Association of Phenotypic Markers of Heat Tolerance with Australian Genomic Estimated Breeding Values and Dairy Cattle Selection Indices
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Genotyping
2.3. Prediction of GEBVs for HT
2.4. Prediction of Australian GEBVs for Other Traits and Selection Indices
- Quality assurance of the genotype-evaluating call rate and genetrain scores for each marker in a batch, lack of variation in the X-chromosome for males, duplicates in a batch indicating sampling issues, and duplicate genotypes for different animals across batches, indicating monozygotic twins or clones (which may cause dependencies in the analysis), Hardy Weinberg equilibrium and genotype inconsistencies given the pedigree.
- Imputation of missing genotypes or genotypes failing to meet the minimum genetrain score.
- Estimation of Direct Genetic Values (DGVs) using BLUP (SNP BLUP) described as RR-BLUP [35], based on an assumption that SNP effects are random and the DGV for bull i called gi is defined as follows:
- iv.
- Blending was based on Harris and Johnson’s [36] estimation of genomic breeding values (GEBVs).
2.5. Statistical Analysis
3. Results
3.1. Variation in Physiological and Production Performance by Relative Thermotolerance
3.2. Variation in Genomic Estimated Breeding Values (GEBVs) by Relative Thermotolerance
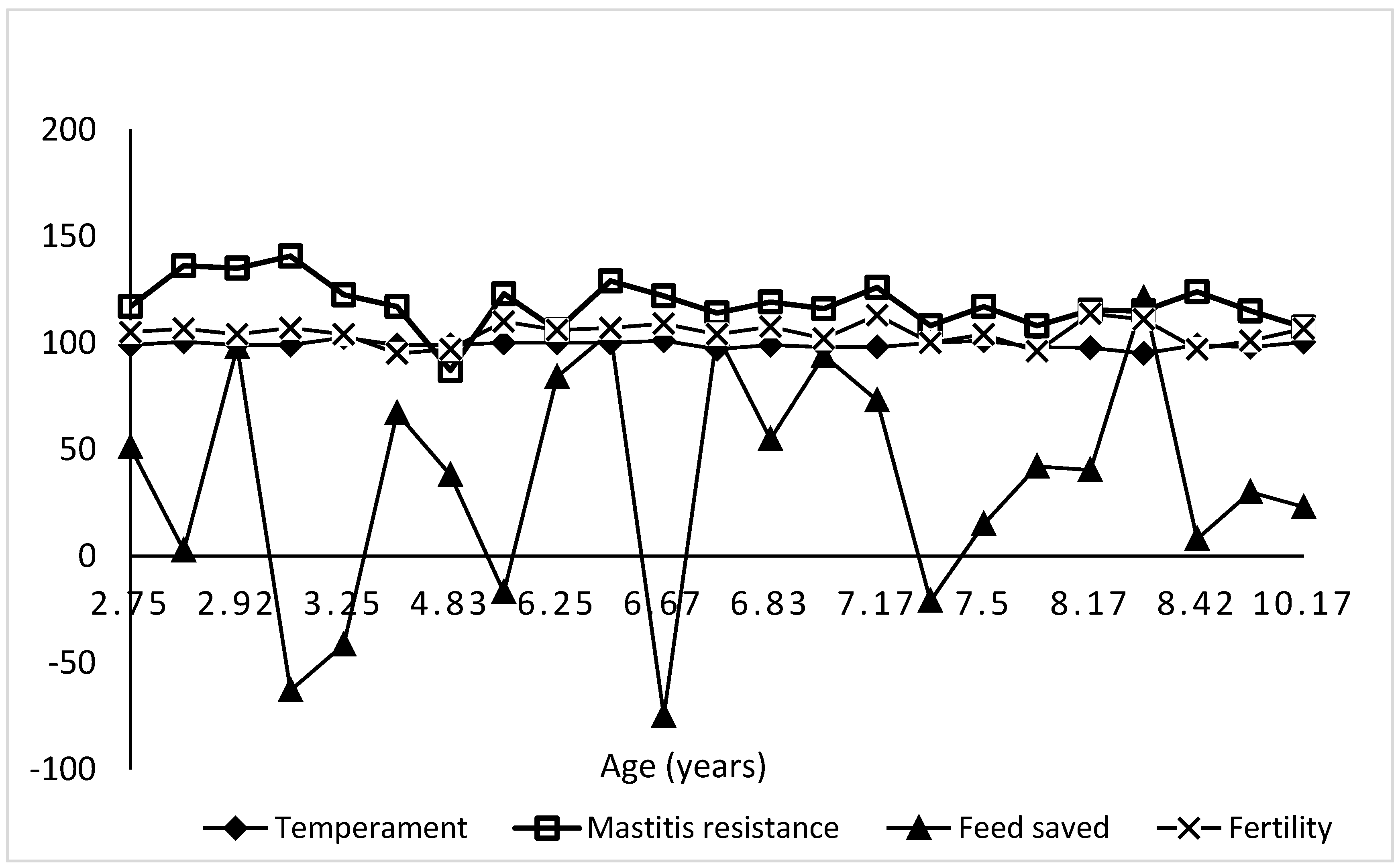
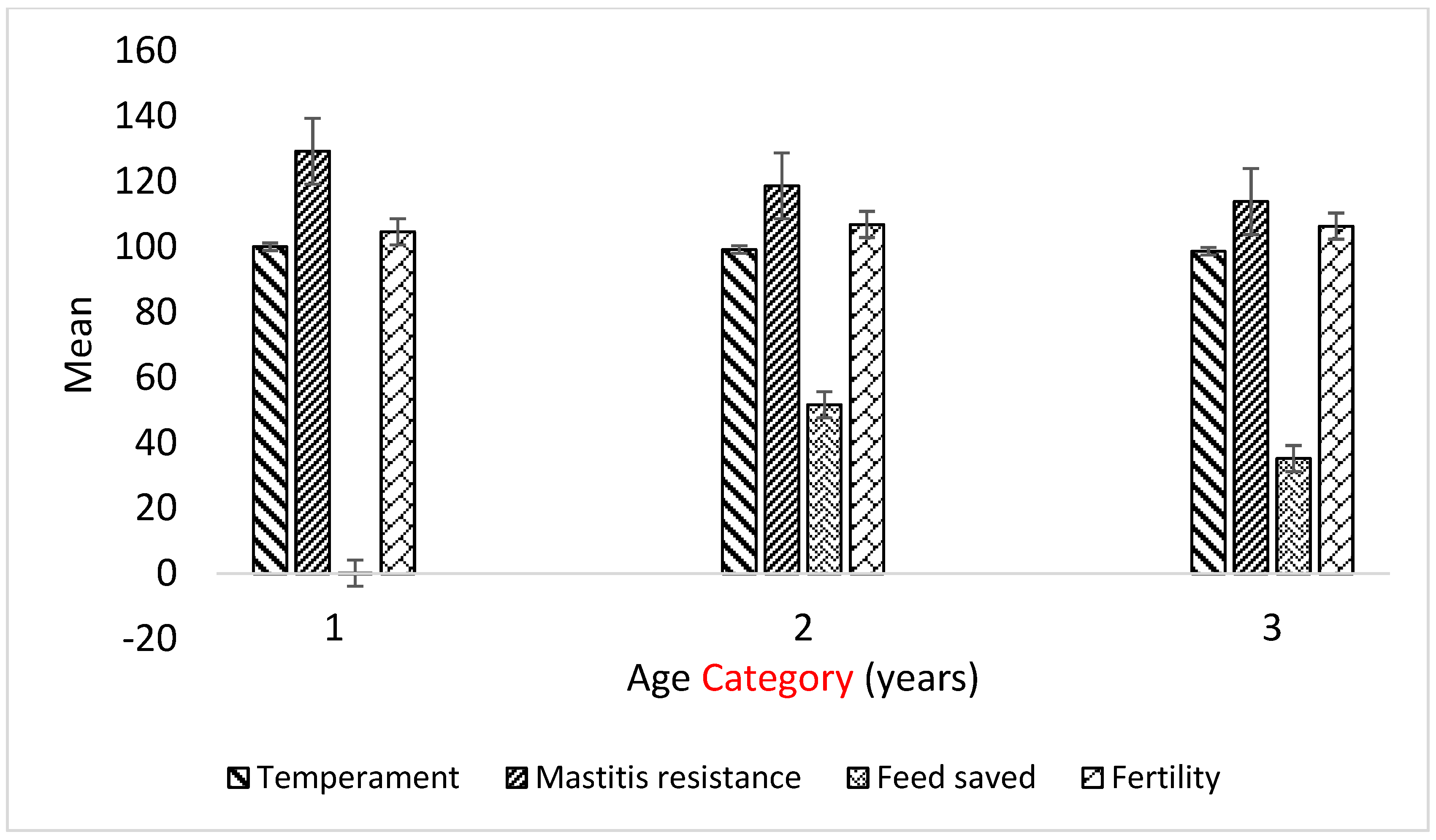
3.3. Variation in GEBVs of Selection Indices by Age Group
3.4. Association of GEBVs of Economic Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AUIBAR. The State of Farm Animal Genetic Resources in Africa; Towards Accelerated Agricultural Growth and Transformation by the Year 2025; Nouala, B.N., Mbole-Kariuki, S., Nengomasha, M., Tchangai, E., Eds.; African Union Interafrican Bureau for Animal Resources (AUIBAR): Nairobi, Kenya, 2019; 293p. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021-Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Rahimi, J.; Mutua, J.Y.; Notenbaert, A.M.O.; Marshall, K.; Butterbach-Bahl, K. Heat stress will detrimentally impact future livestock production in East Africa. Nat. Food 2021, 2, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, J.; Mills, K.E.; Sirovica, L.V.; Sundermann, L.; Bolton, S.E.; von Keyserlingk, M.G. Public perceptions of potential adaptations for mitigating heat stress on Australian dairy farms. J. Dairy Sci. 2022, 105, 5893–5908. [Google Scholar] [CrossRef]
- Osei-Amponsah, R.; Dunshea, F.R.; Leury, B.J.; Cheng, L.; Cullen, B.; Joy, A.; Abhijith, A.; Zhang, M.H.; Chauhan, S.S. Heat Stress Impacts on Lactating Cows Grazing Australian Summer Pastures on an Automatic Robotic Dairy. Animals 2020, 10, 869. [Google Scholar] [CrossRef]
- Zhang, Y.; McCarl, B.; Jones, J. An Overview of Mitigation and Adaptation Needs and Strategies for the Livestock Sector. Climate 2017, 5, 95. [Google Scholar] [CrossRef]
- Freitas, P.H.F.; Wang, Y.; Yan, P.; Oliveira, H.R.; Schenkel, F.S.; Zhang, Y.; Xu, Q.; Brito, L.F. Genetic Diversity and Signatures of Selection for Thermal Stress in Cattle and Other Two Bos Species Adapted to Divergent Climatic Conditions. Front. Genet. 2021, 12, 604823. [Google Scholar] [CrossRef]
- Cheruiyot, E.K.; Nguyen, T.T.T.; Haile-Mariam, M.; Cocks, B.G.; Abdelsayed, M.; Pryce, J.E. Genotype-by-environment (temperature-humidity) interaction of milk production traits in Australian Holstein cattle. J. Dairy Sci. 2020, 103, 2460–2476. [Google Scholar] [CrossRef]
- Luo, H.; Li, X.; Hu, L.; Xu, W.; Chu, Q.; Liu, A.; Guo, G.; Liu, L.; Brito, L.F.; Wang, Y. Genomic analyses and biological validation of candidate genes for rectal temperature as an indicator of heat stress in Holstein cattle. J. Dairy Sci. 2021, 104, 4441–4451. [Google Scholar] [CrossRef]
- Collier, R.J.; Renquist, B.J.; Xiao, Y. A 100-Year Review: Stress physiology including heat stress. J. Dairy Sci. 2017, 100, 10367–10380. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Ferreira, F.C.; Gennari, R.S.; Dahl, G.E.; De Vries, A. Economic feasibility of cooling dry cows across the United States. J. Dairy Sci. 2016, 99, 9931–9941. [Google Scholar] [CrossRef] [PubMed]
- Laporta, J.; Ferreira, F.C.; Ouellet, V.; Dado-Senn, B.; Almeida, A.K.; De Vries, A.; Dahl, G.E. Late-gestation heat stress impairs daughter and granddaughter lifetime performance. J. Dairy Sci. 2020, 103, 7555–7568. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E.; Tao, S.; Laporta, J. Heat Stress Impacts Immune Status in Cows Across the Life Cycle. Front. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef]
- Pryce, J.E.; Haile-Mariam, M. Symposium review: Genomic selection for reducing environmental impact and adapting to climate change. J. Dairy Sci. 2020, 103, 5366–5375. [Google Scholar] [CrossRef]
- Dikmen, S.; Khan, F.A.; Huson, H.J.; Sonstegard, T.S.; Moss, J.I.; Dahl, G.E.; Hansen, P.J. The SLICK hair locus derived from Senepol cattle confers thermotolerance to intensively managed lactating Holstein cows. J. Dairy Sci. 2014, 97, 5508–5520. [Google Scholar] [CrossRef]
- Dikmen, S.; Mateescu, R.G.; Elzo, M.A.; Hansen, P.J. Determination of the optimum contribution of Brahman genetics in an Angus-Brahman multibreed herd for regulation of body temperature during hot weather. J. Anim. Sci. 2018, 96, 2175–2183. [Google Scholar] [CrossRef]
- Jensen, L.M.; Jannaman, E.A.; Pryce, J.E.; De Vries, A.; Hansen, P.J. Effectiveness of the Australian breeding value for heat tolerance at discriminating responses of lactating Holstein cows to heat stress. J. Dairy Sci. 2022, 105, 7820–7828. [Google Scholar] [CrossRef]
- Carmickle, A.T.; Larson, C.C.; Hernandez, F.S.; Pereira, J.M.V.; Ferreira, F.C.; Haimon, M.L.J.; Jensen, L.M.; Hansen, P.J.; Denicol, A.C. Physiological responses of Holstein calves and heifers carrying the SLICK1 allele to heat stress in California and Florida dairy farms. J. Dairy Sci. 2022, 105, 9216–9225. [Google Scholar] [CrossRef]
- Gutierrez-Reinoso, M.A.; Aponte, P.M.; Garcia-Herreros, M. Genomic Analysis, Progress and Future Perspectives in Dairy Cattle Selection: A Review. Animals 2021, 11, 599. [Google Scholar] [CrossRef]
- Garner, J.B.; Douglas, M.L.; Williams, S.R.; Wales, W.J.; Marett, L.C.; Nguyen, T.T.; Reich, C.M.; Hayes, B.J. Genomic Selection Improves Heat Tolerance in Dairy Cattle. Sci. Rep. 2016, 6, 34114. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Bowman, P.J.; Haile-Mariam, M.; Pryce, J.E.; Hayes, B.J. Genomic selection for tolerance to heat stress in Australian dairy cattle. J. Dairy Sci. 2016, 99, 2849–2862. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; JGarner, J.B.; Bowman, P.J.; Haile-Mariam, M.; Hayes, B.J.; Pryce, J.E. Breeding for Heat Tolerance in Australian Dairy Cattle: From Development to Implementation; Agriculture Victoria, AgriBio, Centre for AgriBioscience: Melbourne, Australia, 2017. [Google Scholar]
- Pryce, J.E.; Nguyen, T.T.T.; Axford, M.; Nieuwhof, G.; Shaffer, M. Symposium review: Building a better cow-The Australian experience and future perspectives. J. Dairy Sci. 2018, 101, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T. Advances in dairy cattle breeding to improve heat tolerance. In Advances in Breeding of Dairy Cattle; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 313–336. [Google Scholar]
- DataGene. DataGene Solutions for Herd Improvement; A. DataGene, Ed.; AgriBio, La Trobe University: Melbourne, Australia, 2020; p. 2. [Google Scholar]
- Lowe, G.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Infrared Thermography—A Non-Invasive Method of Measuring Respiration Rate in Calves. Animals 2019, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, J.B.; Mader, T.L.; Holt, S.M.; Lisle, A. A new heat load index for feedlot cattle. J. Anim. Sci. 2008, 86, 226–234. [Google Scholar] [CrossRef]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Jayaprakash, G.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Das, D.N.; Kataktalware, M.A.; Prakash, M.A.; et al. Infrared thermography: A potential noninvasive tool to monitor udder health status in dairy cows. Vet. World 2016, 9, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Zoetis. Zoetis Clarifide. 2019. Available online: https://www.zoetis.com.au/clarifide/index.aspx (accessed on 24 October 2022).
- Nguyen, T.T.; Bowman, P.J.; Haile-Mariam, M.; Nieuwhof, G.J.; Hayes, B.J.; Pryce, J.E. Implementation of a breeding value for heat tolerance in Australian dairy cattle. J. Dairy Sci. 2017, 100, 7362–7367. [Google Scholar] [CrossRef]
- Nieuwhof, G.; Beard, K.; Konstantinov, K.; Bowman, P.; Hayes, B. Implementation of genomics in Australia. Interbull. Bull. 2010, 42, 35. [Google Scholar]
- Moser, G.; Tier, B.; Crump, R.E.; Khatkar, M.S.; Raadsma, H.W. A comparison of five methods to predict genomic breeding values of dairy bulls from genome-wide SNP markers. Genet. Sel. Evol. 2009, 41, 56. [Google Scholar] [CrossRef]
- Harris, B.; Johnson, D. Genomic predictions for New Zealand dairy bulls and integration with national genetic evaluation. J. Dairy Sci. 2010, 93, 1243–1252. [Google Scholar] [CrossRef]
- VSN. Genstat for Windows, 22nd ed.; VSN International: Hemel Hempstead, UK, 2022. [Google Scholar]
- DataGene. Technote 20 Heat Tolerance ABV; Dairy Australia: Melbourne, Austrlia, 2017. [Google Scholar]
- Berihulay, H.; Abied, A.; He, X.; Jiang, L.; Ma, Y. Adaptation Mechanisms of Small Ruminants to Environmental Heat Stress. Animals 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ashraf, S.; Goud, T.S.; Grewal, A.; Singh, S.V.; Yadav, B.R.; Upadhyay, R.C. Expression profiling of major heat shock protein genes during different seasons in cattle (Bos indicus) and buffalo (Bubalus bubalis) under tropical climatic condition. J. Therm. Biol. 2015, 51, 55–64. [Google Scholar] [CrossRef] [PubMed]
- DataGene. Sustainability Index; A. DataGene, Ed.; Dairy Australia: Melbourne, Austrlia, 2020. [Google Scholar]


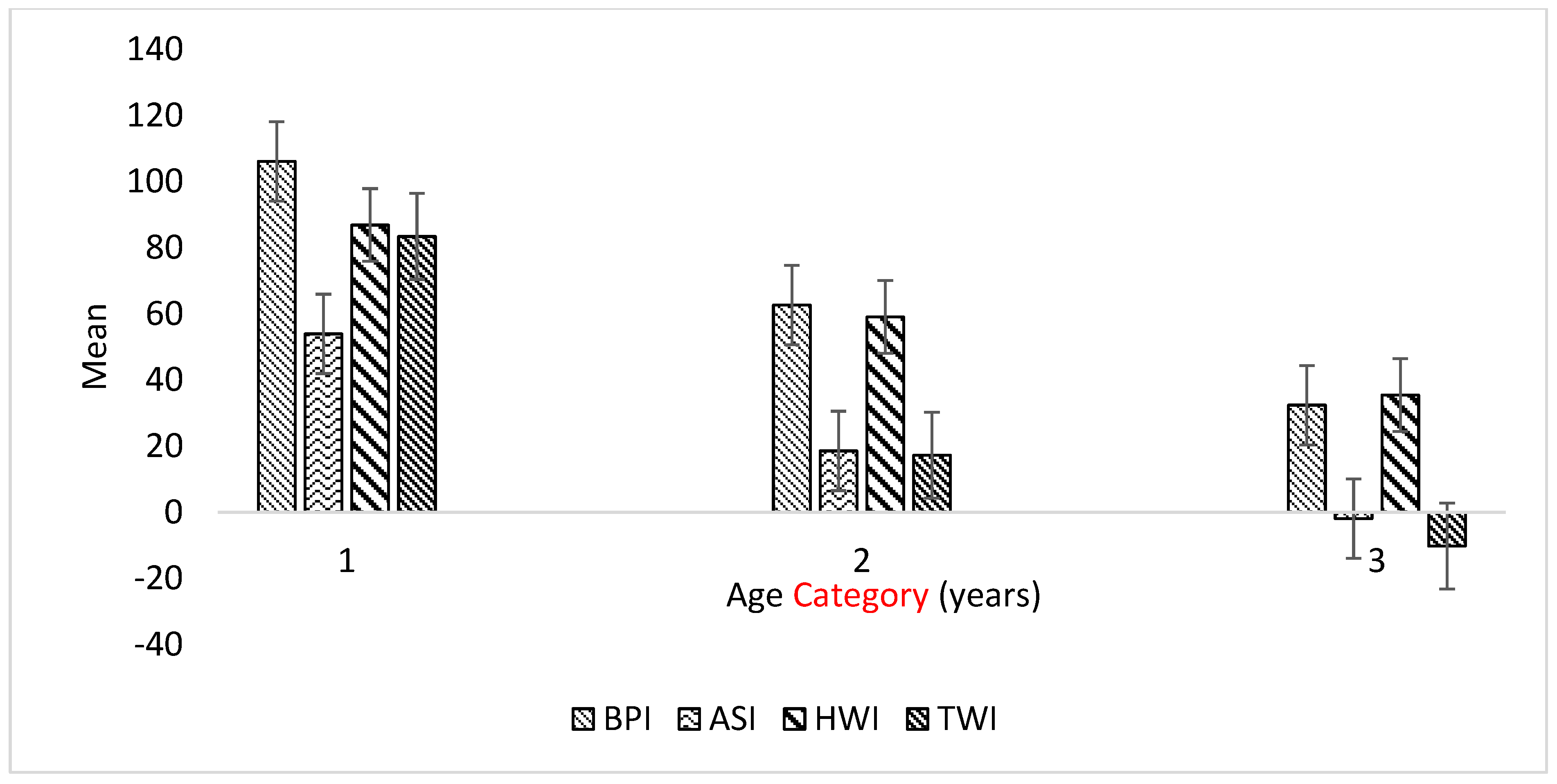
| Parameter | Description | Remarks |
|---|---|---|
| Balanced Performance Index (BPI) | The Balanced Performance Index (BPI) is an economic index that balances the economic contribution of production, health and fertility, type, workability, and feed efficiency. The updated BPI applies greater emphasis to health by adding in survival and mastitis resistance. | The BPI identifies bulls and cows that combine traits that are important to profit. Farmers can track this in their genetic progress report and make appropriate and timely breeding decisions. |
| Health Weighted Index (HWI) | The Health Weighted Index (HWI) allows farmers to fast-track traits such as fertility, mastitis resistance, and feed saved (efficiency). | The HWI puts the greatest emphasis on health and fertility, with production secondary. |
| Type Weighted Index (TWI) | The Type Weighted Index (TWI) allowed farmers to fine-tune type traits to make a good herd even better. | Currently, the TWI has been replaced by good bulls guide tables. |
| Australian Selection Index (ASI) | The ASI is a production-based index that ranks animals (bulls or females) on their ability to produce daughters with the most profitable combination of protein, fat, and milk production. Traits are weighted according to the way Australian dairy farmers are paid for their milk (fat + protein − volume). The ASI is expressed in dollars. An ASI of 200 means this animal is AUD200 per year more profitable from production than average. | The ASI is included in all three indices (the BPI, HWI, and sustainability indices) with the highest waiting on the sustainability index. For example, if an animal has an ASI of 200, then that is the contribution to production. If that same animal has a BPI of 300, then BPI 300 = ASI 200 + 100 from non-production. |
| Feed Saved (FS) ABV | The feed saved ABV allows one to breed cows with reduced maintenance requirements for the same amount of milk produced. It is expressed in kilograms of dry matter of feed saved per cow per year more or less than the average of zero. A positive number represents feed saved; a negative number represents extra feed consumed. In genotyped Holsteins, feed saved ABV utilises maintenance feed requirements predicted from type traits and Residual Feed Intake (RFI). Reliability is a measure of confidence in an ABV. The reliability of an animal’s breeding values improves with age as more information becomes available; for example, genomics, daughters’ performance records, and herd test results. | To improve feed efficiency in your herd, select animals with a feed saved ABV greater than zero. Feed saved is a moderately heritable trait (20–30%), which means that selection for feed saved will make a difference. An updated model for the feed saved ABV was implemented in November 2020, resulting in improved reliability (42–45%). For Holstein bulls, this represented an 11% improvement in reliability. |
| Heat Tolerance (HT) ABV | HT ABV allows farmers to identify animals with a greater ability to tolerate hot, humid conditions with less impact on milk production. It is expressed as a percentage, with a base of 100. An animal with an ABV of 105 is 5% more tolerant to hot, humid conditions than the average, and its drop in production will be 5% less than the average. On the other hand, an ABV of 95 means the animal is 5% less tolerant to hot, humid conditions than the average and its drop in production under heat stress is 5% more than the average. | To improve heat tolerance in your herd, select animals with a heat tolerance ABV of greater than 100. Allow for the lower reliability (36–38%) of the heat tolerance ABV by using a team of bulls. Reliability for HT ABV is expected to increase as more records become available. |
| Parameter | Group 1 (Thermo-Susceptible) | Group 2 (Thermotolerant) |
|---|---|---|
| Respiration rate (breadths min−1) # | 91.8 ± 34.7 (303) * | 90.1 ± 32.1 (313) |
| Panting score λ | 2.0 ± 0.8 (307) | 1.9 ± 0.8 (317) |
| Daily milk production (kg/d) | 21.3 ± 5.6 b (341) | 30.0 ± 6.9 a (340) |
| Fat % | 4.4 ± 0.9 a (340) | 3.9 ± 0.6 b (313) |
| Protein % | 3.2 ± 0.3 a (340) | 3.0 ± 0.2 b (340) |
| Concentrate intake (kg/d) | 5.3 ± 1.8 b (322) | 6.2 ± 1.6 a (320) |
| Rumination time (mins) | 399.4 ± 108 b (320) | 445.9 ± 108.5 a (320) |
| Residual feed (kg/d) | 1.1 ± 0.2 a (322) | 0.7 ± 0.8 b (322) |
| Thermo-Susceptible Group (n = 19) | Thermotolerant Group (n = 20) | Herd Average | |
|---|---|---|---|
| n (sample size) | 19 * | 20 | 39.0 |
| BPI | 75.7 ± 19.2 | 63.2 ± 16.5 | 69.3 |
| ASI | 32.0 ± 12.5 | 19.0 ± 11.9 | 25.3 |
| HWI | 65.6 ± 15.3 | 58.4 ± 13.3 | 61.9 |
| TWI | 37.8 ± 21.5 | 29.5 ± 14.3 | 33.5 |
| Milk | 86.7 ± 68.7 | −14.1 ± 91.2 | 35.0 |
| Milk protein | 4.4 ± 1.8 | 2.4 ± 1.6 | 3.3 |
| Milk fat | 6.1 ± 1.6 | 0.25 ± 2.6 | 3.1 |
| HT | 102.4 ± 0.95 | 104.1 ± 0.93 | 103.2 |
| Feed saved | 20.8 ± 12.0 | 31.5 ± 14.3 | 26.28 |
| Fertility | 106.0 ± 1.05 | 105.8 ± 1.38 | 105.9 |
| Age Category | ||||
|---|---|---|---|---|
| <5 Years | 5–7 Years | >7 Years | Total/Overall | |
| n | 15 | 11 | 13 | 39 |
| BPI | 106.1 a ± 21.3 | 62.6 ab ± 17.6 | 32.4 b ± 20.1 | 69.26 |
| ASI | 53.9 a ± 15.6 | 18.5 ab ± 12.8 | −1.9 b ± 10.4 | 25.33 |
| HWI | 86.9 ± 16.8 | 59.1 ± 13.1 | 35.5 ± 18.1 | 61.9 |
| TWI | 83.4 a ± 17.9 | 17.3 ab ± 20.1 | −10.2 b ± 19.5 | 33.54 |
| Milk | 207.1 a ± 79.0 | −175.3 b ± 131.1 | 14.4 ab ± 68.7 | 35.0 |
| Milk protein | 9.0 a ± 1.7 | −0.18 b ± 2.0 | −0.23 b ± 1.5 | 3.33 |
| Milk fat | 7.0 ± 3.0 | 1.2 ± 2.8 | 0.2 ± 2.2 | 3.10 |
| HT | 100.9 ± 1.2 | 103.9 ± 1.0 | 105.4 ± 0.80 | 103.2 |
| Feed saved | −0.3 ± 15.04 | 51.7 ± 19.7 | 35.2 ± 11.0 | 26.28 |
| Fertility | 104.7 ± 1.43 | 106.91 ± 0.80 | 106.4 ± 1.91 | 105.9 |
| BPI # | ASI | HWI | TWI | Milk | Protein | Fat | FS | Fertility | |
|---|---|---|---|---|---|---|---|---|---|
| ASI | 0.80 ** | ||||||||
| HWI | 0.97 ** | 0.64 ** | |||||||
| TWI | 0.95 ** | 0.77 ** | 0.92 ** | ||||||
| Milk | 0.11 | −0.05 | −0.13 | −0.02 | |||||
| Protein | 0.52 ** | 0.70 ** | 0.40 ** | 0.58 ** | 0.64 ** | ||||
| Fat | 0.61 ** | 0.80 ** | 0.46 ** | 0.56 ** | −0.02 | 0.46 ** | |||
| FS | −0.30 | −0.41 ** | −0.13 | −0.32 * | −0.29 | −0.45 ** | 0.48 ** | ||
| Fertility | 0.51 ** | 0.02 | 0.62 ** | 0.30 | −0.28 | 0.18 | 0.04 | 0.03 | |
| HT | −0.43 ** | −0.70 ** | −0.28 | −0.45 ** | −0.33 * | −0.74 ** | −0.59 ** | 0.45 ** | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osei-Amponsah, R.; Dunshea, F.R.; Leury, B.J.; Abhijith, A.; Chauhan, S.S. Association of Phenotypic Markers of Heat Tolerance with Australian Genomic Estimated Breeding Values and Dairy Cattle Selection Indices. Animals 2023, 13, 2259. https://doi.org/10.3390/ani13142259
Osei-Amponsah R, Dunshea FR, Leury BJ, Abhijith A, Chauhan SS. Association of Phenotypic Markers of Heat Tolerance with Australian Genomic Estimated Breeding Values and Dairy Cattle Selection Indices. Animals. 2023; 13(14):2259. https://doi.org/10.3390/ani13142259
Chicago/Turabian StyleOsei-Amponsah, Richard, Frank R. Dunshea, Brian J. Leury, Archana Abhijith, and Surinder S. Chauhan. 2023. "Association of Phenotypic Markers of Heat Tolerance with Australian Genomic Estimated Breeding Values and Dairy Cattle Selection Indices" Animals 13, no. 14: 2259. https://doi.org/10.3390/ani13142259
APA StyleOsei-Amponsah, R., Dunshea, F. R., Leury, B. J., Abhijith, A., & Chauhan, S. S. (2023). Association of Phenotypic Markers of Heat Tolerance with Australian Genomic Estimated Breeding Values and Dairy Cattle Selection Indices. Animals, 13(14), 2259. https://doi.org/10.3390/ani13142259









