Exploring the Impact of Ampelopsis Grossedentata Flavonoids on Growth Performance, Ruminal Microbiota, and Plasma Physiology and Biochemistry of Kids
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experiment Design
2.2. Growth Performance
2.3. 16S rRNA Gene Sequencing
2.4. Plasma Physiology and Biochemistry
2.5. Data Analysis
3. Results
3.1. Growth Performance
3.2. Composition and Diversity of the Rumen Microbiota
3.2.1. Rumen pH
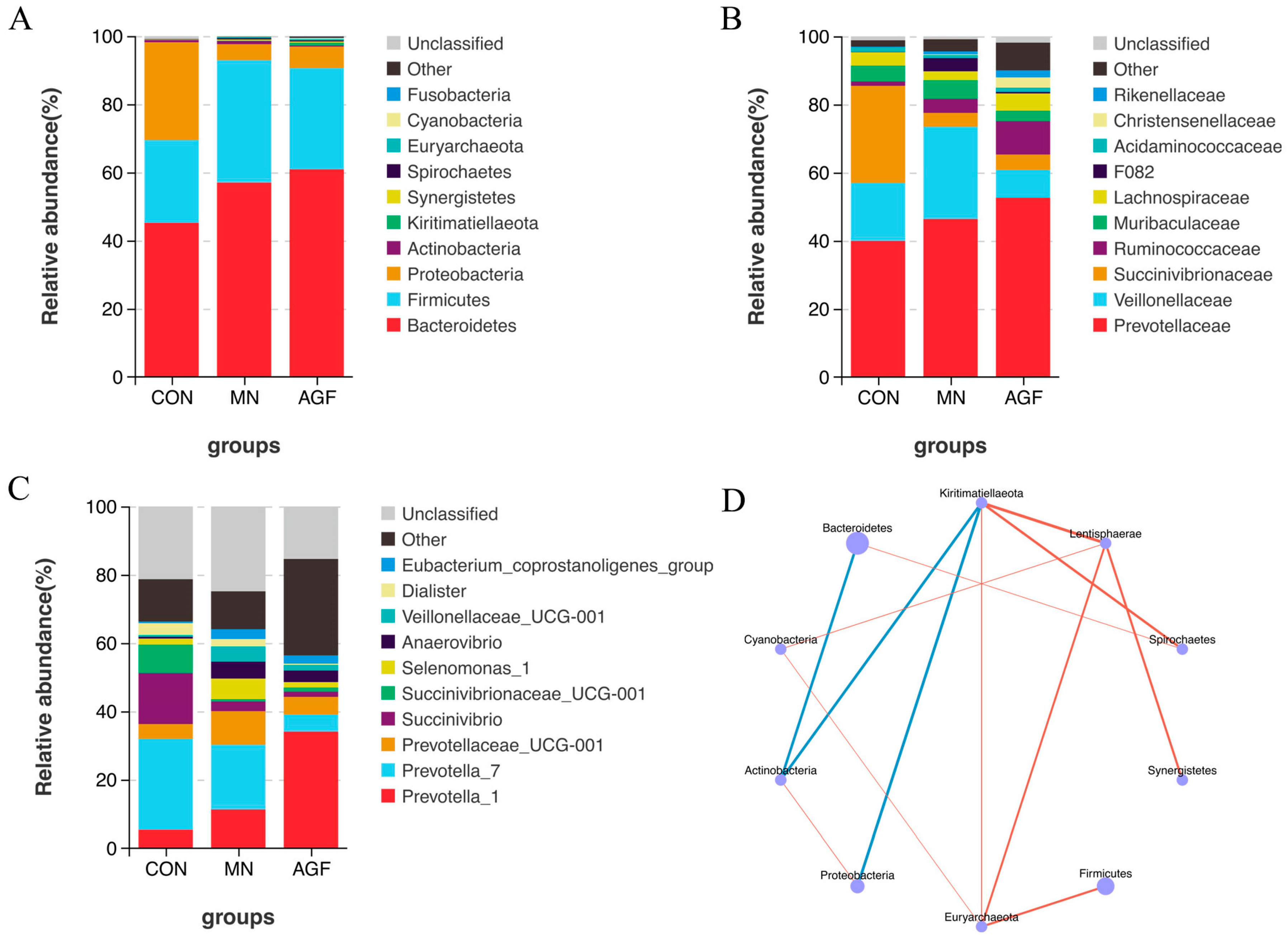
3.2.2. Composition of the Rumen Microbiota
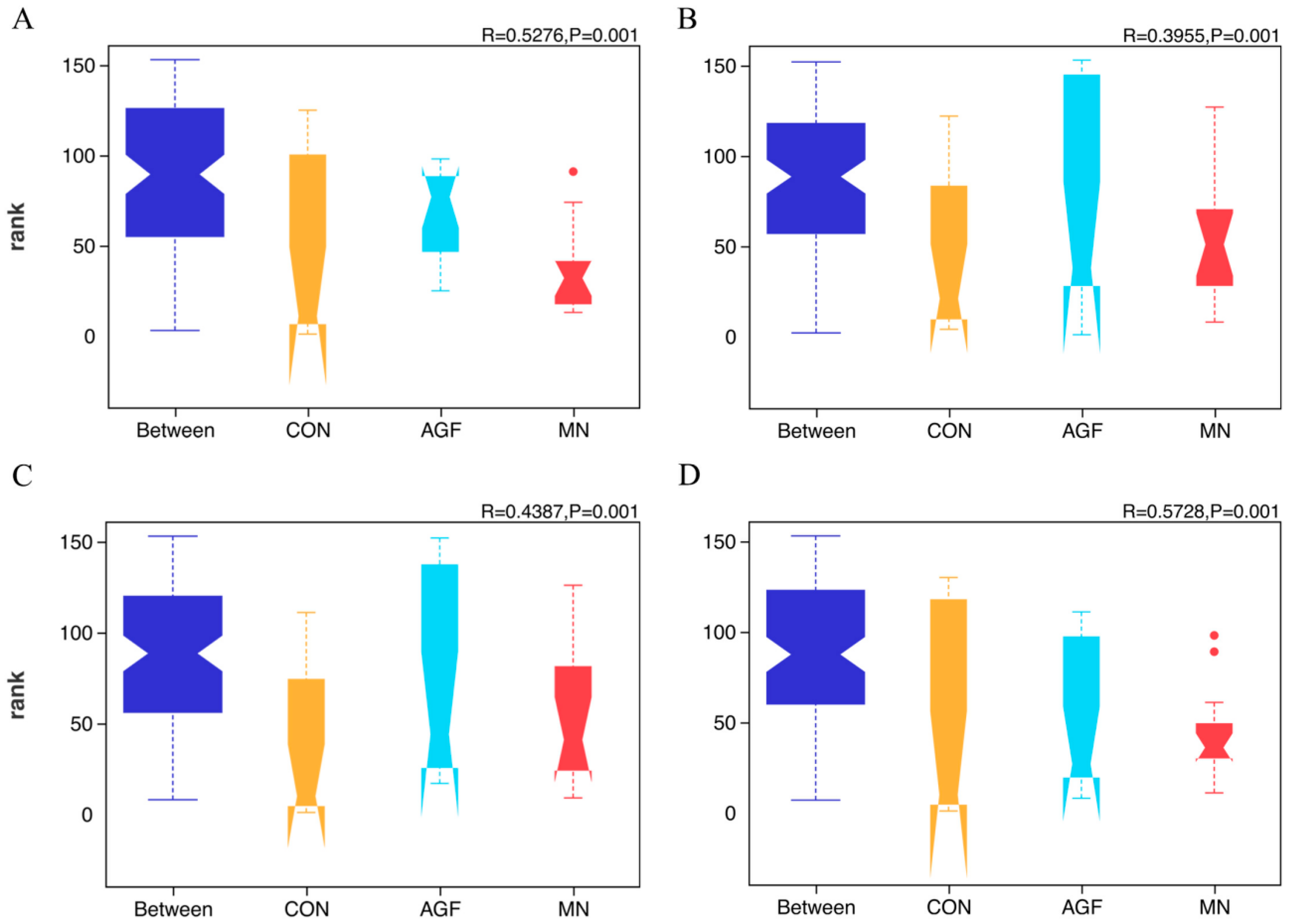
3.2.3. Diversity of the Rumen Microbiota
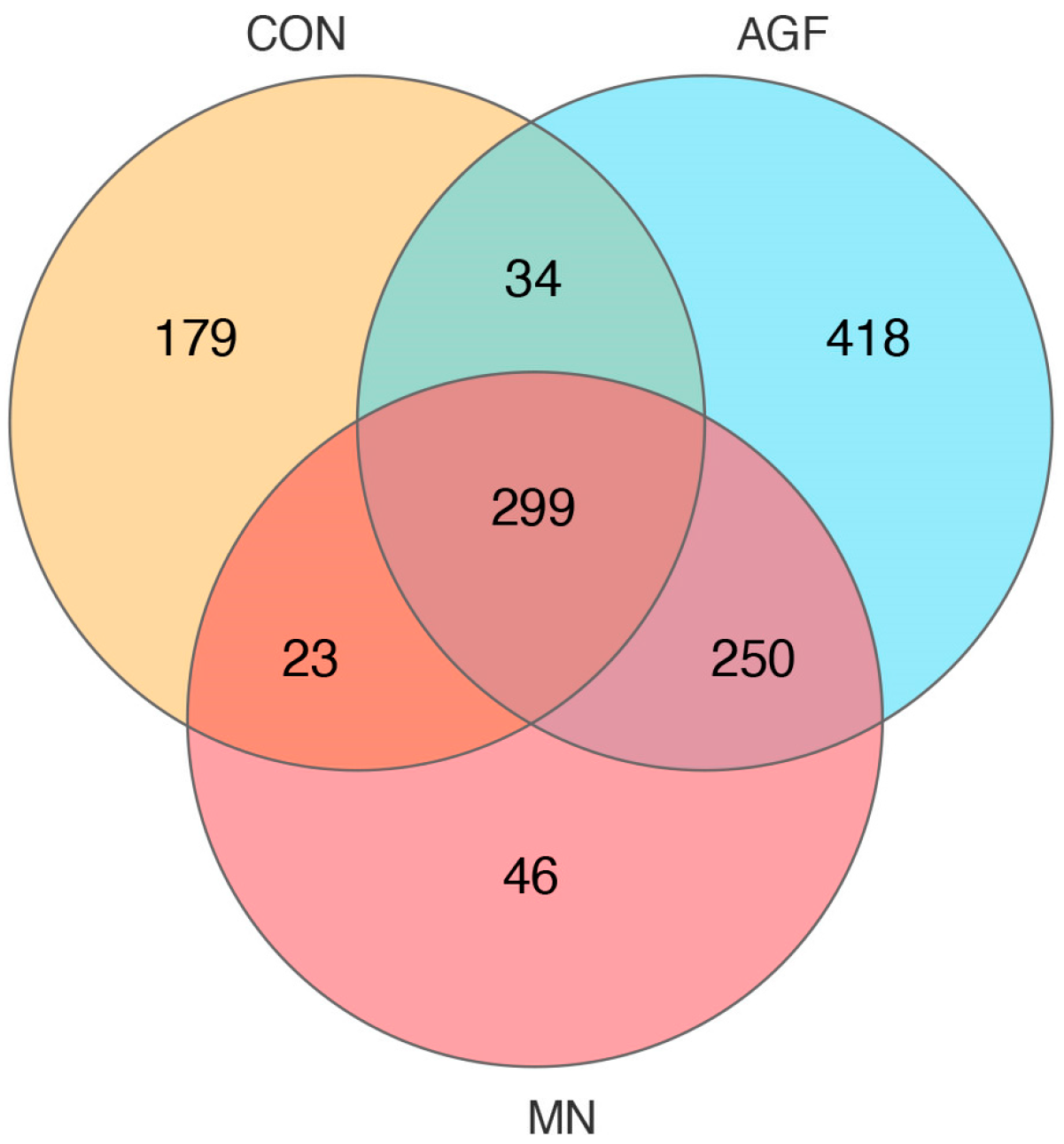
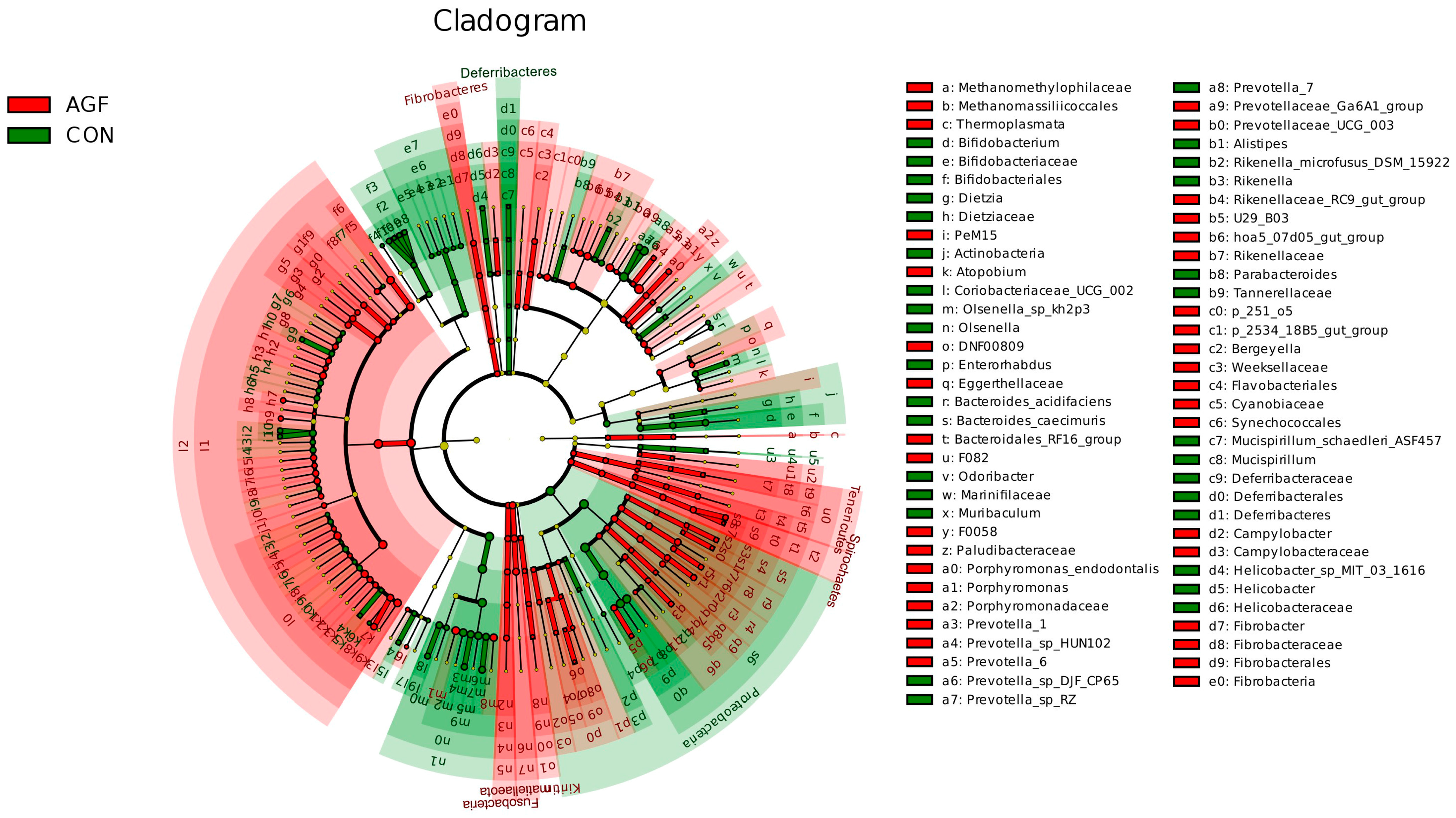
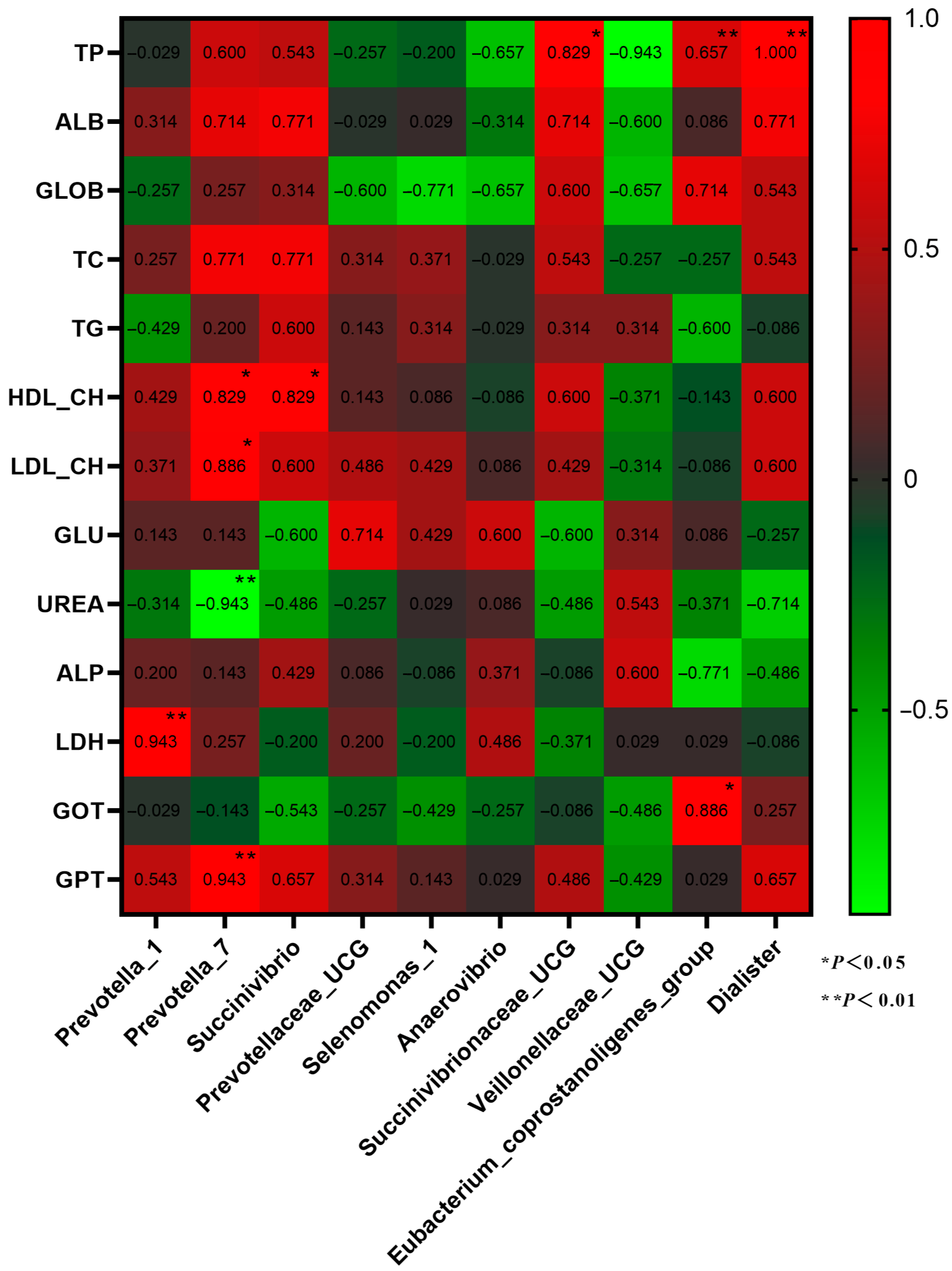
3.2.4. Analysis of Rumen Differential Microbiota
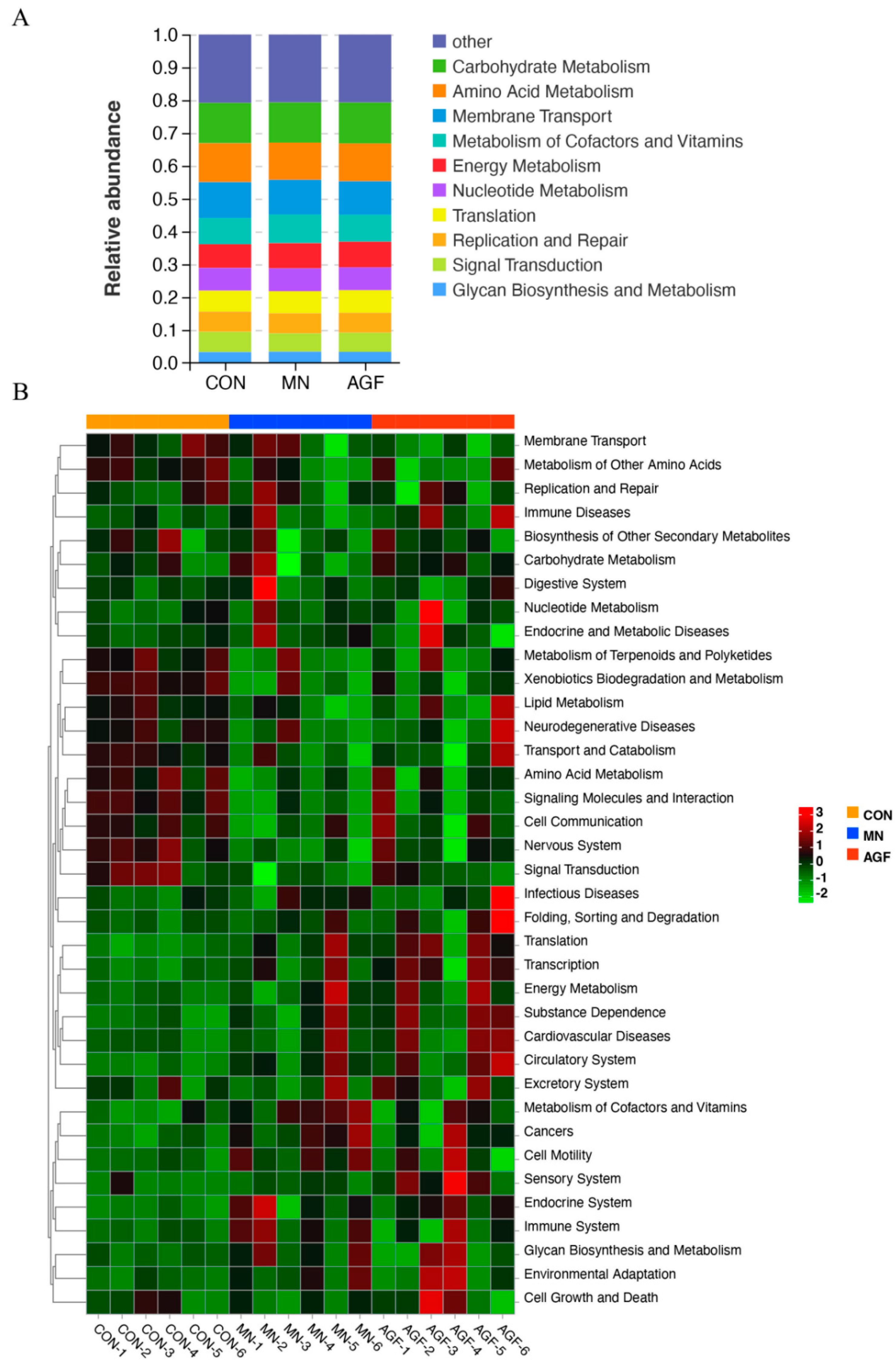
3.2.5. Microbiota Functional Profile Prediction
3.3. Plasma Physiology and Biochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, L.; Wang, H.; Duncan, S.E.; Eigel, W.N.; O’Keefe, S.F. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015, 172, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Xue, H.; Jiang, G.; Liu, X. Antioxidant activity of vine tea (Ampelopsis grossedentata) extract on lipid and protein oxidation in cooked mixed pork patties during refrigerated storage. Food Sci. Nutr. 2019, 7, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Gao, H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, F.; Yuan, Y.T.; Liu, J.; Liu, Y.; Yi, X. Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules 2019, 24, 2831. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhang, M.; Feng, J.; Han, A.; Zhang, S.; Xie, P. Effects of pretreatment by the flavanol ampelopsin on porcine kidney epithelial cell injury induced by hydrogen peroxide. Agric. Sci. China 2010, 9, 598–604. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Yang, S.; Chen, C.; Yang, Y.; Lin, M.; Liu, C.; Wang, W.; Zhou, X.; Ai, Q.; et al. Mechanism of dihydromyricetin on inflammatory diseases. Front. Pharmacol. 2022, 12, 794563. [Google Scholar] [CrossRef]
- Wan, W.; Jiang, B.; Sun, L.; Xu, L.; Xiao, P. Metabolomics reveals that vine tea (Ampelopsis grossedentata) prevents high-fat-diet-induced metabolism disorder by improving glucose homeostasis in rats. PLoS ONE 2017, 12, e0182830. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Liu, Z.; Yang, K.; Chen, Z.; Cheng, Q.; Wu, L. The versatile effects of dihydromyricetin in health. Evid.-Based Complement. Altern. Med. 2017, 2017, 1053617. [Google Scholar] [CrossRef]
- Wu, J.; Miyasaka, K.; Yamada, W.; Takeda, S.; Shimizu, N.; Shimoda, H. The anti-adiposity mechanisms of Ampelopsin and vine tea extract in high fat diet and alcohol-induced fatty liver mouse models. Molecules 2022, 27, 607. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, X.; Tong, Q.; Zhou, X.; Chen, J.; Xiong, W.; Fang, J.; Wang, W.; Shi, C. Interactions of dihydromyricetin, a flavonoid from vine tea (Ampelopsis grossedentata) with gut microbiota. J. Food Sci. 2018, 83, 1444–1453. [Google Scholar] [CrossRef]
- Li, Y.; Hu, H.; Yang, H.; Lin, A.; Xia, H.; Cheng, X.; Kong, M.; Liu, H. Vine tea (Ampelopsis grossedentata) extract attenuates CCl4-induced liver injury by restoring gut microbiota dysbiosis in mice. Mol. Nutr. Food Res. 2022, 66, e2100892. [Google Scholar] [CrossRef]
- Duffield, T.F.; Merrill, J.K.; Bagg, R.N. Meta-analysis of the effects of monensin in beef cattle on feed efficiency, body weight gain, and dry matter intake. J. Anim. Sci. 2012, 90, 4583–4592. [Google Scholar] [CrossRef]
- Ellis, J.L.; Dijkstra, J.; Bannink, A.; Kebreab, E.; Hook, S.E.; Archibeque, S.; France, J. Quantifying the effect of monensin dose on the rumen volatile fatty acid profile in high-grain-fed beef cattle. J. Anim. Sci. 2012, 90, 2717–2726. [Google Scholar] [CrossRef]
- Hecker, J.C.; Neumann, M.; Ueno, R.K.; Falbo, M.K.; Galbeiro, S.; de Souza, A.M.; Venancio, B.J.; Santos, L.C.; Askel, E. Effect of monensin sodium associative to virginiamycin and/or essential oils on the performance of feedlot finished steers. Semin. Ciências Agrárias Londrina 2018, 39, 261–274. [Google Scholar] [CrossRef]
- Goodrich, R.; Garrett, J.; Gast, D.; Kirick, M.A.; Larson, D.A.; Meiske, J.C. Influence of monensin on the performance of cattle. J Anim. Sci. 1984, 58, 1484–1498. [Google Scholar] [CrossRef]
- Vendramini, J.M.B.; Moriel, P.; Cooke, R.F.; Arthington, J.D.; da Silva, H.M.; Piccolo, M.B.; Sanchez, J.M.D.; Gomes, V.; Mamede, P.A. Effects of monensin inclusion into increasing amount of concentrate on growth and physiological parameters of early-weaned beef calves consuming warm-season grasses. J. Anim. Sci. 2018, 96, 5112–5123. [Google Scholar] [CrossRef]
- Bengtsson, B.; Wierup, M. Antimicrobial resistance in Scandinavia after ban of antimicrobial growth promoters. Anim. Biotechnol. 2006, 17, 147–156. [Google Scholar] [CrossRef]
- Gulland, A. Antimicrobial resistance will surge unless use of antibiotics in animal feed is reduced. BMJ 2013, 347, f6050. [Google Scholar] [CrossRef]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. Te European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemoth. 2003, 52, 159–161. [Google Scholar] [CrossRef]
- Zhao, J.; Harper, A.F.; Estienne, M.J.; Webb, K.E., Jr.; McElroy, A.P.; Denbow, D.M. Growth performance and intestinal morphology responses in early weaned pigs to supplementation of antibiotic-free diets with an organic copper complex and spray-dried plasma protein in sanitary and nonsanitary environments. J Anim. Sci. 2007, 85, 1302–1310. [Google Scholar] [CrossRef]
- Giorgino, A.; Raspa, F.; Valle, E.; Bergero, D.; Cavallini, D.; Gariglio, M.; Bongiorno, V.; Bussone, G.; Bergagna, S.; Cimino, F.; et al. Effect of dietary organic acids and botanicals on metabolic status and milk parameters in mid-late lactating goats. Animals 2023, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Girolami, F.; Barbarossa, A.; Badino, P.; Ghadiri, S.; Cavallini, D.; Zaghini, A.; Nebbia, C. Effects of turmeric powder on aflatoxin M1 and aflatoxicol excretion in milk from dairy cows exposed to aflatoxin B1 at the EU maximum tolerable levels. Toxins 2022, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Lodge, S.L.; Browne, J.; Horvath, M.B. Technical note: Bacterial diversity and fermentation end products in rumen fluid samples collected via oral lavage or rumen cannula. J. Anim. Sci. 2009, 87, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. bioRxiv 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, M.; Liu, J.; Zhu, W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: Membership and potential function. Sci. Rep. 2015, 3, 16116. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 1.17-4; The Comprehensive R Archive Network (CRAN): Indianapolis, IN, USA. Available online: http://CRAN.R-project.org/package=vegan (accessed on 1 April 2012).
- Wickham, H.; Chang, W. ggplot2: An Implementation of the Grammar of Graphics, R Package Version 0.7; The Comprehensive R Archive Network (CRAN): Indianapolis, IN, USA. Available online: http://CRAN.R-project.org/package=ggplot2,3 (accessed on 1 December 2016).
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 1. [Google Scholar] [CrossRef]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef]
- Marta, P.; Marco, T.; Andrea, F.; Daniela, F.; Damiano, C. Effect of does parity order on litter homogeneity parameters. Ital. J. Anim. Sci. 2020, 19, 1188–1194. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L.; Humam, A.M.; Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Vet. Res. 2019, 15, 315. [Google Scholar] [CrossRef]
- Moriel, P.; Vendramini, J.M.B.; Carnelos, C.; Piccolo, M.B.; da Silva, H.M. Effects of monensin on growth performance of beef heifers consuming warm-season perennial grass and supplemented with sugarcane molasses. Trop. Anim. Health Prod. 2019, 51, 339–344. [Google Scholar] [CrossRef]
- Jia, P.; Cui, K.; Ma, T.; Wan, F.; Wang, W.; Yang, D.; Wang, Y.; Guo, B.; Zhao, L.; Diao, Q. Influence of dietary supplementation with Bacillus licheniformis and Saccharomyces cerevisiae as alternatives to monensin on growth performance, antioxidant, immunity, ruminal fermentation and microbial diversity of fattening lambs. Sci. Rep. 2018, 8, 16712. [Google Scholar] [CrossRef]
- Xu, P.; Marsafari, M.; Zha, J.; Koffas, M. Microbial Coculture for Flavonoid Synthesis. Trends Biotechnol. 2020, 38, 686–688. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.; Ding, L.; Zeng, X.A. Dihydromyricetin: A review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2019, 91, 586–597. [Google Scholar] [CrossRef]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef]
- Singh, K.M.; Ahir, V.B.; Tripathi, A.K.; Ramani, U.V.; Sajnani, M.; Koringa, P.G.; Jakhesara, S.; Pandya, P.R.; Rank, D.N.; Murty, D.S.; et al. Metagenomic analysis of Surti buffalo (Bubalus bubalis) rumen: A preliminary study. Mol. Biol. Rep. 2012, 39, 4841–4848. [Google Scholar] [CrossRef]
- Hu, X.; Liu, G.; Shafer, A.B.A.; Wei, Y.; Zhou, J.; Lin, S.; Wu, H.B.; Zhou, M.; Hu, D.F.; Liu, S.Q. Comparative analysis of the gut microbial communities in forest and alpine musk deer using high-throughput sequencing. Front. Microbiol. 2017, 8, 572. [Google Scholar] [CrossRef]
- He, S.; Wang, Q.; Li, S.; Ran, C.; Guo, X.; Zhang, Z.; Zhou, Z. Antibiotic growth promoter olaquindox increases pathogen susceptibility in fish by inducing gut microbiota dysbiosis. Sci. China Life Sci. 2017, 60, 1260–1270. [Google Scholar] [CrossRef]
- Zhou, Z.; Fang, L.; Meng, Q.; Li, S.; Chai, S.; Liu, S.; Schonewille, J.T. Assessment of Ruminal Bacterial and Archaeal Community Structure in Yak (Bos grunniens). Front. Microbiol. 2017, 8, 179. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Mansson, M.; Gram, L.; Larsen, T.O. Production of Bioactive Secondary Metabolites by Marine Vibrionaceae. Drugs 2011, 9, 1440–1468. [Google Scholar] [CrossRef]
- Rey, F.E.; Faith, J.J.; Bain, J.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Gordon, J.I. Dissecting the in vivo metabolic potential of two human gut acetogens. J. Biol. Chem. 2010, 285, 22082–22090. [Google Scholar] [CrossRef]
- Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Fan, P.; Bian, B.; Teng, L.; Nelson, C.D.; Driver, J.; Elzo, M.A.; Jeong, K.C. Host genetic effects upon the early gut microbiota in a bovine model with graduated spectrum of genetic variation. ISME J. 2020, 14, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Pérez-Brocal, V.; Moya, A.; Portero-Otin, M.; Ricart, W.; Maldonado, R.; Fernández-Real, J.M. Gut bacterial ClpB-like gene function is associated with decreased body weight and a characteristic microbiota profile. Microbiome 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.A.; Martins, F.M.; Phifer-Rixey, M.; Nachman, M.W. The gut microbiota and Bergmann’s rule in wild house mice. Mol. Ecol. 2020, 29, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Servin, J.A.; Herbold, C.W.; Skophammer, R.G.; Lake, J.A. Evidence excluding the root of the tree of life from the actinobacteria. Mol. Biol. Evol. 2008, 25, 1–4. [Google Scholar] [CrossRef]
- Minseok, K.; James, E.W. A meta-analysis of bacterial diversity in the feces of cattle. Curr. Microbiol. 2016, 72, 145–151. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Pena, E.I.; Krajmalnik-Brown, R. Insights into butyrate production in a controlled fermentation system via gene predictions. mSystems 2017, 2, 13. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Tajasuwan, L.; Kettawan, A.; Rungruang, T.; Wunjuntuk, K.; Prombutara, P. Role of dietary defatted rice bran in the modulation of gut microbiota in AOM/DSS-induced colitis-associated colorectal cancer rat model. Nutrients 2023, 15, 1528. [Google Scholar] [CrossRef]
- Xue, B.; Wu, M.; Yue, S.; Hu, A.; Li, X.; Hong, Q.; Wang, Z.; Wang, L.; Peng, Q.; Xue, B. Changes in rumen bacterial community induced by the dietary physically effective neutral detergent fiber levels in goat diets. Front. Microbiol. 2022, 13, 820509. [Google Scholar] [CrossRef]
- Quan, J.; Cai, G.; Yang, M.; Zeng, Z.; Ding, R.; Wang, X.; Zhuang, Z.; Zhou, S.; Li, S.; Yang, H.; et al. Exploring the fecal microbial composition and metagenomic functional capacities associated with feed efficiency in commercial DLY pigs. Front. Microbiol. 2019, 10, 52. [Google Scholar] [CrossRef]
- Luan, S.; Zhang, S.; Pan, L.; Hu, W.; Cui, H.; Wei, X.; Lin, R.; Li, C.; Zeng, P.; Wang, X.; et al. Salivary microbiota analysis of patients with membranous nephropathy. Mol. Med. Rep. 2022, 25, 90. [Google Scholar] [CrossRef]
- Fonseca, P.P.; Monteiro, R.C.; Araújo, W.L.; Nunes, N.A. Harnessing enzyme cofactors and plant metabolism: An essential partnership. Plant J. 2023, 3, 2. [Google Scholar] [CrossRef]
- Du, Q.; Wang, H.; Xie, J. Thiamin (vitamin B1) biosynthesis and regulation: A rich source of antimicrobial drug targets? Int. J. Biol. Sci. 2011, 7, 41–52. [Google Scholar] [CrossRef]
- Thakur, K.; Tomar, S.K.; Singh, A.K.; Mandal, S.; Arora, S. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef]
- Nkrumah, J.D.; Sherman, E.L.; Li, C.; Marques, E.; Crews, D.H., Jr.; Bartusiak, R.; Murdoch, B.; Wang, Z.; Basarab, J.A.; Moore, S.S. Primary genome scan to identify putative quantitative trait loci for feedlot growth rate, feed intake, and feed efficiency of beef cattle. J. Anim. Sci. 2007, 85, 3170–3181. [Google Scholar] [CrossRef][Green Version]
- Anassori, E.; Dalir-Naghadeh, B.; Pirmohammadi, R.; Hadian, M. Changes in blood profile in sheep receiving raw garlic, garlic oil or monensin. J. Anim. Physiol. Anim. Nutr. 2015, 99, 114–122. [Google Scholar] [CrossRef]
- Zhong, R.; Fang, Y.; Zhou, D.; Sun, X.; Zhou, C.; He, Y. Pelleted total mixed ration improves growth performance of fattening lambs. Anim. Feed Sci. Technol. 2018, 242, 127–134. [Google Scholar] [CrossRef]
- Bauchart, D. Lipid absorption and transport in ruminants. J. Dairy Sci. 1993, 76, 3864–3881. [Google Scholar] [CrossRef]
- Dabbou, S.; Gasco, L.; Rotolo, L.; Pozzo, L.; Tong, J.M.; Dong, X.F.; Rubiolo, P.; Schiavone, A.; Gai, F. Effects of dietary alfalfa flavonoids on the performance, meat quality and lipid oxidation of growing rabbits. Asian-Australas. J. Anim. Sci. 2018, 31, 270–277. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Liu, X.; Wang, N.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; Ouyang, K.H.; et al. Investigation of chemical composition, antioxidant activity, and the effects of alfalfa flavonoids on growth performance. Oxid. Med. Cell. Longev. 2020, 2020, 8569237. [Google Scholar] [CrossRef] [PubMed]
- Gadberry, S.; Lalman, D.; White, F.; Linneen, S.; Beck, P. Meta-analysis of the effects of monensin on growth and bloat of cattle on pasture. Transl. Anim. Sci. 2022, 6, txac031. [Google Scholar] [CrossRef] [PubMed]
- Code of Federal Regulations. Title 21: Food and Drugs. Sub-Chapter E—Animal Drugs, Feeds, and Related Products. 2019. Available online: http://www.ecfr.gov/ (accessed on 22 May 2020).
- Stahl, T.C.; Hatungimana, E.; Klanderman, K.D.; Moreland, S.C.; Erickson, P.S. Sodium butyrate and monensin supplementation to postweaning heifer diets: Effects on growth performance, nutrient digestibility, and health. J. Dairy Sci. 2020, 103, 10207–10218. [Google Scholar] [CrossRef] [PubMed]
- Hales, K.E.; Wells, J.E.; Berry, E.D.; Kalchayanand, N.; Bono, J.L.; Kim, M. The effects of monensin in diets fed to finishing beef steers and heifers on growth performance and fecal shedding of Escherichia coli O157:H7. J. Anim. Sci. 2017, 95, 3738–3744. [Google Scholar] [CrossRef]


| Material | Content (%) | Nutrients | Content |
|---|---|---|---|
| Corn | 30.00 | Digestible energy (MJ/Kg) | 12.57 |
| DDGS | 8.00 | Crude protein | 14.07 |
| Fatty powder | 5.00 | EE | 3.11 |
| Grass meal | 55.00 | CF | 16.83 |
| CaHPO4 | 0.50 | NDF | 27.42 |
| NaCl | 0.50 | ADF | 21.17 |
| Premix | 1.00 | Calcium | 1.14 |
| Total | 100.00 | Total phosphorus | 0.34 |
| Items | CON Group | MN Group | AGF Group |
|---|---|---|---|
| IBW (kg) | 14.18 ± 1.84 | 14.88 ± 1.19 | 14.69 ± 2.06 |
| FBW (kg) | 19.80 ± 1.66 b | 22.13 ± 1.97 a | 22.51 ± 3.50 a |
| ADFI (g) | 683.44 ± 21.29 | 686.85 ± 21.66 | 699.15 ± 21.83 |
| ADG (g) | 62.43 ± 10.39 a | 80.63 ± 16.20 b | 86.77 ± 20.29 b |
| F/G | 13.77 ± 4.91 a | 9.05 ± 1.64 b | 8.44 ± 1.13 b |
| Items | CON Group | MN Group | AGF Group |
|---|---|---|---|
| TP (g/L) | 62.28 ± 1.54 | 65.33 ± 1.95 | 60.21 ± 3.34 |
| ALB (g/L) | 26.05 ± 0.71 | 26.66 ± 0.47 | 24.40 ± 1.61 |
| GLOB (g/L) | 36.23 ± 1.51 | 38.66 ± 2.14 | 35.81 ± 2.47 |
| TC (mmol/L) | 1.79 ± 0.16 | 1.72 ± 0.14 | 1.76 ± 0.20 |
| TG (mmol/L) | 0.30 ± 0.05 | 0.25 ± 0.05 | 0.27 ± 0.08 |
| HDL-CH (mmol/L) | 0.93 ± 0.07 a | 1.0 ±0.08 ab | 1.26 ± 0.14 b |
| LDL-CH (mmol/L) | 0.95 ± 0.12 a | 0.73 ± 0.08 ab | 0.64 ± 0.09 b |
| GLU (mmol/L) | 3.02 ± 0.19 a | 3.32 ± 0.15 ab | 3.62 ± 0.10 b |
| UREA (mmol/L) | 2.95 ± 0.47 Aa | 4.66 ± 0.35 Bb | 4.41 ± 0.30 b |
| ALP (U/L) | 449.00 ± 106.84 a | 206.50 ± 31.29 b | 192.43 ± 60.37 b |
| LDH (U/L) | 238.83 ± 7.89 | 257.88 ± 21.67 | 270.71 ± 17.83 |
| GOT (U/L) | 110.50 ± 11.86 A | 68.63 ± 4.42 B | 85.14 ± 12.94 AB |
| GPT (U/L) | 17.50 ± 1.28 a | 13.25 ± 1.44 b | 13.00 ± 1.23 b |
| GOT/GPT | 6.40 ± 0.66 | 5.52 ± 0.50 | 7.58 ± 2.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Liu, X.; Lu, Y.; Yue, D.; He, X.; Deng, W.; Zhao, S.; Xi, D. Exploring the Impact of Ampelopsis Grossedentata Flavonoids on Growth Performance, Ruminal Microbiota, and Plasma Physiology and Biochemistry of Kids. Animals 2023, 13, 2454. https://doi.org/10.3390/ani13152454
Zhu J, Liu X, Lu Y, Yue D, He X, Deng W, Zhao S, Xi D. Exploring the Impact of Ampelopsis Grossedentata Flavonoids on Growth Performance, Ruminal Microbiota, and Plasma Physiology and Biochemistry of Kids. Animals. 2023; 13(15):2454. https://doi.org/10.3390/ani13152454
Chicago/Turabian StyleZhu, Junhong, Xingneng Liu, Ying Lu, Dan Yue, Xiaoming He, Weidong Deng, Sumei Zhao, and Dongmei Xi. 2023. "Exploring the Impact of Ampelopsis Grossedentata Flavonoids on Growth Performance, Ruminal Microbiota, and Plasma Physiology and Biochemistry of Kids" Animals 13, no. 15: 2454. https://doi.org/10.3390/ani13152454
APA StyleZhu, J., Liu, X., Lu, Y., Yue, D., He, X., Deng, W., Zhao, S., & Xi, D. (2023). Exploring the Impact of Ampelopsis Grossedentata Flavonoids on Growth Performance, Ruminal Microbiota, and Plasma Physiology and Biochemistry of Kids. Animals, 13(15), 2454. https://doi.org/10.3390/ani13152454





