Establishment of Primary Adult Skin Fibroblast Cell Lines from African Savanna Elephants (Loxodonta africana)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
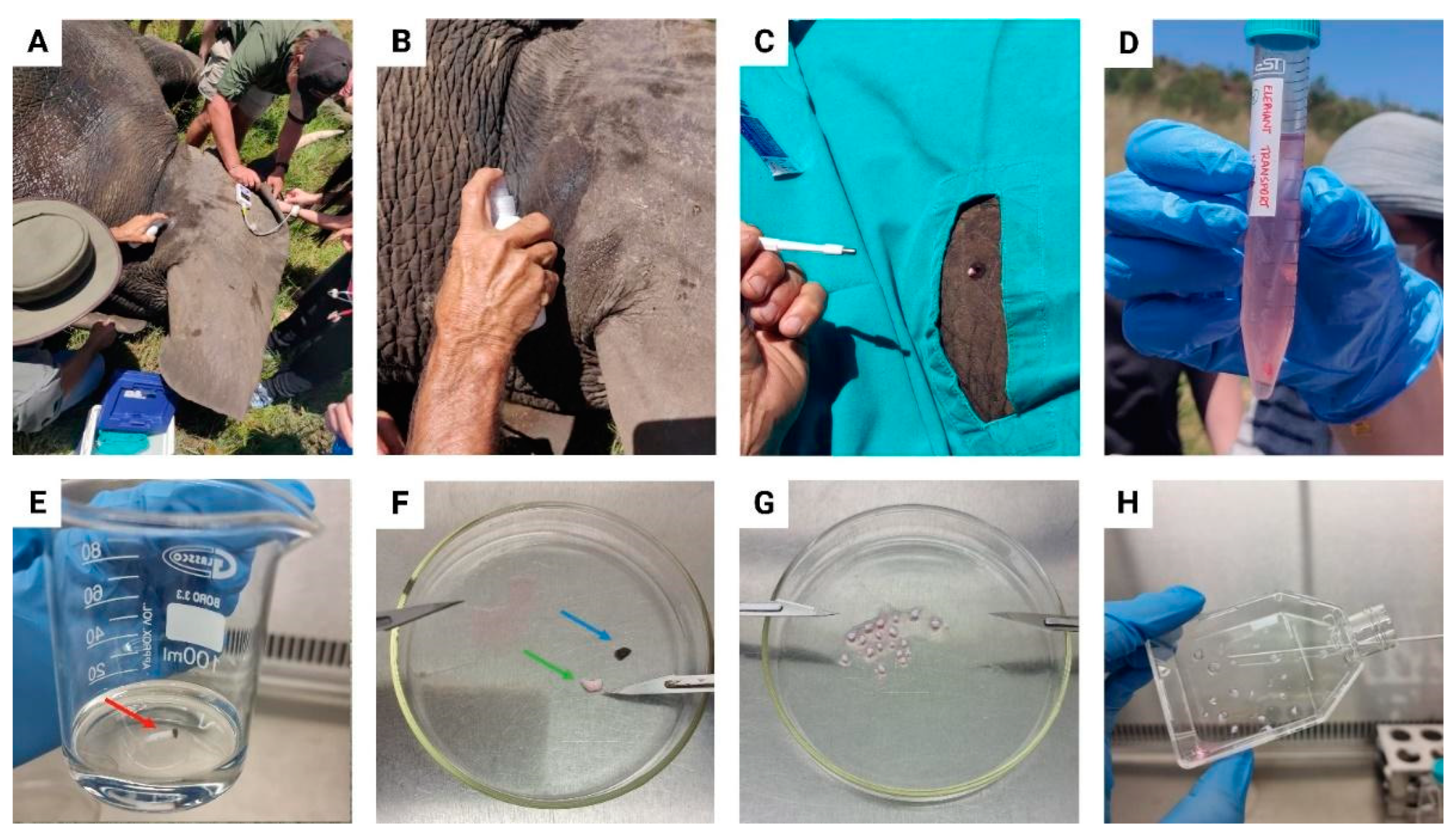
2.1. Tissue Sample Collection
2.2. Biopsy Preparation and Establishment of the Primary EDF Cell Culture
2.3. Primary EDF Growth Curve and Doubling Time (Td) Determination
2.4. Primary EDF Cryopreservation and Thawing
2.5. Metaphase Spreads of the EDFs
2.6. Quantification and Statistical Analysis
3. Results
3.1. Primary EDF Outgrowth and Culturing
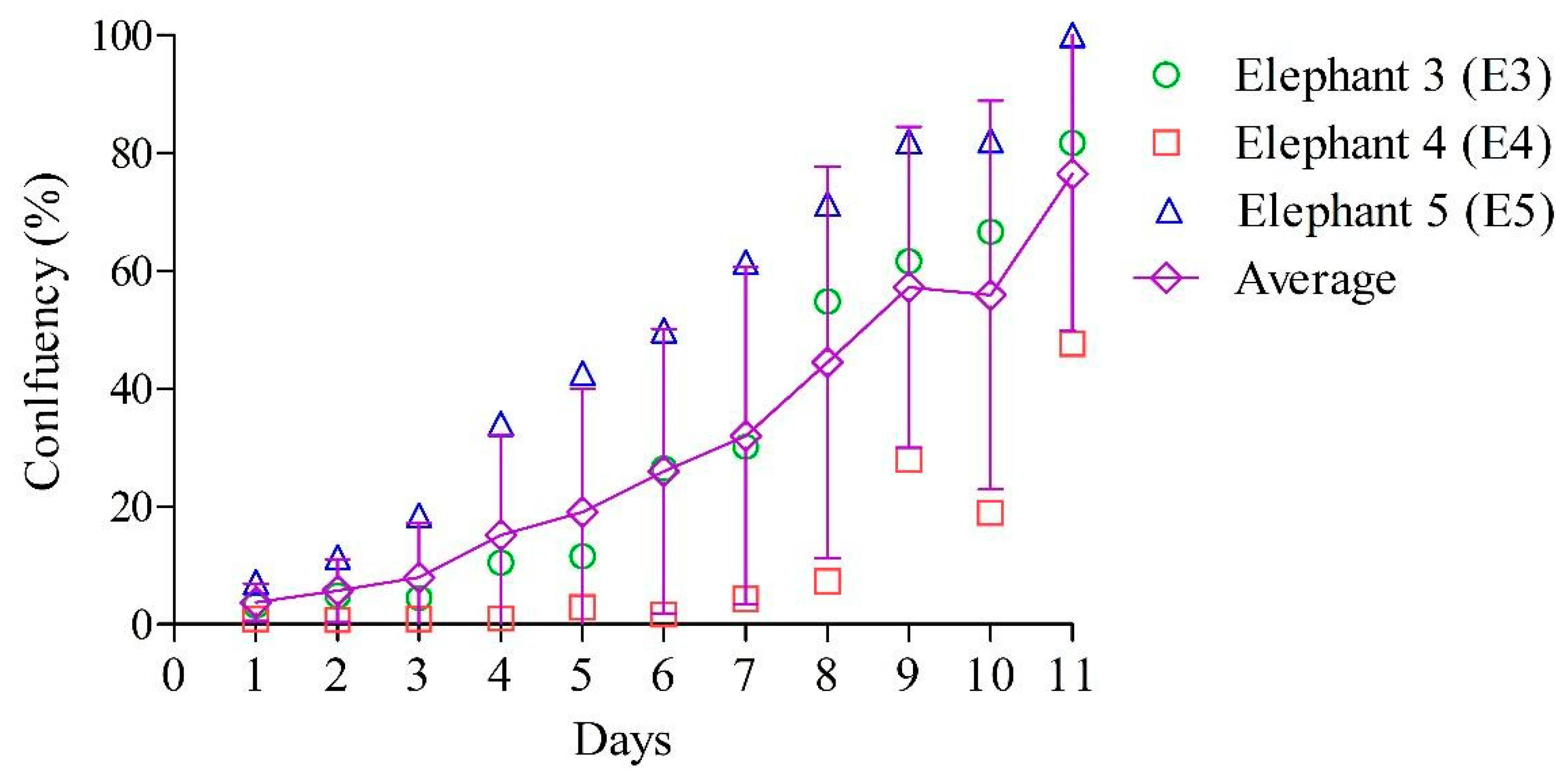
3.2. Primary EDF Growth Curve and Doubling Time (Td)
3.3. Primary EDF Cryopreservation
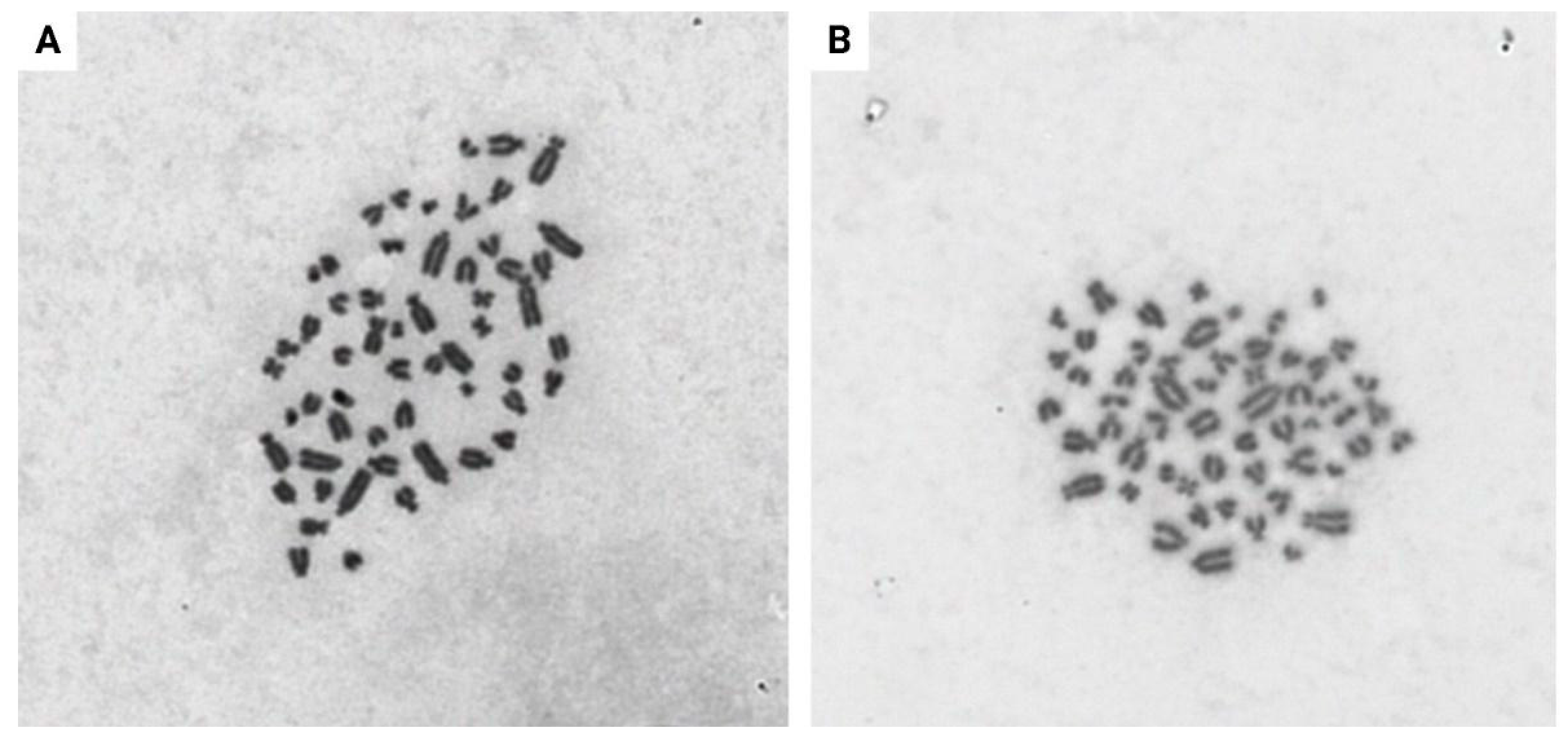
3.4. Metaphase Spreads of the EDFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chase, M.J.; Schlossberg, S.; Griffin, C.R.; Bouché, P.J.; Djene, S.W.; Elkan, P.W.; Ferreira, S.; Grossman, F.; Kohi, E.M.; Landen, K.; et al. Continent-Wide Survey Reveals Massive Decline in African Savannah Elephants. Peer J. 2016, 4, e2354. [Google Scholar] [CrossRef] [PubMed]
- Gobush, K.S.; Edwards, C.T.T.; Balfour, D.; Wittemyer, G.; Maisels, F.; Taylor, R.D. Loxodonta Cyclotis (African Forest Elephant). In The UICN Red List of Threatened Species; IUCN: Gland, Switzerland, 2021; Volume 8235. [Google Scholar] [CrossRef]
- Gobush, K.S.; Edwards, C.T.T.; Balfour, D.; Wittemyer, G.; Maisels, F.; Taylor, R.D. View the UICN Red List of Threatened Species. In The UICN Red List of Threatened Species; IUCN: Gland, Switzerland, 2020; p. 8235. [Google Scholar]
- Williams, C.; Tiwari, S.K.; Goswami, V.R.; De Silva, S.; Kumar, A.; Baskaran, N.; Yoganand, K.; Menon, V. Elephas Maximus. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2020; Volume 2020, pp. 1–29. [Google Scholar]
- Leon-Quinto, T.; Simon, M.A.; Cadenas, R.; Jones, J.; Martinez-Hernandez, F.J.; Moreno, J.M.; Vargas, A.; Martinez, F.; Soria, B. Developing Biological Resource Banks as a Supporting Tool for Wildlife Reproduction and Conservation: The Iberian Lynx Bank as a Model for Other Endangered Species. Anim. Reprod. Sci. 2009, 112, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Bartels, P.; Kotze, A. Wildlife Biomaterial Banking in Africa for Now and the Future. J. Environ. Monit. 2006, 8, 779–781. [Google Scholar] [CrossRef]
- Ryder, O.A.; Onuma, M. Viable Cell Culture Banking for Biodiversity Characterization and Conservation. Annu. Rev. Anim. Biosci. 2018, 6, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Wiidt, D.E. Genetic Resource Banks for Conserving Wildlife Species: Justification, Examples and Becoming Organized on a Global Basis. Anim. Reprod. Sci. 1992, 28, 247–257. [Google Scholar] [CrossRef]
- Clarke, A.G. The Frozen Ark Project: The Role of Zoos and Aquariums in Preserving the Genetic Material of Threatened Animals. Int. Zoo Yearb. 2009, 43, 222–230. [Google Scholar] [CrossRef]
- Mestre-Citrinovitz, A.C.; Sestelo, A.J.; Ceballos, M.B.; Barañao, J.L.; Saragüeta, P. Isolation of Primary Fibroblast Culture from Wildlife: The Panthera onca Case to Preserve a South American Endangered Species. Curr. Protoc. Mol. Biol. 2016, 116, 28.7.1–28.7.14. [Google Scholar] [CrossRef]
- Borges, A.A.; Lira, G.P.D.O.; Nascimento, L.E.; Santos, M.V.D.O.; De Oliveira, M.F.; Silva, A.R.; Pereira, A.F. Isolation, Characterization, and Cryopreservation of Collared Peccary Skin-Derived Fibroblast Cell Lines. Peer J. 2020, 8, e9136. [Google Scholar] [CrossRef]
- Silva, M.B.; Praxedes, A.; Borges, A.A.; Oliveira, L.R.M.; Nascimento, M.B.; Silva, H.V.R.; Silva, A.R.; Pereira, A.F. Evaluation of the Damage Caused by In Vitro Culture and Cryopreservation to Dermal Fibroblasts Derived from Jaguars: An Approach to Conservation through Biobanks. Zoo Biol. 2021, 40, 288–296. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Yan, X.; Guo, X.; Song, Y.; Wu, B.; Bao, S.; Cao, G.; Guo, J.; Sun, Q. Establishment and biological characteristics of fibroblast cell lines obtained from wild corsac fox. Vitr. Cell Dev. Biol. Anim. 2020, 56, 837–841. [Google Scholar] [CrossRef]
- Wang, T.; Li, Z.; Wei, J.; Zheng, D.; Wang, C.; Xu, C.; Chen, W.; Wang, B. Establishment and Characterization of Fibroblast Cultures Derived from a Female Common Hippopotamus (Hippopotamus amphibius) Skin Biopsy. Cell Biol. Int. 2021, 45, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Z.; Zheng, D.; Liu, W.; Huang, P.; Zeng, Z.; Xu, C.; Wang, B.; Wei, J. Establishment and Characterization of a Fibroblast Cell Line from Postmortem Skin of an Adult Chinese Muntjac (Muntiacus reevesi). Vitr. Cell Dev. Biol. Anim. 2020, 56, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Jenuit, M.; International Islamic University Malaysia; Zainuddin, Z.Z.; Payne, J.; Ahmad, A.H.; Yusof, A.M.; Isa, M.L.; Ibrahim, M.; Sabah, U.M.; Benha University. Establishment and cryopreservation of fibroblast cell line from a Sumatran rhinoceros (Dicerorhinus sumatrensis). J. Sustain. Sci. Manag. 2021, 16, 85–98. [Google Scholar] [CrossRef]
- Siengdee, P.; Klinhom, S.; Thitaram, C.; Nganvongpanit, K. Isolation and culture of primary adult skin fibroblasts from the Asian elephant (Elephas maximus). Peer J. 2018, 6, e4302. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Inoue, K.; Wakayama, T. Recent advancements in cloning by somatic cell nuclear transfer. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110329. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Ping, J.; Ali, S.; Zhen, G.; Kang, J.Z.; Yi, P.Z.; Huixian, L.; Zhihui, Z. Conservation of endangered species through somatic cell nuclear transfer (SCNT). Conserv. Genet. Resour. 2021, 13, 349–357. [Google Scholar] [CrossRef]
- Abegglen, L.M.; Caulin, A.F.; Chan, A.; Lee, K.; Robinson, R.; Campbell, M.S.; Kiso, W.K.; Schmitt, D.L.; Waddell, P.J.; Bhaskara, S.; et al. Potential Mechanisms for Cancer Resistance in Elephants and Comparative Cellular Response to DNA Damage in Humans. JAMA 2015, 314, 1850–1860. [Google Scholar] [CrossRef]
- Sulak, M.; Fong, L.; Mika, K.; Chigurupati, S.; Yon, L.; Mongan, N.P.; Emes, R.D.; Lynch, V.J. TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. eLife 2016, 5, e11994. [Google Scholar] [CrossRef]
- Tollis, M.; Boddy, A.M.; Maley, C.C. Peto’s Paradox: How has evolution solved the problem of cancer prevention? BMC Biol. 2017, 15, 60. [Google Scholar] [CrossRef]
- Nunney, L. Cancer suppression and the evolution of multiple retrogene copies of TP53 in elephants: A re-evaluation. Evol. Appl. 2022, 15, 891–901. [Google Scholar] [CrossRef]
- Abegglen, L.M.; Harrison, T.M.; Moresco, A.; Fowles, J.S.; Troan, B.V.; Kiso, W.K.; Schmitt, D.; Boddy, A.M.; Schiffman, J.D. Of Elephants and Other Mammals: A Comparative Review of Reproductive Tumors and Potential Impact on Conservation. Animals 2022, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Doll, R. The Age Distribution of Cancer and a Multi-Stage Theory of Carcinogenesis. Br. J. Cancer 1954, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nordling, C.O. A New Theory on the Cancer-Inducing Mechanism. Br. J. Cancer 1953, 7, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Caulin, A.F.; Maley, C.C. Peto’s Paradox: Evolution’s prescription for cancer prevention. Trends Ecol. Evol. 2011, 26, 175–182. [Google Scholar] [CrossRef]
- Peto, R.; Roe, F.J.; Lee, P.N.; Levy, L.; Clack, J. Cancer and ageing in mice and men. Br. J. Cancer 1975, 32, 411–426. [Google Scholar] [CrossRef]
- Gaughran, S.J.; Pless, E.; Stearns, S.C. How elephants beat cancer. eLife 2016, 5, e21864. [Google Scholar] [CrossRef]
- Vazquez, J.M.; Sulak, M.; Chigurupati, S.; Lynch, V.J. A Zombie LIF Gene in Elephants Is Upregulated by TP53 to Induce Apoptosis in Response to DNA Damage. Cell Rep. 2018, 24, 1765–1776. [Google Scholar] [CrossRef]
- Haupt, S.; Haupt, Y. P53 at the start of the 21st century: Lessons from elephants. F1000Research 2017, 6, 2041. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Tyner, S.D.; Venkatachalam, S.; Choi, J.; Jones, S.; Ghebranious, N.; Igelmann, H.; Lu, X.; Soron, G.; Cooper, B.; Brayton, C.; et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002, 415, 45–53. [Google Scholar] [CrossRef]
- Gomes, N.M.V.; Ryder, O.A.; Houck, M.L.; Charter, S.J.; Walker, W.; Forsyth, N.R.; Austad, S.N.; Venditti, C.; Pagel, M.; Shay, J.W.; et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 2011, 10, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Pearson, V.R.; Bosse, J.B.; Koyuncu, O.O.; Scherer, J.; Toruno, C.; Robinson, R.; Abegglen, L.M.; Schiffman, J.D.; Enquist, L.W.; Rall, G.F. Identification of African Elephant Polyomavirus in wild elephants and the creation of a vector expressing its viral tumor antigens to transform elephant primary cells. PLoS ONE 2021, 16, e0244334. [Google Scholar] [CrossRef] [PubMed]
- Mijele, D.; Omondi, P.; Gakuya, F.; Rossi, L.; Chiyo, P.I.; Soriguer, R.; Angelone-Alasaad, S. A practical guideline to remote biopsy darting of wildebeests for genetic sampling. Int. J. Vet. Sci. Med. 2016, 4, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Smith, F. Histology of the Skin of the Elephant. J. Anat. Physiol. 1890, 24, 493–503. [Google Scholar] [PubMed]
- Cytosmart, Lonza. Available online: https://cytosmart.com/applications/cell-confluence (accessed on 5 February 2023).
- Rodman, T.; Flehinger, B.; Rohlf, F. Metaphase chromosome associations: Colcemid distorts the pattern. Cytogenet. Genome Res. 1980, 27, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Vangipuram, M.; Ting, D.; Kim, S.; Diaz, R.; Schüle, B. Skin Punch Biopsy Explant Culture for Derivation of Primary Human Fibroblasts. J. Vis. Exp. 2013, 77, e3779. [Google Scholar] [CrossRef]
- Villegas, J.; McPhaul, M. Establishment and Culture of Human Skin Fibroblasts. Curr. Protoc. Mol. Biol. 2005, 71, 28.3.1–28.3.9. [Google Scholar] [CrossRef]
- Frönicke, L.; Wienberg, J.; Stone, G.; Adams, L.; Stanyon, R. Towards the delineation of the ancestral eutherian genome organization: Comparative genome maps of human and the African elephant (Loxodonta africana) generated by chromosome painting. Proc. R. Soc. B Boil. Sci. 2003, 270, 1331–1340. [Google Scholar] [CrossRef]
- Yang, F.; Alkalaeva, E.Z.; Perelman, P.L.; Pardini, A.T.; Harrison, W.R.; O’Brien, P.C.M.; Fu, B.; Graphodatsky, A.S.; Ferguson-Smith, M.A.; Robinson, T.J. Reciprocal chromosome painting among human, aardvark, and elephant (superorder Afrotheria) reveals the likely eutherian ancestral karyotype. Proc. Natl. Acad. Sci. USA 2003, 100, 1062–1066. [Google Scholar] [CrossRef]
- Khan, M.; Gasser, S. Generating Primary Fibroblast Cultures from Mouse Ear and Tail Tissues. J. Vis. Exp. 2016, 2016, e53565. [Google Scholar] [CrossRef]
- Grela, E.; Piet, M.; Luchowski, R.; Grudzinski, W.; Paduch, R.; Gruszecki, W.I. Imaging of human cells exposed to an antifungal antibiotic amphotericin B reveals the mechanisms associated with the drug toxicity and cell defence. Sci. Rep. 2018, 8, 14067. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Gao, D.; Baust, J.M. Cryopreservation: An Emerging Paradigm Change. Organogenesis 2009, 5, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, E.; Tinh, N.H.; Kues, W.; Vien, N.T. A protocol for the cryoconservation of breeds by low-cost emergency cell banks—A pilot study. Animal 2008, 2, 869. [Google Scholar] [CrossRef] [PubMed]
- Ryu, A.H.; Eckalbar, W.L.; Kreimer, A.; Yosef, N.; Ahituv, N. Use antibiotics in cell culture with caution: Genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci. Rep. 2017, 7, 7533. [Google Scholar] [CrossRef]
- Elliott, R.L.; Jiang, X.-P. The adverse effect of gentamicin on cell metabolism in three cultured mammary cell lines: “Are cell culture data skewed?”. PLoS ONE 2019, 14, e0214586. [Google Scholar] [CrossRef]
- Gao, D.; Critser, J.K. Mechanisms of Cryoinjury in Living Cells. ILAR J. 2000, 41, 187–196. [Google Scholar] [CrossRef]
- Yap, N.Y.; Ong, T.A.; Morais, C.; Pailoor, J.; Gobe, G.C.; Rajandram, R. Establishment of epithelial and fibroblast cell cultures and cell lines from primary renal cancer nephrectomies. Cell Biol. Int. 2019, 43, 715–725. [Google Scholar] [CrossRef]
- Hungerford, D.A.; Chandra, H.S.; Snyder, R.L.; Ulmer, F.A., Jr. Chromosomes of Three Elephants, Two Asian (Elephas maximus) and One African (Loxodonta africana). Cytogenetics 1966, 5, 243–246. [Google Scholar] [CrossRef]
- Houck, M.; Kumamoto, A.; Gallagher, D.S., Jr.; Benirschke, K. Comparative cytogenetics of the African elephant (Loxodonta africana) and Asiatic elephant (Elephas maximus). Cytogenet. Genome Res. 2001, 93, 249–252. [Google Scholar] [CrossRef]
- Palkopoulou, E.; Lipson, M.; Mallick, S.; Nielsen, S.; Rohland, N.; Baleka, S.; Karpinski, E.; Ivancevic, A.M.; To, T.-H.; Kortschak, R.D.; et al. A comprehensive genomic history of extinct and living elephants. Proc. Natl. Acad. Sci. USA 2018, 115, E2566–E2574. [Google Scholar] [CrossRef]
- Kim, J.; Farré, M.; Auvil, L.; Capitanu, B.; Larkin, D.M.; Ma, J.; Lewin, H.A. Reconstruction and evolutionary history of eutherian chromosomes. Proc. Natl. Acad. Sci. USA 2017, 114, E5379–E5388. [Google Scholar] [CrossRef] [PubMed]
- Mitelman, F.; Mertens, F.; Johansson, B. A breakpoint map of recurrent chromosomal rearrangements in human neoplasia. Nat. Genet. 1997, 15, 417–474. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, U.; Winter, B.; Gugerell, A.; Hegedus, B.; Brostjan, C.; Laufer, G.; Messner, B. Primary Human Fibroblasts in Culture Switch to a Myofibroblast-Like Phenotype Independently of TGF Beta. Cells 2019, 8, 721. [Google Scholar] [CrossRef] [PubMed]
- Strand, J.; Callesen, H.; Pertoldi, C.; Purup, S. Establishing Cell Lines from Fresh or Cryopreserved Tissue from the Great Crested Newt (Triturus cristatus): A Preliminary Protocol. Animals 2021, 11, 367. [Google Scholar] [CrossRef]


| Elephant Code Name | Gender (Male/Female) | Age | Date of Sample Collection | Location of Sample Collection | Type of Analysis |
|---|---|---|---|---|---|
| E1 | M | 25 | 25.09.2021 | Botlierskop Game Reserve | Morphological outgrowth; explant outgrowth; metaphase spread |
| E2 | M | 14 | 19.11.2021 | Botlierskop Game Reserve | n/a |
| E3 | F | 35 | 16.12.2021 | Sanbona Wildlife Reserve | Explant outgrowth; growth curve and Td *; cryopreservation |
| E4 | M | 25 | 16.12.2021 | Sanbona Wildlife Reserve | Explant outgrowth; growth curve and Td * |
| E5 | M | 26 | 15.01.2022 | Botlierskop Game Reserve | Explant outgrowth; growth curve and Td *; cryopreservation |
| Elephant Code Name | Biopsy | Days for Outgrowth Confirmation | Successful Fibroblast Outgrowth/Total Number of Biopsies | Average Days until Explant Outgrowth of EDFs * (Average ± SD) |
|---|---|---|---|---|
| E1 | 1 2 3 4 | 16 9 - 12 | 4/5 | 13.25 ± 3.40 |
| E2 | 1–5 | - | 0/5 | n/a |
| E3 | 1 2 3 4 5 | 18 - 25 - - | 2/6 | 19 ± 1.20 |
| E4 | 1 2 3 4 5 6 | 25 - 25 - - - | 2/6 | 25 ± 0 |
| E5 | 1 2 3 4 5 6 | 11 - 11 - - - | 2/6 | 11 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen van Vuuren, A.; Bolcaen, J.; Engelbrecht, M.; Burger, W.; De Kock, M.; Durante, M.; Fisher, R.; Martínez-López, W.; Miles, X.; Rahiman, F.; et al. Establishment of Primary Adult Skin Fibroblast Cell Lines from African Savanna Elephants (Loxodonta africana). Animals 2023, 13, 2353. https://doi.org/10.3390/ani13142353
Jansen van Vuuren A, Bolcaen J, Engelbrecht M, Burger W, De Kock M, Durante M, Fisher R, Martínez-López W, Miles X, Rahiman F, et al. Establishment of Primary Adult Skin Fibroblast Cell Lines from African Savanna Elephants (Loxodonta africana). Animals. 2023; 13(14):2353. https://doi.org/10.3390/ani13142353
Chicago/Turabian StyleJansen van Vuuren, Amèlia, Julie Bolcaen, Monique Engelbrecht, Willem Burger, Maryna De Kock, Marco Durante, Randall Fisher, Wilner Martínez-López, Xanthene Miles, Farzana Rahiman, and et al. 2023. "Establishment of Primary Adult Skin Fibroblast Cell Lines from African Savanna Elephants (Loxodonta africana)" Animals 13, no. 14: 2353. https://doi.org/10.3390/ani13142353
APA StyleJansen van Vuuren, A., Bolcaen, J., Engelbrecht, M., Burger, W., De Kock, M., Durante, M., Fisher, R., Martínez-López, W., Miles, X., Rahiman, F., Tinganelli, W., & Vandevoorde, C. (2023). Establishment of Primary Adult Skin Fibroblast Cell Lines from African Savanna Elephants (Loxodonta africana). Animals, 13(14), 2353. https://doi.org/10.3390/ani13142353







