Reference Range of Kaolin-Activated Thromboelastography (TEG) Values in Healthy Pet Rabbits (Oryctolagus cuniculus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Selected
2.2. Sample Collection and Processing
2.3. Thromboelastography
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, M.B.; Harr, K.E.; Seelig, D.M.; Wardrop, K.J.; Weiss, D.J. (Eds.) Schalm’s Veterinary Hematology, 7th ed.; Wiley: Hoboken, NJ, USA, 2022; Available online: https://www.wiley.com/en-ie/Schalm’s+Veterinary+Hematology,+7th+Edition-p-9781119500520 (accessed on 5 April 2023).
- McMichael, M. New Models of Hemostasis. Top. Companion Anim. Med. 2012, 27, 40–45. [Google Scholar] [CrossRef]
- Smith, S.A. The Cell-Based Model of Coagulation: State-Of-The-Art Review. J. Vet. Emerg. Crit. Care 2009, 19, 3–10. [Google Scholar] [CrossRef]
- Hoffman, M.; Monroe, D.M. A Cell-Based Model of Hemostasis. Thromb. Haemost. 2001, 85, 958–965. [Google Scholar] [CrossRef]
- Wiinberg, B.; Kristensen, A.T. Thromboelastography in Veterinary Medicine. Semin. Thromb. Hemost. 2010, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.M.; Naik-Mathuria, B.J.; Gay, A.N.; Olutoye, O.O.; Teruya, J. Parameters of Thromboelastography in Healthy Newborns. Am. J. Clin. Pathol. 2008, 130, 99–102. [Google Scholar] [CrossRef]
- Mcmichael, M.; Goggs, R.; Smith, S.; Wagg, C.; Warman, S.; Wiinberg, B. Systematic Evaluation of Evidence on Veterinary Viscoelastic Testing Part 1: System Comparability. J. Vet. Emerg. Crit. Care 2014, 24, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.J.; Rozanski, E.A.; Brainard, B.M.; de Laforcade, A.M.; Brooks, M.B. Assessment of the Relationships among Coagulopathy, Hyperfibrinolysis, Plasma Lactate, and Protein C in Dogs with Spontaneous Hemoperitoneum. J. Vet. Emerg. Crit. Care 2016, 26, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Rieser, T.M.; Brooks, M.B.; Russell, M.W. Evidence of Hypercoagulability in Dogs with Parvoviral Enteritis. J. Am. Vet. Med. Assoc. 2000, 217, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.T.; Wiinberg, B.; Jessen, L.R.; Andreasen, E.; Jensen, A.L. Evaluation of Human Recombinant Tissue Factor-Activated Thromboelastography in 49 Dogs with Neoplasia. J. Vet. Intern. Med. 2008, 22, 140–147. [Google Scholar] [CrossRef]
- Shimokawa, M.; Kitaguchi, K.; Kawaguchi, M.; Sakamoto, T.; Kakimoto, M.; Furuya, H. The Influence of Induced Hypothermia for Hemostatic Function on Temperature-Adjusted Measurements in Rabbits. Anesth. Analg. 2003, 96, 1209–1213. [Google Scholar] [CrossRef]
- Tranholm, M.; Rojkjaer, R.; Pyke, C.; Kristensen, A.T.; Klitgaard, B.; Lollike, K.; Blajchman, M.A. Recombinant Factor VIIa Reduces Bleeding in Severely Thrombocytopenic Rabbits. Thromb. Res. 2003, 109, 217–223. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Baird, M.S. Extreme Hemodilution in Rabbits: An in Vitro and in Vivo Thrombelastographic® Analysis. Anesth. Analg. 2000, 90, 541–545. [Google Scholar] [CrossRef]
- Wiinberg, B.; Lundorff Jensen, A.; Rojkjaer, R.; Johansson, P.; Kjelgaard-Hansen, M.; Kristensen, A.T. Validation of Human Recombinant Tissue Factor-Activated Thromboelastography on Citrated Whole Blood from Clinically Healthy Dogs. Vet. Clin. Pathol. 2005, 34, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Jessen, L.R.; Wiinberg, B.; Jensen, A.L.; Kjelgaard-Hansen, M.; Jensen, K.H.; Pedersen, L.B.; Kristensen, A.T. In Vitro Heparinization of Canine Whole Blood with Low Molecular Weight Heparin (Dalteparin) Significantly and Dose-Dependently Prolongs Heparinase-Modified Tissue Factor-Activated Thromboelastography Parameters and Prothrombinase-Induced Clotting Time. Vet. Clin. Pathol. 2008, 37, 363–372. [Google Scholar] [CrossRef]
- Bauer, N.; Eralp, O.; Moritz, A. Establishment of Reference Intervals for Kaolin-Activated Thromboelastography in Dogs Including an Assessment of the Effects of Sex and Anticoagulant Use. J. Vet. Diagn. Invest 2009, 21, 641–648. [Google Scholar] [CrossRef]
- Marschner, C.B.; Bjørnvad, C.R.; Kristensen, A.T.; Wiinberg, B. Thromboelastography Results on Citrated Whole Blood from Clinically Healthy Cats Depend on Modes of Activation. Acta Vet. Scand. 2010, 52, 38. [Google Scholar] [CrossRef]
- Mendez-Angulo, J.L.; Mudge, M.C.; Vilar-Saavedra, P.; Stingle, N.; Couto, C.G. Thromboelastography in Healthy Horses and Horses with Inflammatory Gastrointestinal Disorders and Suspected Coagulopathies. J. Vet. Emerg. Crit. Care 2010, 20, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Wohlauer, M.V.; Moore, E.E.; Harr, J.; Gonzalez, E.; Fragoso, M.; Silliman, C.C. A Standardized Technique for Performing Thromboelastography in Rodents. Shock 2011, 36, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Jankun, J.; Selman, S.H.; Keck, R.W.; Łysiak-Szydłowska, W.; Skrzypczak-Jankun, E. Very Long Half-Life Plasminogen Activator Inhibitor Type 1 Reduces Bleeding in a Mouse Model. BJU Int. 2010, 105, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Flight, S.M.; Masci, P.P.; Lavin, M.F.; Gaffney, P.J. Resistance of Porcine Blood Clots to Lysis Relates to Poor Activation of Porcine Plasminogen by Tissue Plasminogen Activator. Blood Coagul. Fibrinolysis 2006, 17, 417–420. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Geary, B.T.; Baird, M.S. Evaluation of the Contribution of Platelets to Clot Strength by Thromboelastography in Rabbits: The Role of Tissue Factor and Cytochalasin D. Anesth. Analg. 2000, 91, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Peng, L.; Zhao, H. Diverse Coagulopathies in a Rabbit Model with Different Abdominal Injuries. World J. Emerg. Med. 2017, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G. The Detection of Changes in Heparin Activity in the Rabbit: A Comparison of Anti-Xa Activity, Thrombelastography®, Activated Partial Thromboplastin Time, and Activated Coagulation Time. Anesth. Analg. 2002, 95, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Sánchez, E.E.; Redford, D.T. Characterization of the Rabbit as an In Vitro and In Vivo Model to Assess the Effects of Fibrinogenolytic Activity of Snake Venom on Coagulation. Basic Clin. Pharmacol. Toxicol. 2018, 122, 157–164. [Google Scholar] [CrossRef] [PubMed]
- McCammon, A.T.; Wright, J.P.; Figueroa, M.; Nielsen, V.G. Hemodilution with Albumin, but Not Hextend, Results in Hypercoagulability as Assessed by Thrombelastography in Rabbits: Role of Heparin-Dependent Serpins and Factor VIII Complex. Anesth. Analg. 2002, 95, 844–850. [Google Scholar] [CrossRef]
- Burton, A.G.; Jandrey, K.E. Use of Thromboelastography in Clinical Practice. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 1397–1409. [Google Scholar] [CrossRef]
- Hanel, R.M.; Chan, D.L.; Conner, B.; Gauthier, V.; Holowaychuk, M.; Istvan, S.; Walker, J.M.; Wood, D.; Goggs, R.; Wiinberg, B. Systematic Evaluation of Evidence on Veterinary Viscoelastic Testing Part 4: Definitions and Data Reporting. J. Vet. Emerg. Crit. Care 2014, 24, 47–56. [Google Scholar] [CrossRef]
- De Laforcade, A.; Goggs, R.; Wiinberg, B. Systematic Evaluation of Evidence on Veterinary Viscoelastic Testing Part 3: Assay Activation and Test Protocol. J. Vet. Emerg. Crit. Care 2014, 24, 37–46. [Google Scholar] [CrossRef]
- Flatland, B.; Koenigshof, A.M.; Rozanski, E.A.; Goggs, R.; Wiinberg, B. Systematic Evaluation of Evidence on Veterinary Viscoelastic Testing Part 2: Sample Acquisition and Handling. J. Vet. Emerg. Crit. Care 2014, 24, 30–36. [Google Scholar] [CrossRef]
- Leclere, M.; Lavoie, J.P.; Dunn, M.; Bédard, C. Evaluation of a Modified Thrombelastography Assay Initiated with Recombinant Human Tissue Factor in Clinically Healthy Horses. Vet. Clin. Pathol. 2009, 38, 462–466. [Google Scholar] [CrossRef]
- Johansson, P.I.; Bochsen, L.; Andersen, S.; Viuff, D. Investigation of the Effect of Kaolin and Tissue-Factor-Activated Citrated Whole Blood, on Clot-Forming Variables, as Evaluated by Thromboelastography. Transfusion 2008, 48, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Thalheimer, U.; Triantos, C.K.; Samonakis, D.N.; Zambruni, A.; Senzolo, M.; Leandro, G.; Patch, D.; Burroughs, A.K. A Comparison of Kaolin-Activated versus Nonkaolin-Activated Thromboelastography in Native and Citrated Blood. Blood Coagul. Fibrinolysis 2008, 19, 495–501. [Google Scholar] [CrossRef]
- Flint, S.K.; Wood, R.D.; Abrams-Ogg, A.C.G.; Kruth, S.A.; Bersenas, A. Comparison of Citrated Native and Kaolin-Activated Samples for Thrombelastographic Analysis in Healthy Dogs. Vet. Clin. Pathol. 2012, 41, 249–255. [Google Scholar] [CrossRef]
- Chan, K.L.; Summerhayes, R.G.; Ignjatovic, V.; Horton, S.B.; Monagle, P.T. Reference Values for Kaolin-Activated Thromboelastography in Healthy Children. Anesth. Analg. 2007, 105, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP Reference Interval Guidelines: Determination of de Novo Reference Intervals in Veterinary Species and Other Related Topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G. Crotalus Atrox Venom Exposed to Carbon Monoxide Has Decreased Fibrinogenolytic Activity In Vivo in Rabbits. Basic Clin. Pharmacol. Toxicol. 2018, 122, 82–86. [Google Scholar] [CrossRef]
- Nielsen, V.G. Hemodilution with Lactated Ringer’s Solution Causes Hypocoagulability in Rabbits. Blood Coagul. Fibrinolysis 2004, 15, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G. Effects of PentaLyte® and Voluven® Hemodilution on Plasma Coagulation Kinetics in the Rabbit: Role of Thrombin-Fibrinogen and Factor XIII-Fibrin Polymer Interactions. Acta Anaesthesiol. Scand. 2005, 49, 1263–1271. [Google Scholar] [CrossRef]
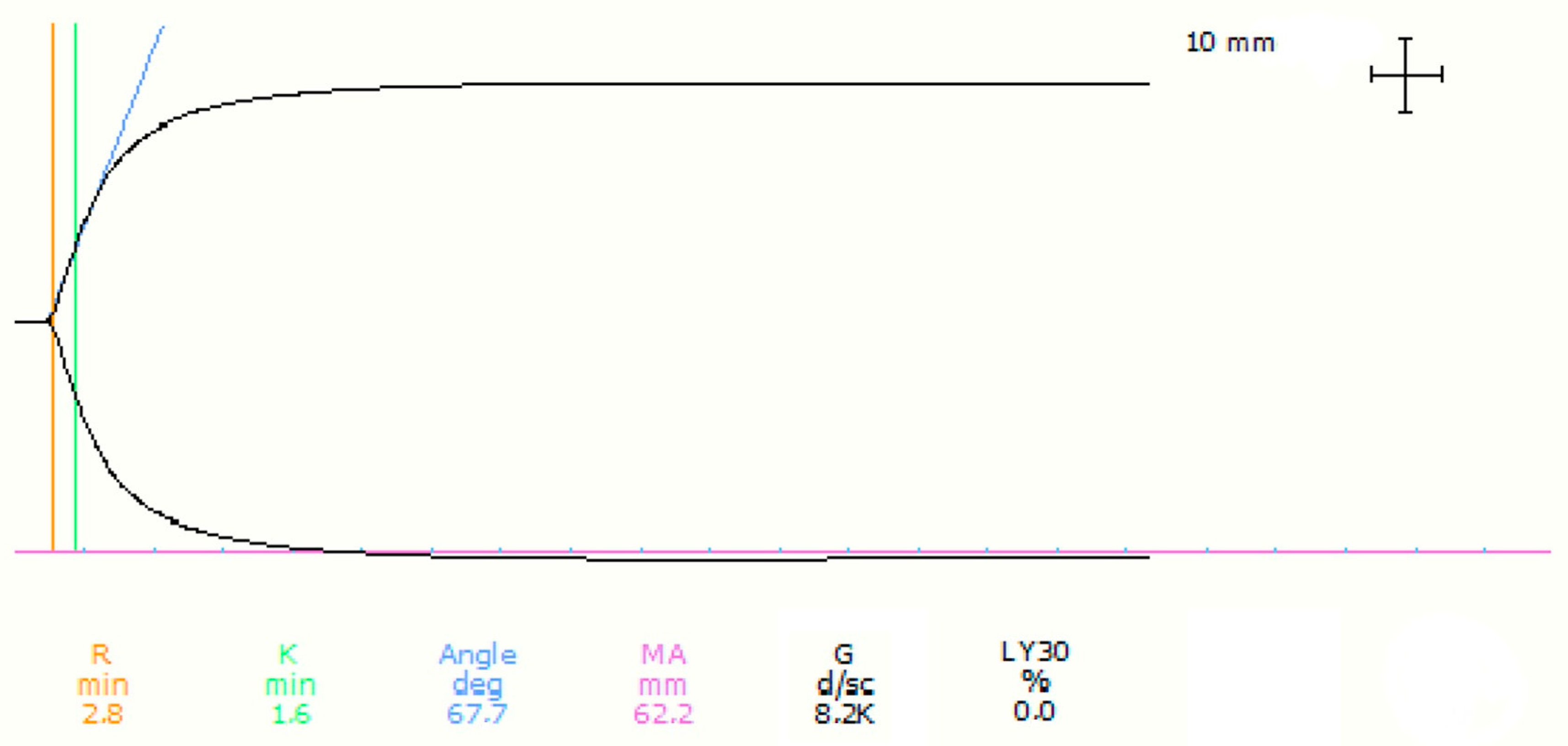
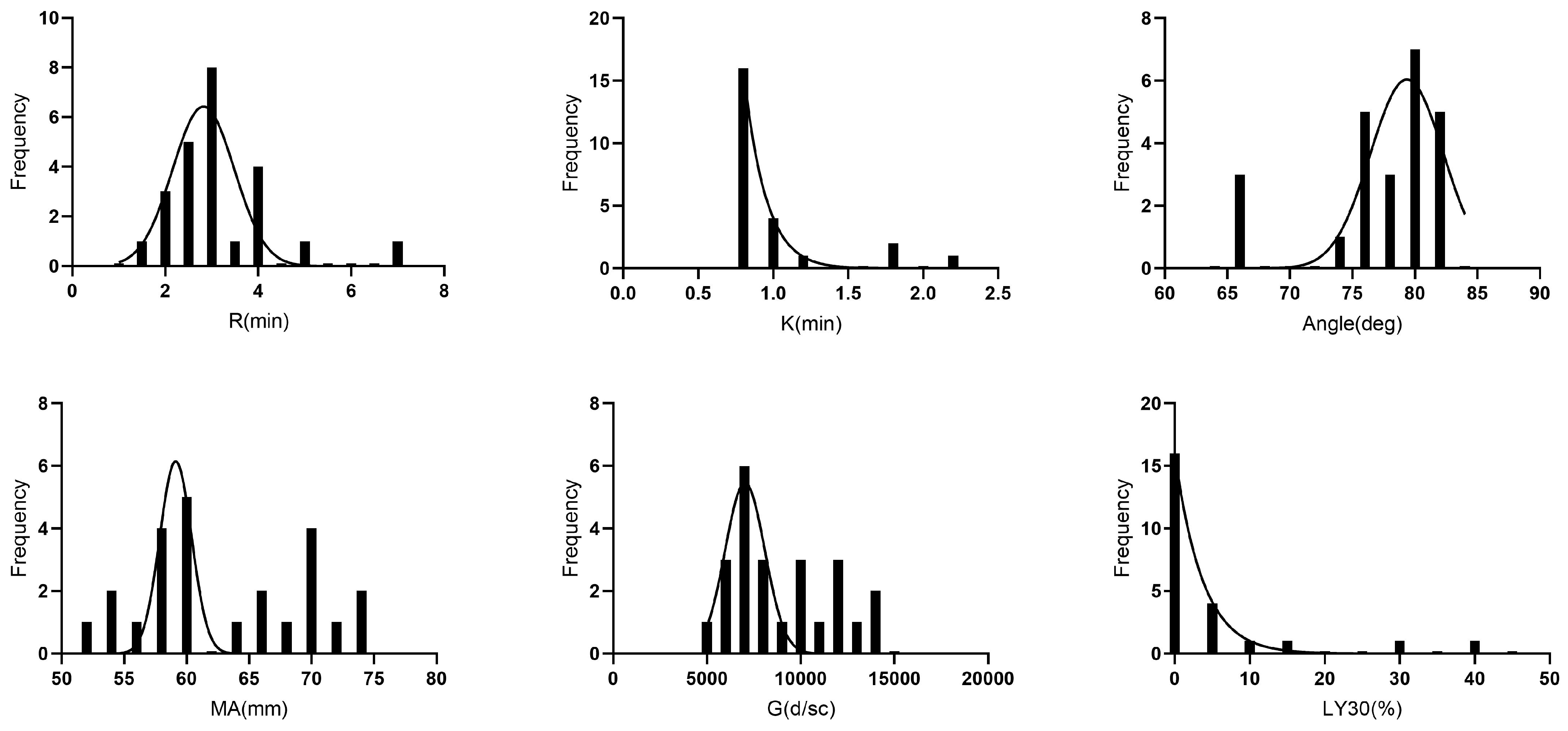
| Parameter (Unit) | Mean | Standard Deviation | Range |
|---|---|---|---|
| Haematocrit (%) | 34 | 2.7 | 31.3–43.3 |
| Erythrocytes (×106/µL) | 5.4 | 0.5 | 4.5–6.9 |
| Haemoglobin (g/dL) | 11.4 | 1 | 11.0–14.4 |
| Mean corpuscular haemoglobin (Pg/cell) | 21.2 | 1.2 | 19.4–23.8 |
| Mean corpuscular haemoglobin concentration (g/dL) | 33.6 | 0.8 | 32.3–34.5 |
| Mean corpuscular volume (g/µm3) | 63 | 2.6 | 59.0–70.1 |
| Platelets (×103/µL) | 484.6 | 122.1 | 134–567 |
| White blood cells (×103/µL) | 7.5 | 2.7 | 4.1–10.8 |
| Neutrophils (×103/µL) | 2.8 | 1.3 | 1.1–7.4 |
| Lymphocytes (×103/µL) | 4.1 | 1.6 | 0.5–6.5 |
| Monocytes (×103/µL) | 0.2 | 0.1 | 0–3.7 |
| Eosinophils (×103/µL) | 0.01 | 0.01 | 0–0.03 |
| Basophils (×103/µL) | 0.4 | 0.3 | 0–0.4 |
| Parameter (Unit) | Mean | Standard Deviation | Range |
|---|---|---|---|
| Alanine transferase (U/L) | 75.6 | 25.5 | 52–80 |
| Bilirubin (total) (mg/dL) | 0.14 | 0.03 | 0.1–0.5 |
| Chloride (mmol/L) | 106 | 2.8 | 96–109 |
| Cholesterol (mg/dL) | 35.2 | 10.9 | 6–65 |
| Creatinine (mg/dL) | 1.1 | 0.2 | 1.0–2.2 |
| Glucose (mg/dL) | 150.5 | 15.7 | 109–161 |
| Phosphorous (mg/dL) | 3.4 | 0.9 | 3.0–6.2 |
| Potassium (mmol/L) | 4.5 | 0.4 | 3.4–5.1 |
| Sodium (mmol/L) | 142.8 | 2.4 | 138–148 |
| Total protein (g/dL) | 6.3 | 0.4 | 6.1–7.7 |
| Parameter (Unit) | Mean | Standard Deviation | Range |
|---|---|---|---|
| Fibrinogen (mg/dL) | 348 | 138.8 | 289.0–393.3 |
| PT (s) | 8.7 | 0.5 | 4.5–10.5 |
| PTTa (s) | 16.3 | 2.3 | 15.7–42.7 |
| Variable (Unit) | Distribution of Data | Mean | Standard Deviation | Median Range (Min–Max) | Lower 5% Reference Limit | Upper 95% Reference Limit | Method | Out of RI n/tot (%) |
|---|---|---|---|---|---|---|---|---|
| R (min) | Not normal | 3.6 | 1.2 | 3 (1.4–6.9) | 1.9 | 5 | RUD | 1/24 (4.2) |
| K (min) | Not normal | 1 | 0.4 | 0.8 (0.8–2.2) | 0.9 | 1.8 | RUD | 3/24 (12.5) |
| α Angle (degrees) | Not normal | 77.4 | 5 | 79.0 (65.8–82.2) | 65.9 | 82.1 | RUD | 3/24 (12.5) |
| MA (mm) | Normal | 62.9 | 6.7 | 60.6 (52.4–73.54) | 53.7 | 73.5 | SUD | 0/24 |
| G (dyn/cm2) | Normal | 8969.1 | 2711.7 | 7688.6 (5497.0–14,213.4) | 5796.6 | 13,885.9 | SUD | 0/24 |
| LY30 (%) | Not normal | 4.7 | 10.1 | 0 (0–41.5) | 0 | 25.7 | RUD | 3/24 (12.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassan, T.; Pastor, J.; Agulla, B.; Jornet, O.; Martorell, J. Reference Range of Kaolin-Activated Thromboelastography (TEG) Values in Healthy Pet Rabbits (Oryctolagus cuniculus). Animals 2023, 13, 2389. https://doi.org/10.3390/ani13142389
Bassan T, Pastor J, Agulla B, Jornet O, Martorell J. Reference Range of Kaolin-Activated Thromboelastography (TEG) Values in Healthy Pet Rabbits (Oryctolagus cuniculus). Animals. 2023; 13(14):2389. https://doi.org/10.3390/ani13142389
Chicago/Turabian StyleBassan, Tiziana, Josep Pastor, Beatriz Agulla, Oriol Jornet, and Jaume Martorell. 2023. "Reference Range of Kaolin-Activated Thromboelastography (TEG) Values in Healthy Pet Rabbits (Oryctolagus cuniculus)" Animals 13, no. 14: 2389. https://doi.org/10.3390/ani13142389
APA StyleBassan, T., Pastor, J., Agulla, B., Jornet, O., & Martorell, J. (2023). Reference Range of Kaolin-Activated Thromboelastography (TEG) Values in Healthy Pet Rabbits (Oryctolagus cuniculus). Animals, 13(14), 2389. https://doi.org/10.3390/ani13142389





