Metabolic Changes Associated with Different Levels of Energy Deficits in Mediterranean Buffaloes during the Early Lactation Stage: Type and Role of the Main Lipid Fractions Involved
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Farm
2.2. Clinical Procedures and Experimental Design
2.3. Biochemical Analysis and Group Division
2.4. Thin Layer Chromatography Associated with Gas Chromatography (TLC-GC)
2.5. Statistical Analysis
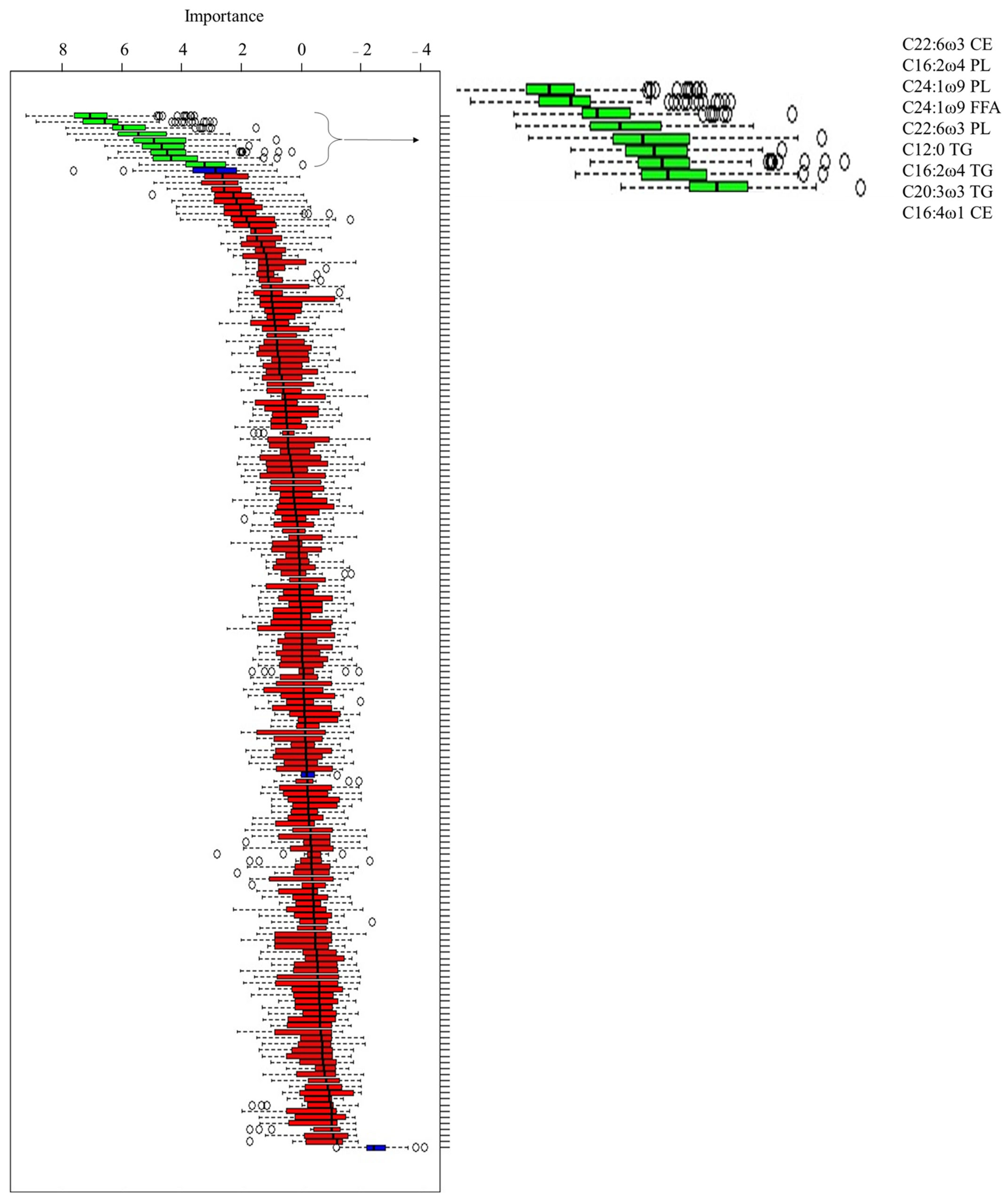
3. Results
- Only 1 FA was not significant (C16:2 ω 4 TG; p-value = 0.063);
- One FA was a good marker: the C16:2 ω 4 PL (AUC: 0.80, CI: 0.68 to 0.89, cut-off > 0.526, Se: 96%, Sp: 52.6%; p-value < 0.001);
- Six FAs were moderate markers: C24:1 ω 9 FFA (AUC: 0.76, CI: 0.64 to 0.86, cut-off > 0.021, Se: 76%, Sp:79%; p-value < 0.001), C20:3 ω 3 TG (AUC: 0.76, CI: 0.63 to 0.86, cut-off ≤ 0.102, Se: 80%, Sp: 68.4%; p-value < 0.001), C24:1 ω 9 PL (AUC: 0.74, CI: 0.61 to 0.84, cut-off ≥ 0.374, Se: 72%, Sp: 68.4%; p-value < 0.001), C12:0 TG (AUC: 0.74, CI: 0.61 to 0.84, cut-off ≤ 0.522, Se: 56%, Sp: 86.8%; p-value < 0.001), C22:6 ω 3 CE (AUC: 0.70, CI: 0.58 to 0.81, cut-off > 0.159, Se: 60%, Sp: 84.2%; p-value = 0.007), and C22:6 ω 3 PL (AUC: 0.70, CI: 0.57 to 0.81, cut-off > 0.106, Se: 64%, Sp: 81.6%; p-value = 0.008);
- One FA was a poor marker: C16:4 ω 1 CE (AUC: 0.68, CI: 0.55 to 0.79, cut-off > 0.095, Se: 64%, Sp: 7 9%; p-value = 0.023).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rosa, G.; Napolitano, F.; Grasso, F.; Pacelli, C.; Bordi, A. On the Development of a Monitoring Scheme of Buffalo Welfare at Farm Level. Ital. J. Anim. Sci. 2005, 4, 115–125. [Google Scholar] [CrossRef]
- Alarcon, P.; Manosalva, C.; Carretta, M.D.; Hidalgo, A.I.; Figueroa, C.D.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Burgos, R.A. Fatty and Hydroxycarboxylic Acid Receptors: The Missing Link of Immune Response and Metabolism in Cattle. Vet. Immunol. Immunopathol. 2018, 201, 77–87. [Google Scholar] [CrossRef]
- Gianesella, M.; Fiore, E.; Arfuso, F.; Vecchio, D.; Curone, G.; Morgante, M.; Mazzotta, E.; Badon, T.; Rossi, P.; Bedin, S.; et al. Serum Haptoglobin and Protein Electrophoretic Fraction Modifications in Buffaloes (Bubalus bubalis) around Calving and during Early Lactation. J. Dairy Res. 2019, 86, 291–295. [Google Scholar] [CrossRef]
- Fiore, E.; Lisuzzo, A.; Laghi, L.; Harvatine, K.J.; Mazzotta, E.; Alterisio, M.C.; Ciaramella, P.; Zhu, C.; Contiero, B.; Faillace, V.; et al. Serum Metabolomics Assessment of Etiological Processes Predisposing Ketosis in Water Buffalo during Early Lactation. J. Dairy Sci. 2023, 106, 3465–3476. [Google Scholar] [CrossRef]
- Horst, E.A.; Kvidera, S.K.; Baumgard, L.H. Invited Review: The Influence of Immune Activation on Transition Cow Health and Performance—A Critical Evaluation of Traditional Dogmas. J. Dairy Sci. 2021, 104, 8380–8410. [Google Scholar] [CrossRef]
- Lisuzzo, A.; Laghi, L.; Fiore, F.; Harvatine, K.; Mazzotta, E.; Faillace, V.; Spissu, N.; Zhu, C.; Moscati, L.; Fiore, E. Evaluation of the Metabolomic Profile through 1H-NMR Spectroscopy in Ewes Affected by Postpartum Hyperketonemia. Sci. Rep. 2022, 12, 16463. [Google Scholar] [CrossRef]
- Fiore, E.; Arfuso, F.; Gianesella, M.; Vecchio, D.; Morgante, M.; Mazzotta, E.; Badon, T.; Rossi, P.; Bedin, S.; Piccione, G. Metabolic and Hormonal Adaptation in Bubalus Bubalis around Calving and Early Lactation. PLoS ONE 2018, 13, e0193803. [Google Scholar] [CrossRef]
- Fiore, E.; Tessari, R.; Morgante, M.; Gianesella, M.; Badon, T.; Bedin, S.; Mazzotta, E.; Berlanda, M. Identification of Plasma Fatty Acids in Four Lipid Classes to Understand Energy Metabolism at Different Levels of Ketonemia in Dairy Cows Using Thin Layer Chromatography and Gas Chromatographic Techniques (TLC-GC). Animals 2020, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; O’Boyle, N.J.; Herdt, T.H.; Sordillo, L.M. Lipomobilization in Periparturient Dairy Cows Influences the Composition of Plasma Nonesterified Fatty Acids and Leukocyte Phospholipid Fatty Acids. J. Dairy Sci. 2010, 93, 2508–2516. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Hervey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals; Academic Press: San Diego, CA, USA, 2008; ISBN 9781626239777. [Google Scholar]
- Friedman, H. Simplified Determinations of Statistical Power, Magnitude of Effect and Research Sample Sizes. Educ. Psychol. Meas. 1982, 42, 521–526. [Google Scholar] [CrossRef]
- De Rosa, G.; Grasso, F.; Winckler, C.; Bilancione, A.; Pacelli, C.; Masucci, F.; Napolitano, F. Application of the Welfare Quality Protocol to Dairy Buffalo Farms: Prevalence and Reliability of Selected Measures. J. Dairy Sci. 2015, 98, 6886–6896. [Google Scholar] [CrossRef] [PubMed]
- Fadul, M.; D’Andrea, L.; Alsaaod, M.; Borriello, G.; Di Lori, A.; Stucki, D.; Ciaramella, P.; Steiner, A.; Guccione, J. Assessment of Feeding, Ruminating and Locomotion Behaviors in Dairy Cows around Calving—A Retrospective Clinical Study to Early Detect Spontaneous Disease Appearance. PLoS ONE 2022, 17, e0264834. [Google Scholar] [CrossRef] [PubMed]
- Guccione, J.; Carcasole, C.; Alsaaod, M.; D’Andrea, L.; Di Loria, A.; De Rosa, A.; Ciaramella, P.; Steiner, A. Assessment of Foot Health and Animal Welfare: Clinical Findings in 229 Dairy Mediterranean Buffaloes (Bubalus bubalis) Affected by Foot Disorders. BMC Vet. Res. 2016, 12, 107. [Google Scholar] [CrossRef]
- Lisuzzo, A.; Fiore, F.; Harvatine, K.; Mazzotta, E.; Berlanda, M.; Spissu, N.; Badon, T.; Contiero, B.; Moscati, L.; Fiore, E. Changes in Plasma Fatty Acids Profile in Hyperketonemic Ewes during Early Lactation: A Preliminary Study. Sci. Rep. 2022, 12, 17017. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.; Giambelluca, S.; Morgante, M.; Contiero, B.; Mazzotta, E.; Vecchio, D.; Vazzana, I.; Rossi, P.; Arfuso, F.; Piccione, G.; et al. Changes in Some Blood Parameters, Milk Composition and Yield of Buffaloes (Bubalus bubalis) during the Transition Period. Anim. Sci. J. 2017, 88, 2025–2032. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational Biomarker Discovery in Clinical Metabolomics: An Introductory Tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]
- Contreras, G.A.; Sordillo, L.M. Lipid Mobilization and Inflammatory Responses during the Transition Period of Dairy Cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef]
- Russell, K.E.; Roussel, A.J. Evaluation of the Ruminant Serum Chemistry Profile. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 403–426. [Google Scholar] [CrossRef]
- Youssef, M.A.; El-Khodery, S.A.; El-deeb, W.M.; El-Amaiem, W.E.E.A. Ketosis in Buffalo (Bubalus Bubalis): Clinical Findings and the Associated Oxidative Stress Level. Trop. Anim. Health Prod. 2010, 42, 1771–1777. [Google Scholar] [CrossRef]
- Watts, J.S.; Rezamand, P.; Sevier, D.L.; Price, W.; McGuire, M.A. Short-Term Effects of Dietary Trans Fatty Acids Compared with Saturated Fatty Acids on Selected Measures of Inflammation, Fatty Acid Profiles, and Production in Early Lactating Holstein Dairy Cows. J. Dairy Sci. 2013, 96, 6932–6943. [Google Scholar] [CrossRef] [PubMed]
- Tyburczy, C.; Lock, A.L.; Dwyer, D.A.; Destaillats, F.; Mouloungui, Z.; Candy, L.; Bauman, D.E. Uptake and Utilization of Trans Octadecenoic Acids in Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 3850–3861. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Franci, O.; Scalia, D.; Bernabucci, U.; Ronchi, B.; Nardone, A. Effects on Functions of Ovine Blood Mononuclear Cells for Each of Several Fatty Acids at Concentrations Found in Plasma on Healthy and Ketotic Ewes. Am. J. Vet. Res. 2002, 63, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Weiss-Hersh, K.; Garcia, A.L.; Marosvölgyi, T.; Szklenár, M.; Decsi, T.; Rühl, R. Saturated and Monounsaturated Fatty Acids in Membranes Are Determined by the Gene Expression of Their Metabolizing Enzymes SCD1 and ELOVL6 Regulated by the Intake of Dietary Fat. Eur. J. Nutr. 2020, 59, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, G.; Du, X.; Shi, Z.; Jin, M.; Sha, X.; Li, X.; Wang, Z.; Li, X. Expression Patterns of Hepatic Genes Involved in Lipid Metabolism in Cows with Subclinical or Clinical Ketosis. J. Dairy Sci. 2019, 102, 1725–1735. [Google Scholar] [CrossRef]
- Bernal-Santos, G.; O’Donnell, A.M.; Vicini, J.L.; Hartnell, G.F.; Bauman, D.E. Hot Topic: Enhancing Omega-3 Fatty Acids in Milk Fat of Dairy Cows by Using Stearidonic Acid-Enriched Soybean Oil from Genetically Modified Soybeans. J. Dairy Sci. 2010, 93, 32–37. [Google Scholar] [CrossRef]
- Siard-Altman, M.H.; Harris, P.A.; Moffett-Krotky, A.D.; Ireland, J.L.; Betancourt, A.; Barker, V.D.; McMurry, K.E.; Reedy, S.E.; Adams, A.A. Relationships of Inflamm-Aging with Circulating Nutrient Levels, Body Composition, Age, and Pituitary Pars Intermedia Dysfunction in a Senior Horse Population. Vet. Immunol. Immunopathol. 2020, 221, 110013. [Google Scholar] [CrossRef]
- Singh, N.; Barnych, B.; Wagner, K.M.; Wan, D.; Morisseau, C.; Hammock, B.D. Adrenic Acid-Derived Epoxy Fatty Acids Are Naturally Occurring Lipids and Their Methyl Ester Prodrug Reduces Endoplasmic Reticulum Stress and Inflammatory Pain. ACS Omega 2021, 6, 7165–7174. [Google Scholar] [CrossRef]
- Wang, X.; Lin, H.; Gu, Y. Multiple Roles of Dihomo-γ-Linolenic Acid against Proliferation Diseases. Lipids Health Dis. 2012, 11, 25. [Google Scholar] [CrossRef]
- Peter, A.; Cegan, A.; Wagner, S.; Lehmann, R.; Stefan, N.; Königsrainer, A.; Königsrainer, I.; Häring, H.U.; Schleicher, E. Hepatic Lipid Composition and Stearoyl-Coenzyme A Desaturase 1 MRNA Expression Can Be Estimated from Plasma VLDL Fatty Acid Ratios. Clin. Chem. 2009, 55, 2113–2120. [Google Scholar] [CrossRef]
- Zhang, G.; Ametaj, B.N. Ketosis an Old Story Under a New Approach. Dairy 2020, 1, 42–60. [Google Scholar] [CrossRef]
- Oikawa, S.; Mizunuma, Y.; Iwasaki, Y.; Tharwat, M. Changes of Very Low-Density Lipoprotein Concentration in Hepatic Blood from Cows with Fasting-Induced Hepatic Lipidosis. Can. J. Vet. Res. 2010, 74, 317–320. [Google Scholar]
- McFadden, J.W. Review: Lipid Biology in the Periparturient Dairy Cow: Contemporary Perspectives. Animal 2020, 14, S165–S175. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.N.; Rehage, J.; Beaulieu, A.D.; Bahaa, A.O.; Drackley, J.K. Prepartum Nutrition Alters Fatty Acid Composition in Plasma, Adipose Tissue, and Liver Lipids of Periparturient Dairy Cows. J. Dairy Sci. 2007, 90, 2941–2959. [Google Scholar] [CrossRef] [PubMed]
- Tessari, R.; Berlanda, M.; Morgante, M.; Badon, T.; Gianesella, M.; Mazzotta, E.; Contiero, B.; Fiore, E. Changes of Plasma Fatty Acids in Four Lipid Classes to Understand Energy Metabolism at Different Levels of Non-Esterified Fatty Acid (Nefa) in Dairy Cows. Animals 2020, 10, 1410. [Google Scholar] [CrossRef]
- Gross, J.J.; Kessler, E.C.; Albrecht, C.; Bruckmaier, R.M. Response of the Cholesterol Metabolism to a Negative Energy Balance in Dairy Cows Depends on the Lactational Stage. PLoS ONE 2015, 10, e0121956. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-Inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]

| Parameters | Group H (n = 38) | Group K (n = 25) | SEM | p-Values |
|---|---|---|---|---|
| BCS 1 | 4.58 | 5.08 | 0.18 | 0.058 |
| Parity | 3.53 | 3.89 | 0.37 | 0.506 |
| DIM 2 | 30.4 | 33.5 | 2.19 | 0.330 |
| Milk production (Kg/d) | 14.3 | 14.9 | 0.58 | 0.461 |
| BHB 3 (mmol/L) | 0.47 | 0.74 | 0.02 | <0.001 |
| NEFA 4 (mEq/L) | 0.24 | 0.25 | 0.02 | 0.883 |
| CHO 5 (mg/dL) | 75.0 | 86.2 | 5.48 | 0.161 |
| TGR 6 (mg/dL) | 9.35 | 10.09 | 0.49 | 0.302 |
| GLU 7 (mg/dL) | 62.4 | 64.3 | 1.28 | 0.313 |
| GGT 8 (U/L) | 19.6 | 21.8 | 1.06 | 0.170 |
| AST 9 (U/L) | 140.0 | 164.0 | 7.12 | 0.017 |
| ALT 10 (U/L) | 48.0 | 49.4 | 2.07 | 0.638 |
| PL | Nomenclature | Group H (n = 38) | Group K (n = 25) | SEM | p-Values |
|---|---|---|---|---|---|
| C6:0 | Caproic acid | 0.05 | 0.06 | 0.004 | 0.510 |
| C8:0 | Caprylic acid | 0.03 | 0.05 | 0.005 | 0.017 |
| C10:0 | Capric acid | 0.05 | 0.05 | 0.006 | 0.650 |
| C12:0 | Lauric acid | 0.41 | 0.46 | 0.068 | 0.750 |
| C14:0 | Myristic acid | 0.43 | 0.47 | 0.045 | 0.760 |
| C14:1 ω 5 | Myristelaidic acid | 0.14 | 0.17 | 0.016 | 0.560 |
| C16:0 | Palmitic acid | 15.8 | 17.1 | 0.868 | 0.840 |
| C16:1 ω 9 | Hypogeic acid | 0.15 | 0.16 | 0.010 | 0.810 |
| C16:1 ω 7 | Palmitoleic acid | 0.32 | 0.38 | 0.022 | 0.290 |
| C16:2 ω 4 | Hexadecadienoic acid | 0.43 | 1.24 | 0.121 | <0.001 |
| C16:3 ω 4 | Hexadecatrienoic acid | 0.03 | 0.04 | 0.003 | 0.140 |
| C16:4 ω 1 | Hexadecatetraenoic acid | 0.04 | 0.04 | 0.006 | 0.400 |
| C17:0 | Margaric acid | 0.84 | 0.85 | 0.060 | 0.850 |
| C17:1 ω 7 | Heptadecenoic acid | 0.14 | 0.15 | 0.011 | 0.830 |
| C18:0 | Stearic acid | 18.2 | 20.6 | 1.125 | 0.380 |
| C18:1 ω 9 | Oleic acid | 9.15 | 10.9 | 0.508 | 0.059 |
| C18:1 ω 7 | Vaccenic acid | 1.13 | 1.47 | 0.092 | 0.020 |
| C18:2 ω 6 | Linoleic acid | 20.0 | 22.3 | 1.515 | 0.750 |
| C18:3 ω 6 | γ-Linolenic acid (GLA) | 0.12 | 0.14 | 0.011 | 0.360 |
| C18:3 ω 3 | α-Linolenic acid (ALA) | 0.97 | 1.01 | 0.081 | 0.910 |
| C18:4 ω 3 | Stearidonic acid (SDA) | 0.01 | 0.01 | 0.002 | 0.710 |
| C20:0 | Arachidic acid | 0.12 | 0.13 | 0.006 | 0.310 |
| C20:1 ω 11 | Gadoleic acid | 0.06 | 0.08 | 0.006 | 0.083 |
| C20:1 ω 9 | Gondoic acid | 0.04 | 0.05 | 0.004 | 0.021 |
| C20:2 ω 6 | Eicosadienoic acid | 0.11 | 0.12 | 0.009 | 0.420 |
| C20:3 ω 9 | Mead acid | 0.26 | 0.27 | 0.030 | 0.850 |
| C20:3 ω 6 | Dihomo-γ-Linolenic acid (DGLA) | 1.44 | 1.64 | 0.108 | 0.440 |
| C20:4 ω 6 | Arachidonic acid | 2.41 | 2.62 | 0.162 | 0.620 |
| C20:3 ω 3 | Eicosatrienoic acid (ETE) | 0.03 | 0.03 | 0.003 | 0.480 |
| C20:4 ω 3 | Eicosatetranoic acid (ETA) | 0.09 | 0.11 | 0.008 | 0.190 |
| C20:5 ω 3 | Eicosapentanoic acid (EPA) | 0.54 | 0.65 | 0.044 | 0.250 |
| C22:0 | Behenic acid | 0.47 | 0.54 | 0.024 | 0.170 |
| C22:1 ω 9 | Erucic acid | 0.01 | 0.01 | 0.001 | 0.510 |
| C22:1 ω 7 | 15-Docosenoic acid | 0.01 | 0.01 | 0.001 | 0.470 |
| C22:2 ω 6 | Docosadienoic acid | 0.05 | 0.05 | 0.004 | 0.530 |
| C22:4 ω 6 | Adrenic acid (ADA) | 0.29 | 0.33 | 0.027 | 0.660 |
| C22:5 ω 3 | Docosapentaenoic acid (DPA) | 0.61 | 0.65 | 0.041 | 0.690 |
| C22:6 ω 3 | Docosahexaenoic acid (DHA) | 0.09 | 0.23 | 0.034 | 0.003 |
| C24:0 | Lignoceric acid | 0.50 | 0.55 | 0.038 | 0.560 |
| C24:1 ω 9 | Nervonic acid | 0.26 | 0.44 | 0.035 | <0.001 |
| PLFA mg/dL | / | 76.0 | 85.9 | 4.47 | 0.420 |
| PL mg/dL | / | 105.0 | 119.0 | 6.18 | 0.410 |
| FFA | Nomenclature | Group H (n = 38) | Group K (n = 25) | SEM | p-Values |
|---|---|---|---|---|---|
| C6:0 | Caproic acid | 0.03 | 0.03 | 0.002 | 0.450 |
| C8:0 | Caprylic acid | 0.02 | 0.02 | 0.003 | 0.670 |
| C10:0 | Capric acid | 0.03 | 0.03 | 0.003 | 0.750 |
| C12:0 | Lauric acid | 0.37 | 0.33 | 0.026 | 0.540 |
| C14:0 | Myristic acid | 0.19 | 0.20 | 0.020 | 0.680 |
| C14:1 ω 5 | Myristelaidic acid | 0.02 | 0.01 | 0.001 | 0.240 |
| C15:0 | Pentadecanoic Acid | 0.06 | 0.07 | 0.005 | 0.690 |
| C16:0 | Palmitic acid | 1.37 | 1.42 | 0.097 | 0.920 |
| C16:1 ω 9 | Hypogeic acid | 0.02 | 0.02 | 0.002 | 0.300 |
| C16:1 ω 7 | Palmitoleic acid | 0.04 | 0.04 | 0.006 | 0.940 |
| C16:2 ω 4 | Hexadecadienoic acid | 0.01 | 0.01 | 0.001 | 0.300 |
| C16:3 ω 4 | Hexadecatrienoic acid | 0.004 | 0.003 | 0.001 | 0.540 |
| C16:4 ω 1 | Hexadecatetraenoic acid | 0.010 | 0.012 | 0.001 | 0.080 |
| C17:1 | Heptadecenoic acid | 0.01 | 0.01 | 0.002 | 0.730 |
| C18:0 | Stearic acid | 1.68 | 0.78 | 0.113 | 0.770 |
| C18:1 ω 9 | Oleic acid | 0.76 | 0.82 | 0.117 | 0.910 |
| C18:1 ω 7 | Vaccenic acid | 0.06 | 0.07 | 0.007 | 0.480 |
| C18:2 ω 6 | Linoleic acid | 0.12 | 0.12 | 0.012 | 0.670 |
| C18:3 ω 6 | γ-Linolenic acid (GLA) | 0.042 | 0.039 | 0.001 | 0.030 |
| C18:3 ω 3 | α-Linolenic acid (ALA) | 0.01 | 0.01 | 0.001 | 0.280 |
| C18:4 ω 3 | Stearidonic acid (SDA) | 0.004 | 0.004 | 0.001 | 0.840 |
| C20:0 | Arachidic acid | 0.02 | 0.02 | 0.001 | 0.940 |
| C20:1 ω 11 | Gadoleic acid | 0.004 | 0.005 | 0.001 | 0.190 |
| C20:1 ω 9 | Gondoic acid | 0.006 | 0.004 | 0.001 | 0.016 |
| C20:2 ω 6 | Eicosadienoic acid | 0.005 | 0.004 | 0.001 | 0.320 |
| C20:3 ω 9 | Mead acid | 0.003 | 0.003 | 0.001 | 0.630 |
| C20:3 ω 6 | Dihomo-γ-Linolenic acid (DGLA) | 0.01 | 0.01 | 0.001 | 0.520 |
| C20:4 ω 6 | Arachidonic acid | 0.01 | 0.01 | 0.001 | 0.670 |
| C20:3 ω 3 | Eicosatrienoic acid (ETE) | 0.007 | 0.008 | 0.0001 | 0.095 |
| C20:4 ω 3 | Eicosatetranoic acid (ETA) | 0.004 | 0.003 | 0.001 | 0.220 |
| C20:5 ω 3 | Eicosapentanoic acid (EPA) | 0.02 | 0.04 | 0.006 | 0.039 |
| C22:0 | Behenic acid | 0.01 | 0.01 | 0.001 | 0.150 |
| C22:1 ω 9 | Erucic acid | 0.004 | 0.002 | 0.001 | 0.088 |
| C22:1 ω 7 | 15-Docosenoic acid | 0.003 | 0.002 | 0.001 | 0.490 |
| C22:2 ω 6 | Docosadienoic acid | 0.016 | 0.019 | 0.001 | 0.044 |
| C22:4 ω 6 | Adrenic acid (ADA) | 0.02 | 0.03 | 0.003 | 0.018 |
| C22:5 ω 3 | Docosapentaenoic acid (DPA) | 0.01 | 0.27 | 0.070 | 0.018 |
| C22:6 ω 3 | Docosahexaenoic acid (DHA) | 0.02 | 0.06 | 0.053 | 0.590 |
| C24:0 | Lignoceric acid | 0.02 | 0.03 | 0.006 | 0.120 |
| C24:1 ω 9 | Nervonic acid | 0.02 | 0.04 | 0.005 | 0.001 |
| FFA mg/dL | 4.99 | 5.36 | 0.369 | 0.740 |
| TG | Nomenclature | Group H (n = 38) | Group K (n = 25) | SEM | p-Values |
|---|---|---|---|---|---|
| C6:0 | Caproic acid | 0.03 | 0.03 | 0.002 | 0.890 |
| C8:0 | Caprylic acid | 0.04 | 0.05 | 0.006 | 0.320 |
| C10:0 | Capric acid | 0.07 | 0.05 | 0.006 | 0.036 |
| C12:0 | Lauric acid | 0.76 | 0.54 | 0.063 | 0.025 |
| C14:0 | Myristic acid | 0.26 | 0.19 | 0.040 | 0.240 |
| C14:1 ω 5 | Myristelaidic acid | 0.02 | 0.03 | 0.001 | 0.380 |
| C15:0 | Pentadecanoic Acid | 0.17 | 0.18 | 0.016 | 0.840 |
| C16:0 | Palmitic acid | 2.28 | 2.20 | 0.117 | 0.360 |
| C16:1 ω 9 | Hypogeic acid | 0.03 | 0.04 | 0.005 | 0.260 |
| C16:1 ω 7 | Palmitoleic acid | 0.04 | 0.04 | 0.004 | 1.000 |
| C16:2 ω 4 | Hexadecadienoic acid | 0.04 | 0.04 | 0.004 | 0.120 |
| C16:3 ω 4 | Hexadecatrienoic acid | 0.004 | 0.01 | 0.001 | 0.014 |
| C16:4 ω 1 | Hexadecatetraenoic acid | 0.01 | 0.02 | 0.001 | 0.450 |
| C17:1 ω 7 | Heptadecenoic acid | 0.01 | 0.01 | 0.001 | 1.000 |
| C18:0 | Stearic acid | 3.53 | 3.98 | 0.244 | 0.280 |
| C18:1 ω 9 | Oleic acid | 0.46 | 0.53 | 0.053 | 0.470 |
| C18:1 ω 7 | Vaccenic acid | 0.18 | 0.26 | 0.024 | 0.025 |
| C18:2 ω 6 | Linoleic acid | 0.20 | 0.44 | 0.101 | 0.095 |
| C18:3 ω 6 | γ-Linolenic acid (GLA) | 0.05 | 0.05 | 0.001 | 0.830 |
| C18:3 ω 3 | α-Linolenic acid (ALA) | 0.01 | 0.01 | 0.001 | 0.450 |
| C18:4 ω 3 | Stearidonic acid (SDA) | 0.01 | 0.01 | 0.001 | 0.120 |
| C20:0 | Arachidic acid | 0.06 | 0.06 | 0.004 | 0.630 |
| C20:1 ω 11 | Gadoleic acid | 0.01 | 0.12 | 0.002 | 0.150 |
| C20:1 ω 9 | Gondoic acid | 0.01 | 0.01 | 0.001 | 0.101 |
| C20:2 ω 6 | Eicosadienoic acid | 0.004 | 0.001 | 0.001 | 0.340 |
| C20:3 ω 9 | Mead acid | 0.004 | 0.005 | 0.001 | 0.260 |
| C20:3 ω 6 | Dihomo-γ-Linolenic acid (DGLA) | 0.02 | 0.02 | 0.001 | 0.910 |
| C20:4 ω 6 | Arachidonic acid | 0.01 | 0.01 | 0.002 | 0.220 |
| C20:3 ω 3 | Eicosatrienoic acid (ETE) | 0.11 | 0.09 | 0.004 | 0.001 |
| C20:4 ω 3 | Eicosatetranoic acid (ETA) | 0.01 | 0.01 | 0.001 | 0.400 |
| C20:5 ω 3 | Eicosapentanoic acid (EPA) | 0.02 | 0.03 | 0.005 | 0.580 |
| C22:0 | Behenic acid | 0.03 | 0.04 | 0.002 | 0.130 |
| C22:1 ω 9 | Erucic acid | 0.003 | 0.004 | 0.001 | 0.530 |
| C22:1 ω 7 | 15-Docosenoic acid | 0.004 | 0.004 | 0.001 | 0.410 |
| C22:2 ω 6 | Docosadienoic acid | 0.01 | 0.01 | 0.001 | 0.560 |
| C22:4 ω 6 | Adrenic acid (ADA) | 0.01 | 0.01 | 0.001 | 0.160 |
| C22:5 ω 3 | Docosapentaenoic acid (DPA) | 0.01 | 0.01 | 0.001 | 0.930 |
| C22:6 ω 3 | Docosahexaenoic acid (DHA) | 0.01 | 0.01 | 0.002 | 0.520 |
| C24:0 | Lignoceric acid | 0.03 | 0.04 | 0.003 | 0.130 |
| C24:1 ω 9 | Nervonic acid | 0.04 | 0.10 | 0.012 | 0.002 |
| TGFA mg/dL | / | 8.59 | 9.12 | 0.51 | 0.600 |
| TG mg/dL | / | 9.03 | 9.58 | 0.54 | 0.600 |
| CE | Nomenclature | Group H (n = 38) | Group K (n = 25) | SEM | p-Values |
|---|---|---|---|---|---|
| C6:0 | Caproic acid | 0.06 | 0.07 | 0.004 | 0.160 |
| C8:0 | Caprylic acid | 0.08 | 0.06 | 0.021 | 0.630 |
| C10:0 | Capric acid | 0.14 | 0.12 | 0.012 | 0.400 |
| C12:0 | Lauric acid | 0.73 | 0.65 | 0.075 | 0.610 |
| C14:0 | Myristic acid | 0.61 | 0.57 | 0.077 | 0.480 |
| C14:1 ω 5 | Myristelaidic acid | 0.11 | 0.12 | 0.006 | 0.990 |
| C15:0 | Pentadecanoic Acid | 0.49 | 0.50 | 0.050 | 0.700 |
| C16:0 | Palmitic acid | 8.52 | 8.81 | 0.594 | 0.760 |
| C16:1 ω 9 | Hypogeic acid | 0.69 | 0.90 | 0.142 | 0.560 |
| C16:1 ω 7 | Palmitoleic acid | 1.41 | 1.11 | 0.109 | 0.012 |
| C16:2 ω 4 | Hexadecadienoic acid | 0.17 | 0.18 | 0.020 | 0.810 |
| C16:3 ω 4 | Hexadecatrienoic acid | 0.14 | 0.16 | 0.019 | 0.680 |
| C16:4 ω 1 | Hexadecatetraenoic acid | 0.09 | 0.12 | 0.010 | 0.024 |
| C17:1 ω 7 | Heptadecenoic acid | 0.54 | 0.61 | 0.040 | 0.410 |
| C18:0 | Stearic acid | 1.88 | 1.93 | 0.907 | 0.890 |
| C18:1 ω 9 | Oleic acid | 18.1 | 19.8 | 0.161 | 0.470 |
| C18:1 ω 7 | Vaccenic acid | 0.66 | 0.61 | 0.052 | 0.280 |
| C18:2 ω 6 | Linoleic acid | 42.7 | 45.4 | 3.405 | 0.860 |
| C18:3 ω 6 | γ-Linolenic acid (GLA) | 1.85 | 1.86 | 0.200 | 0.600 |
| C18:3 ω 3 | α-Linolenic acid (ALA) | 1.72 | 1.85 | 0.118 | 0.980 |
| C18:4 ω 3 | Stearidonic acid (SDA) | 0.04 | 0.04 | 0.004 | 0.340 |
| C20:0 | Arachidic acid | 0.05 | 0.05 | 0.011 | 0.780 |
| C20:1 ω 11 | Gadoleic acid | 0.10 | 0.10 | 0.012 | 0.780 |
| C20:1 ω 9 | Gondoic acid | 0.04 | 0.05 | 0.005 | 0.420 |
| C20:2 ω 6 | Eicosadienoic acid | 0.20 | 0.20 | 0.005 | 0.570 |
| C20:3 ω 9 | Mead acid | 0.07 | 0.07 | 0.007 | 0.970 |
| C20:3 ω 6 | Dihomo-γ-Linolenic acid (DGLA) | 0.27 | 0.30 | 0.023 | 0.540 |
| C20:4 ω 6 | Arachidonic acid | 0.94 | 0.98 | 0.065 | 0.960 |
| C20:3 ω 3 | Eicosatrienoic acid (ETE) | 0.11 | 0.13 | 0.005 | 0.029 |
| C20:4 ω 3 | Eicosatetranoic acid (ETA) | 0.10 | 0.10 | 0.005 | 0.700 |
| C20:5 ω 3 | Eicosapentanoic acid (EPA) | 0.49 | 0.51 | 0.026 | 0.940 |
| C22:0 | Behenic acid | 0.210 | 0.22 | 0.015 | 0.700 |
| C22:1 ω 9 | Erucic acid | 0.05 | 0.07 | 0.009 | 0.330 |
| C22:1 ω 7 | 15-Docosenoic acid | 0.04 | 0.04 | 0.009 | 0.460 |
| C22:2 ω 6 | Docosadienoic acid | 0.04 | 0.03 | 0.015 | 0.160 |
| C22:4 ω 6 | Adrenic acid (ADA) | 0.06 | 0.06 | 0.013 | 0.970 |
| C22:5 ω 3 | Docosapentaenoic acid (DPA) | 0.14 | 0.68 | 0.134 | 0.010 |
| C22:6 ω 3 | Docosahexaenoic acid (DHA) | 0.10 | 0.23 | 0.021 | <0.001 |
| C24:0 | Lignoceric acid | 0.70 | 0.60 | 0.082 | 0.150 |
| C24:1 ω 9 | Nervonic acid | 0.16 | 0.16 | 0.016 | 0.640 |
| CEFA mg/dL | / | 84.4 | 89.7 | 5.25 | 0.950 |
| CE mg/dL | / | 197.0 | 210.0 | 12.25 | 0.940 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisuzzo, A.; Alterisio, M.C.; Mazzotta, E.; Ciaramella, P.; Guccione, J.; Gianesella, M.; Badon, T.; Fiore, E. Metabolic Changes Associated with Different Levels of Energy Deficits in Mediterranean Buffaloes during the Early Lactation Stage: Type and Role of the Main Lipid Fractions Involved. Animals 2023, 13, 2333. https://doi.org/10.3390/ani13142333
Lisuzzo A, Alterisio MC, Mazzotta E, Ciaramella P, Guccione J, Gianesella M, Badon T, Fiore E. Metabolic Changes Associated with Different Levels of Energy Deficits in Mediterranean Buffaloes during the Early Lactation Stage: Type and Role of the Main Lipid Fractions Involved. Animals. 2023; 13(14):2333. https://doi.org/10.3390/ani13142333
Chicago/Turabian StyleLisuzzo, Anastasia, Maria Chiara Alterisio, Elisa Mazzotta, Paolo Ciaramella, Jacopo Guccione, Matteo Gianesella, Tamara Badon, and Enrico Fiore. 2023. "Metabolic Changes Associated with Different Levels of Energy Deficits in Mediterranean Buffaloes during the Early Lactation Stage: Type and Role of the Main Lipid Fractions Involved" Animals 13, no. 14: 2333. https://doi.org/10.3390/ani13142333
APA StyleLisuzzo, A., Alterisio, M. C., Mazzotta, E., Ciaramella, P., Guccione, J., Gianesella, M., Badon, T., & Fiore, E. (2023). Metabolic Changes Associated with Different Levels of Energy Deficits in Mediterranean Buffaloes during the Early Lactation Stage: Type and Role of the Main Lipid Fractions Involved. Animals, 13(14), 2333. https://doi.org/10.3390/ani13142333






