mears, G.J.; Schwartzkopf-Genswein, K.S. Long-haul transport and lack of preconditioning increases fecal shedding of Escherichia coli and Escherichia coli O157:H7 by calves. J. Food Prot. 2004, 67, 672–678. [Google Scholar] [CrossRef]
- Chase-Topping, M.E.; McKendrick, I.J.; Pearce, M.C.; MacDonald, P.; Matthews, L.; Halliday, J.; Allison, L.; Fenlon, D.; Low, J.C.; Gunn, G.; et al. Risk factors for the presence of high-level shedders of Escherichia coli O157 on Scottish farms. J. Clin. Microbiol. 2007, 45, 1594–1603. [Google Scholar] [CrossRef] [Green Version]
- Garber, L.P.; Wells, S.J.; Hancock, D.D.; Doyle, M.P.; Tuttle, J.; Shere, J.A.; Zhao, T. Risk factors for fecal shedding of Escherichia coli O157:H7 in dairy calves. J. Am. Vet. Med. Assoc. 1995, 207, 46–49. [Google Scholar] [PubMed]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [Green Version]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.G.; Sperandio, V. The epinephrine/norepinephrine/autoinducer-3 interkingdom signaling system in Escherichia coli O157:H7. Microb. Endoc. 2010, 874, 213–227. [Google Scholar] [CrossRef]
- Carlson-Banning, K.M.; Sperandio, V. Enterohemorrhagic Escherichia coli outwits hosts through sensing small molecules. Curr. Opin. Microbiol. 2018, 41, 83–88. [Google Scholar] [CrossRef]
- Wadolkowski, E.A.; Burris, J.A.; O’Brien, A.D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 1990, 58, 2438–2445. [Google Scholar] [CrossRef]
- Leatham, M.P.; Banerjee, S.; Autieri, S.M.; Mercado-Lubo, R.; Conway, T.; Cohen, P.S. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the streptomycin-treated mouse intestine. Infect. Immun. 2009, 77, 2876–2886. [Google Scholar] [CrossRef] [Green Version]
- Mundy, R.; Girard, F.; FitzGerald, A.J.; Frankel, G. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol. Lett. 2006, 265, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, K.; Taguchi, K.; Hara, T.; Yokoyama, S.; Kawada, K.; Mori, H. Adhesion and colonization of enterohemorrhagic Escherichia coli O157:H7 in cecum of mice. Microbiol. Immunol. 2003, 47, 125–132. [Google Scholar]
- Lange, M.E.; Uwiera, R.R.E.; Inglis, G.D. Enteric Escherichia coli O157:H7 in cattle, and the use of mice as a model to elucidate key aspects of the host-pathogen-microbiota interaction: A review. Front. Vet. Sci. 2022, 9, 937866. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Taguchi, H.; Yamaguchi, H.; Osaki, T.; Komatsu, A.; Kamiya, S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 2004, 41, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Goswami, K.; Chen, C.; Xiaoli, L.; Eaton, K.A.; Dudley, E.G. Coculture of Escherichia coli O157:H7 with a nonpathogenic E. coli strain increases toxin production and virulence in a germfree mouse model. Infect. Immun. 2015, 83, 4185–4193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, K.A.; Friedman, D.I.; Francis, G.J.; Tyler, J.S.; Young, V.B.; Haeger, J.; Abu-Ali, G.; Whittam, T.S. Pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ-free mice. Infect. Immun. 2008, 76, 3054–3063. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, H.; Takahashi, M.; Yamaguchi, H.; Osaki, T.; Komatsu, A.; Fujioka, Y.; Kamiya, S. Experimental infection of germ-free mice with hyper-toxigenic enterohaemorrhagic Escherichia coli O157:H7, strain 6. J. Med. Microbiol. 2002, 51, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, M.E.; Uwiera, R.R.E.; Inglis, G.D. Housing gnotobiotic mice in conventional animal facilities. Curr. Protoc. Mouse Biol. 2019, 9, e59. [Google Scholar] [CrossRef] [Green Version]
- CDC. Standard Operating Procedure for PulseNet PFGE of Escherichia coli O157:H7, Escherichia coli Non-O157(STEC), Salmonella Serotypes, Shigella sonnei and Shigella flexneri; CDC: Atlanta, GA, USA, 2013. [Google Scholar]
- Strachan, N.J.; Rotariu, O.; Lopes, B.; MacRae, M.; Fairley, S.; Laing, C.; Gannon, V.; Allison, L.J.; Hanson, M.F.; Dallman, T.; et al. Whole genome sequencing demonstrates that geographic variation of Escherichia coli O157 genotypes dominates host association. Sci. Rep. 2015, 5, 14145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessonov, K.; Laing, C.; Robertson, J.; Yong, I.; Ziebell, K.; Gannon, V.P.J.; Nichani, A.; Arya, G.; Nash, J.H.E.; Christianson, S. ECTyper: In silico Escherichia coli serotype and species prediction from raw and assembled whole-genome sequence data. Microb. Genom. 2021, 7, 000728. [Google Scholar] [CrossRef]
- Waters, N.R.; Abram, F.; Brennan, F.; Holmes, A.; Pritchard, L. Easy phylotyping of Escherichia coli via the EzClermont web app and command-line tool. Access Microbiol. 2020, 2, acmi000143. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Petkau, A.; Avery, B.P.; Chen, J.C.; Folster, J.P.; Carson, C.A.; Kearney, A.; Nadon, C.; Mabon, P.; Thiessen, J.; et al. Correlation between phenotypic and in silico detection of antimicrobial resistance in Salmonella enterica in Canada using Staramr. Microorganisms 2022, 10, 292. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Maiden, M.C. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, F.; Smith, D.W.; Hutson, P.H. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur. J. Pharmacol. 2008, 583, 115–127. [Google Scholar] [CrossRef]
- Sturm, M.; Becker, A.; Schroeder, A.; Bilkei-Gorzo, A.; Zimmer, A. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes Brain Behav. 2015, 14, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Ardayfio, P.; Kim, K.S. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav. Neurosci. 2006, 120, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.G.; Jung, H.S.; Kim, K.J.; Min, S.S.; Yoon, B.J. Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice. Neurosci. Lett. 2013, 555, 137–142. [Google Scholar] [CrossRef]
- Stavric, S.; Gleeson, T.M.; Blanchfield, B.; Pivnick, H. Competitive exclusion of Salmonella from newly hatched chicks by mixtures of pure bacterial cultures isolated from fecal and cecal contents of adult birds. J. Food Prot. 1985, 48, 778–782. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Bhagat, S.M.; Bowles, N.P.; Weil, Z.M.; Pfaff, D.W.; McEwen, B.S. Endocrine and physiological changes in response to chronic corticosterone: A potential model of the metabolic syndrome in mouse. Endocrinology 2010, 151, 2117–2127. [Google Scholar]
- Deacon, R.M. Assessing nest building in mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Koelink, P.J.; Wildenberg, M.E.; Stitt, L.W.; Feagan, B.G.; Koldijk, M.; van’t Wout, A.B.; Atreya, R.; Vieth, M.; Brandse, J.F.; Duijst, S.; et al. Development of reliable, valid and responsive scoring systems for endoscopy and histology in animal models for inflammatory bowel disease. J. Crohn’s Colitis 2018, 12, 794–803. [Google Scholar] [CrossRef] [Green Version]
- Eaton, K.A.; Fontaine, C.; Friedman, D.I.; Conti, N.; Alteri, C.J. Pathogenesis of colitis in germ-free mice infected with EHEC O157:H7. Vet. Pathol. 2017, 54, 710–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar] [PubMed]
- Abnous, K.; Brooks, S.P.; Kwan, J.; Matias, F.; Green-Johnson, J.; Selinger, L.B.; Thomas, M.; Kalmokoff, M. Diets enriched in oat bran or wheat bran temporally and differentially alter the composition of the fecal community of rats. J. Nutr. 2009, 139, 2024–2031. [Google Scholar] [CrossRef] [Green Version]
- Bescucci, D.M.; Clarke, S.T.; Brown, C.L.J.; Boras, V.F.; Montina, T.; Uwiera, R.R.E.; Inglis, G.D. The absence of murine cathelicidin-related antimicrobial peptide impacts host responses enhancing Salmonella enterica serovar Typhimurium infection. Gut Pathog. 2020, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Paxman, E.J.; Boora, N.S.; Kiss, D.; Laplante, D.P.; King, S.; Montina, T.; Metz, G.A.S. Prenatal maternal stress from a natural disaster alters urinary metabolomic profiles in project ice storm participants. Sci. Rep. 2018, 8, 12932. [Google Scholar] [CrossRef]
- Veselkov, K.A.; Lindon, J.C.; Ebbels, T.M.; Crockford, D.; Volynkin, V.V.; Holmes, E.; Davies, D.B.; Nicholson, J.K. Recursive segment-wise peak alignment of biological 1H NMR spectra for improved metabolic biomarker recovery. Anal. Chem. 2009, 81, 56–66. [Google Scholar] [CrossRef]
- Anderson, P.E.; Mahle, D.A.; Doom, T.E.; Reo, N.V.; DelRaso, N.J.; Raymer, M.L. Dynamic adaptive binning: An improved quantification technique for NMR spectroscopic data. Metabolomics 2011, 7, 179–190. [Google Scholar]
- Chong, J.; Yamamoto, M.; Xia, J. MetaboAnalystR 2.0: From raw spectra to biological insights. Metabolites 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, A.M.; Romick-Rosendale, L.E.; Kennedy, M.A. Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal. Biochem. 2010, 401, 134–143. [Google Scholar] [CrossRef]
- Meale, S.J.; Chaucheyras-Durand, F.; Berends, H.; Guan, L.L.; Steele, M.A. From pre- to postweaning: Transformation of the young calf’s gastrointestinal tract. J. Dairy Sci. 2017, 100, 5984–5995. [Google Scholar] [CrossRef]
- Wadolkowski, E.A.; Sung, L.M.; Burris, J.A.; Samuel, J.E.; O’Brien, A.D. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce shiga-like toxin type II. Infect. Immun. 1990, 58, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.; Ziebell, K.; Zhang, Y.; Benson, A.; Konczy, P.; Johnson, R.; Gannon, V. Identification of Escherichia coli O157:H7 genomic regions conserved in strains with a genotype associated with human infection. Appl. Environ. Microbiol. 2007, 73, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziebell, K.; Steele, M.; Zhang, Y.; Benson, A.; Taboada, E.N.; Laing, C.; McEwen, S.; Ciebin, B.; Johnson, R.; Gannon, V. Genotypic characterization and prevalence of virulence factors among Canadian Escherichia coli O157:H7 strains. Appl. Environ. Microbiol. 2008, 74, 4314–4323. [Google Scholar] [PubMed] [Green Version]
- Laing, C.R.; Zhang, Y.; Gilmour, M.W.; Allen, V.; Johnson, R.; Thomas, J.E.; Gannon, V.P. A comparison of shiga-toxin 2 bacteriophage from classical enterohemorrhagic Escherichia coli serotypes and the German E. coli O104:H4 outbreak strain. PLoS ONE 2012, 7, e37362. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Laing, C.; Zhang, Z.; Hallewell, J.; You, C.; Ziebell, K.; Johnson, R.P.; Kropinski, A.M.; Thomas, J.E.; Karmali, M.; et al. Lineage and host source are both correlated with levels of shiga toxin 2 production by Escherichia coli O157:H7 strains. Appl. Environ. Microbiol. 2010, 76, 474–482. [Google Scholar]
- Naylor, S.W.; Low, J.C.; Besser, T.E.; Mahajan, A.; Gunn, G.J.; Pearce, M.C.; McKendrick, I.J.; Smith, D.G.; Gally, D.L. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 2003, 71, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Betzen, C.; Plotnicki, K.; Fathalizadeh, F.; Pappan, K.; Fleming, T.; Bielaszewska, M.; Karch, H.; Tonshoff, B.; Rafat, N. Shiga toxin 2a-induced endothelial injury in hemolytic uremic syndrome: A metabolomic analysis. J. Infect. Dis. 2016, 213, 1031–1040. [Google Scholar] [CrossRef] [Green Version]
- Barth, M.C.; Ahluwalia, N.; Anderson, T.J.; Hardy, G.J.; Sinha, S.; Alvarez-Cardona, J.A.; Pruitt, I.E.; Rhee, E.P.; Colvin, R.A.; Gerszten, R.E. Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J. Biol. Chem. 2009, 284, 19189–19195. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, S.M.; Hazen, T.H.; Rasko, D.A.; Eppinger, M. EHEC genomics: Past, present, and future. Microbiol. Spectr. 2014, 2, EHEC-0020-2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.L.J.; Zaytsoff, S.J.M.; Montina, T.; Inglis, G.D. Corticosterone-mediated physiological stress alters liver, kidney, and breast muscle metabolomic profiles in chickens. Animals 2021, 11, 3056. [Google Scholar] [CrossRef]
- Zaytsoff, S.J.M.; Brown, C.L.J.; Montina, T.; Metz, G.A.S.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Corticosterone-mediated physiological stress modulates hepatic lipid metabolism, metabolite profiles, and systemic responses in chickens. Sci. Rep. 2019, 9, 19225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The open field test. In Mood and Anxiety Related Phenotypes in Mice; Gould, T.D., Wolfgang Walz, U.o.S., Saskatoon, S.K., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.P.; et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef] [PubMed]
- Vlisidou, I.; Lyte, M.; van Diemen, P.M.; Hawes, P.; Monaghan, P.; Wallis, T.S.; Stevens, M.P. The neuroendocrine stress hormone norepinephrine augments Escherichia coli O157:H7-induced enteritis and adherence in a bovine ligated ileal loop model of infection. Infect. Immun. 2004, 72, 5446–5451. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, V.; Torres, A.G.; Jarvis, B.; Nataro, J.P.; Kaper, J.B. Bacteria-host communication: The language of hormones. Proc. Natl. Acad. Sci. USA 2003, 100, 8951–8956. [Google Scholar] [CrossRef]
- Lyte, M.; Vulchanova, L.; Brown, D.R. Stress at the intestinal surface: Catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011, 343, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Frank, C.D.; Green, B.T. Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157:H7. FEMS Microbiol. Lett. 1996, 139, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Moberg, G.P.; Mench, J.A. (Eds.) Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CAB International: Wallingford, UK, 2000; pp. 1–22. [Google Scholar]
- Sharara-Chami, R.I.; Joachim, M.; Pacak, K.; Majzoub, J.A. Glucocorticoid treatment–effect on adrenal medullary catecholamine production. Shock 2010, 33, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Letterio, J.J.; Roberts, A.B. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 1998, 16, 137–161. [Google Scholar] [CrossRef] [Green Version]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.S.; Hartland, E.L. The inflammatory response during enterohemorrhagic Escherichia coli infection. Microbiol. Spectr. 2014, 2, EHEC-0012-2013. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Isogai, E.; Isogai, H.; Kimura, K.; Hayashi, S.; Kubota, T.; Fujii, N.; Takeshi, K. Role of tumor necrosis factor alpha in gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Infect. Immun. 1998, 66, 197–202. [Google Scholar] [CrossRef]
- LeJeune, J.T.; Wetzel, A.N. Preharvest control of Escherichia coli O157 in cattle. J. Anim. Sci. 2007, 85, E73–E80. [Google Scholar] [CrossRef] [PubMed]
- Maltby, R.; Leatham-Jensen, M.P.; Gibson, T.; Cohen, P.S.; Conway, T. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS ONE 2013, 8, e53957. [Google Scholar] [CrossRef] [Green Version]
- Freter, R.; Brickner, H.; Fekete, J.; Vickerman, M.M.; Carey, K.E. Survival and implantation of Escherichia coli in the intestinal tract. Infect. Immun. 1983, 39, 686–703. [Google Scholar] [CrossRef]
- Iversen, H.; Abée-Lund, T.M.L.; Aspholm, M.; Arnesen, L.P.; Lindback, T. Commensal E. coli Stx2 lysogens produce high levels of phages after spontaneous prophage induction. Front. Cell Infect. Microbiol. 2015, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: Resistance is futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Dean-Nystrom, E.A.; Bosworth, B.T.; Cray, W.C., Jr.; Moon, H.W. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 1997, 65, 1842–1848. [Google Scholar] [CrossRef]
- Zhao, T.; Tkalcic, S.; Doyle, M.P.; Harmon, B.G.; Brown, C.A.; Zhao, P. Pathogenicity of enterohemorrhagic Escherichia coli in neonatal calves and evaluation of fecal shedding by treatment with probiotic Escherichia coli. J. Food Prot. 2003, 66, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Nart, P.; Naylor, S.W.; Huntley, J.F.; McKendrick, I.J.; Gally, D.L.; Low, J.C. Responses of cattle to gastrointestinal colonization by Escherichia coli O157:H7. Infect. Immun. 2008, 76, 5366–5372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, J.K.; Keepers, T.R.; Gross, L.K.; Seaner, R.M.; Obrig, T.G. CXCL1/KC and CXCL2/MIP-2 are critical effectors and potential targets for therapy of Escherichia coli O157:H7-associated renal inflammation. Am. J. Pathol. 2007, 170, 526–537. [Google Scholar] [CrossRef] [Green Version]
- Wang, O.; Liang, G.; McAllister, T.A.; Plastow, G.; Stanford, K.; Selinger, B.; Guan, L.L. Comparative transcriptomic analysis of rectal tissue from beef steers revealed reduced host immunity in Escherichia coli O157:H7 super-shedders. PLoS ONE 2016, 11, e0151284. [Google Scholar] [CrossRef] [Green Version]
- Naude, P.J.; den Boer, J.A.; Luiten, P.G.; Eisel, U.L. Tumor necrosis factor receptor cross-talk. FEBS J. 2011, 278, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Bellmeyer, A.; Cotton, C.; Kanteti, R.; Koutsouris, A.; Viswanathan, V.K.; Hecht, G. Enterohemorrhagic Escherichia coli suppresses inflammatory response to cytokines and its own toxin. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G576–G581. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Zhang, H.; Wang, H.; Hu, J.; Du, M.; Zhu, M.J. Host inflammatory response inhibits Escherichia coli O157:H7 adhesion to gut epithelium through augmentation of mucin expression. Infect. Immun. 2014, 82, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Corbishley, A.; Ahmad, N.I.; Hughes, K.; Hutchings, M.R.; McAteer, S.P.; Connelley, T.K.; Brown, H.; Gally, D.L.; McNeilly, T.N. Strain-dependent cellular immune responses in cattle following Escherichia coli O157:H7 colonization. Infect. Immun. 2014, 82, 5117–5131. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, M.E.; Clarke, S.T.; Boras, V.F.; Brown, C.L.J.; Zhang, G.; Laing, C.R.; Uwiera, R.R.E.; Montina, T.; Kalmokoff, M.L.; Taboada, E.N.; et al. Commensal Escherichia coli Strains of Bovine Origin Competitively Mitigated Escherichia coli O157:H7 in a Gnotobiotic Murine Intestinal Colonization Model with or without Physiological Stress. Animals 2023, 13, 2577. https://doi.org/10.3390/ani13162577
Lange ME, Clarke ST, Boras VF, Brown CLJ, Zhang G, Laing CR, Uwiera RRE, Montina T, Kalmokoff ML, Taboada EN, et al. Commensal Escherichia coli Strains of Bovine Origin Competitively Mitigated Escherichia coli O157:H7 in a Gnotobiotic Murine Intestinal Colonization Model with or without Physiological Stress. Animals. 2023; 13(16):2577. https://doi.org/10.3390/ani13162577
Chicago/Turabian StyleLange, Maximo E., Sandra T. Clarke, Valerie F. Boras, Catherine L. J. Brown, Guangzhi Zhang, Chad R. Laing, Richard R. E. Uwiera, Tony Montina, Martin L. Kalmokoff, Eduardo N. Taboada, and et al. 2023. "Commensal Escherichia coli Strains of Bovine Origin Competitively Mitigated Escherichia coli O157:H7 in a Gnotobiotic Murine Intestinal Colonization Model with or without Physiological Stress" Animals 13, no. 16: 2577. https://doi.org/10.3390/ani13162577
APA StyleLange, M. E., Clarke, S. T., Boras, V. F., Brown, C. L. J., Zhang, G., Laing, C. R., Uwiera, R. R. E., Montina, T., Kalmokoff, M. L., Taboada, E. N., Gannon, V. P. J., Metz, G. A. S., Church, J. S., & Inglis, G. D. (2023). Commensal Escherichia coli Strains of Bovine Origin Competitively Mitigated Escherichia coli O157:H7 in a Gnotobiotic Murine Intestinal Colonization Model with or without Physiological Stress. Animals, 13(16), 2577. https://doi.org/10.3390/ani13162577





