Seasonal Variation in Gut Microbiota of the Wild Daurian Ground Squirrel (Spermophilus dauricus): Metagenomic Insights into Seasonal Breeding
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal Acquisition and Sample Collection
2.2. DNA Extraction and Metagenomic Sequencing
2.3. Data Processing, Species Composition Analysis, and Database Annotation
2.4. Differentially Expressed Gene Analysis
2.5. Statistical Analysis
3. Results
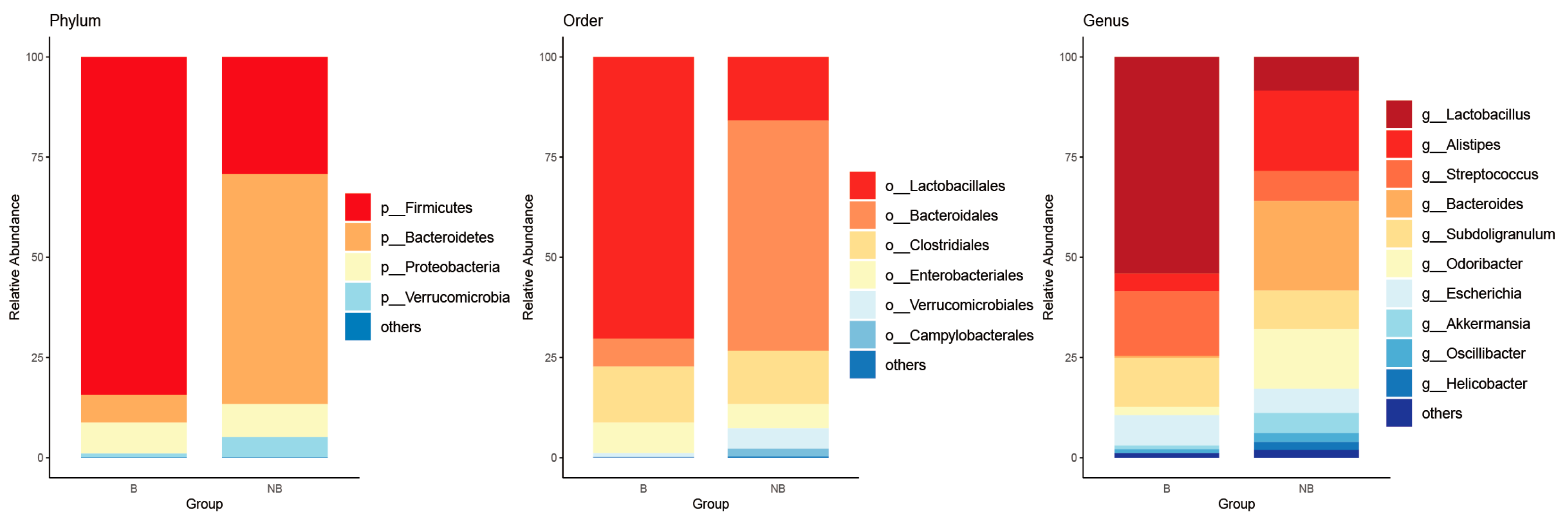
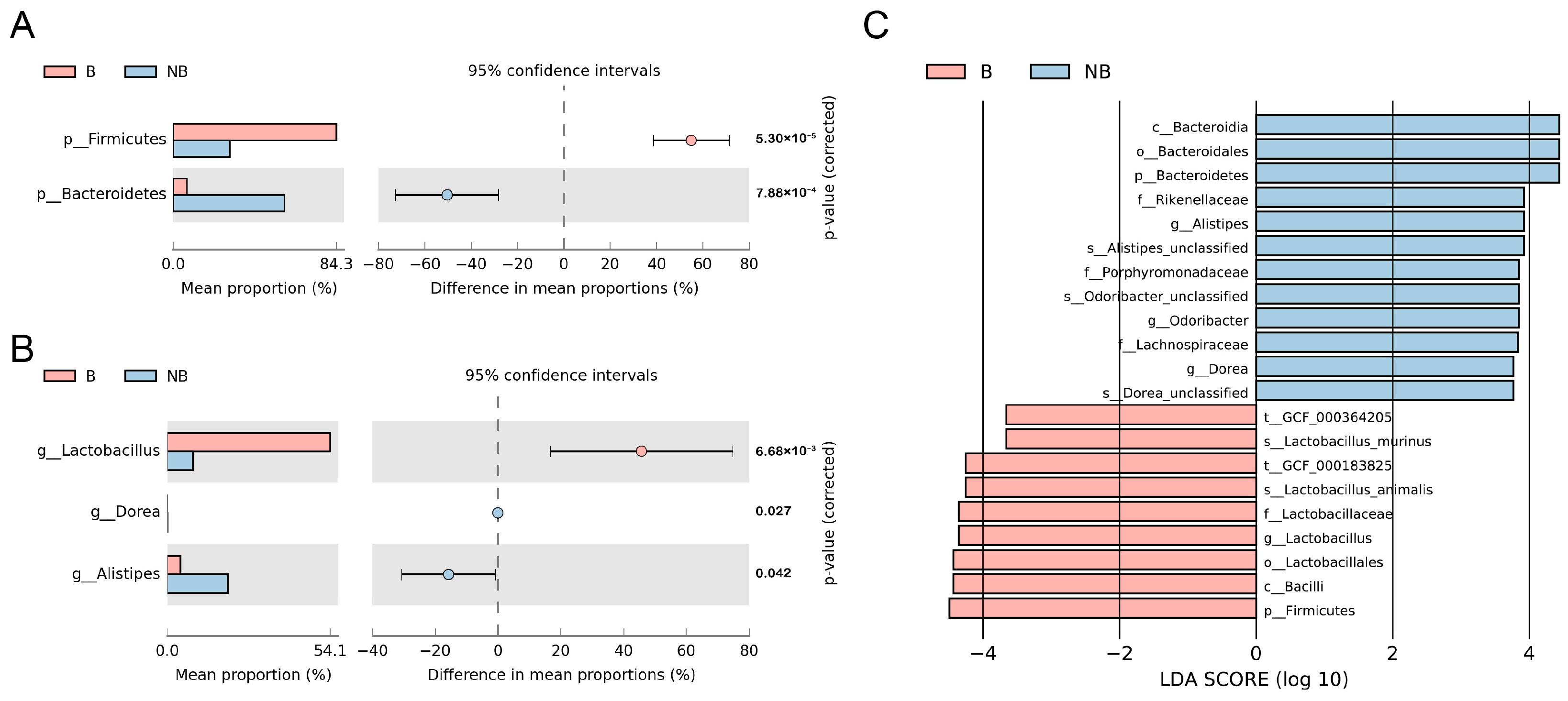
3.1. Species Composition and Variation of the Gut Microbiota
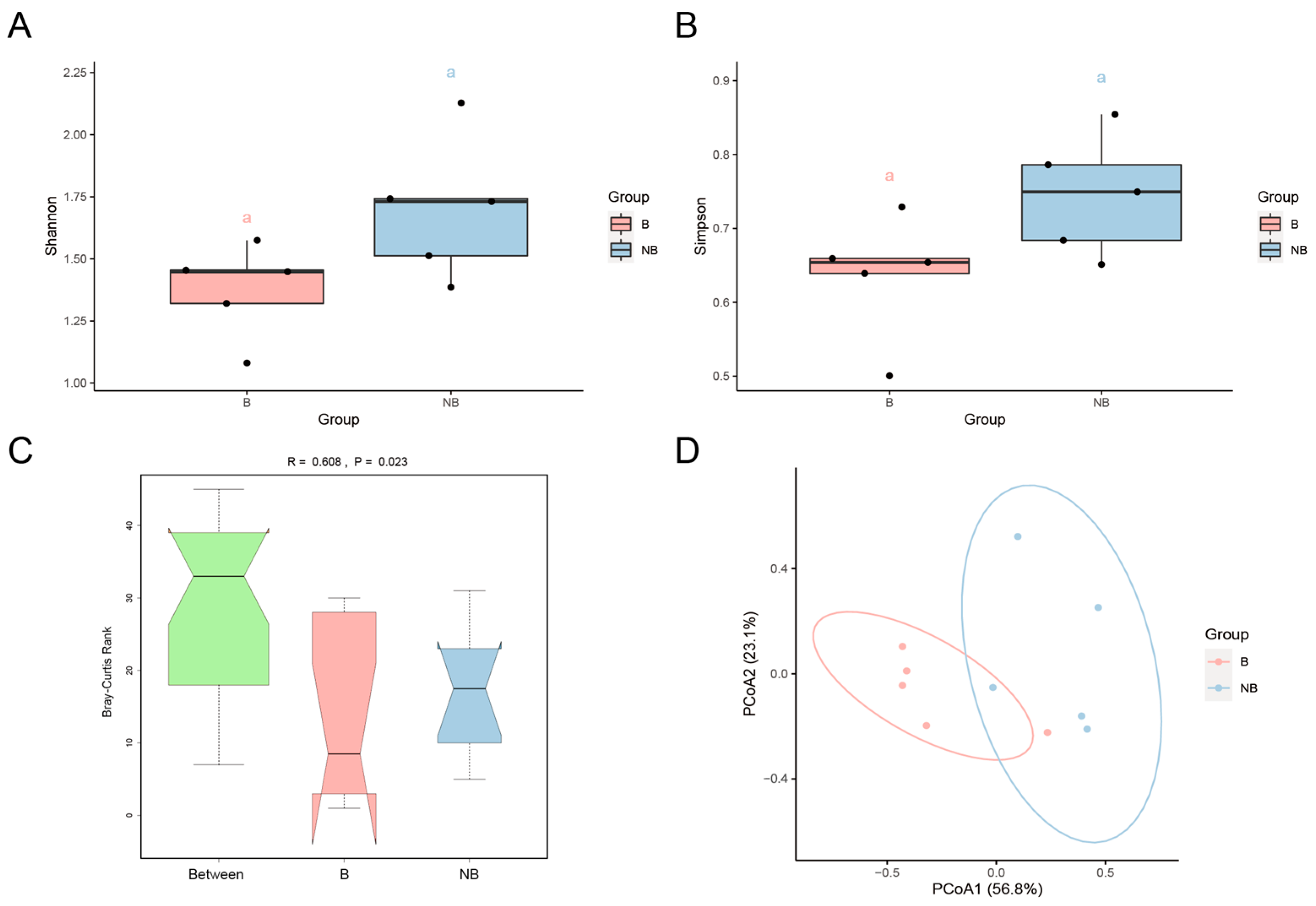
3.2. Diversity of the Gut Microbiota
3.3. Functional Annotation of the Gut Microbiota
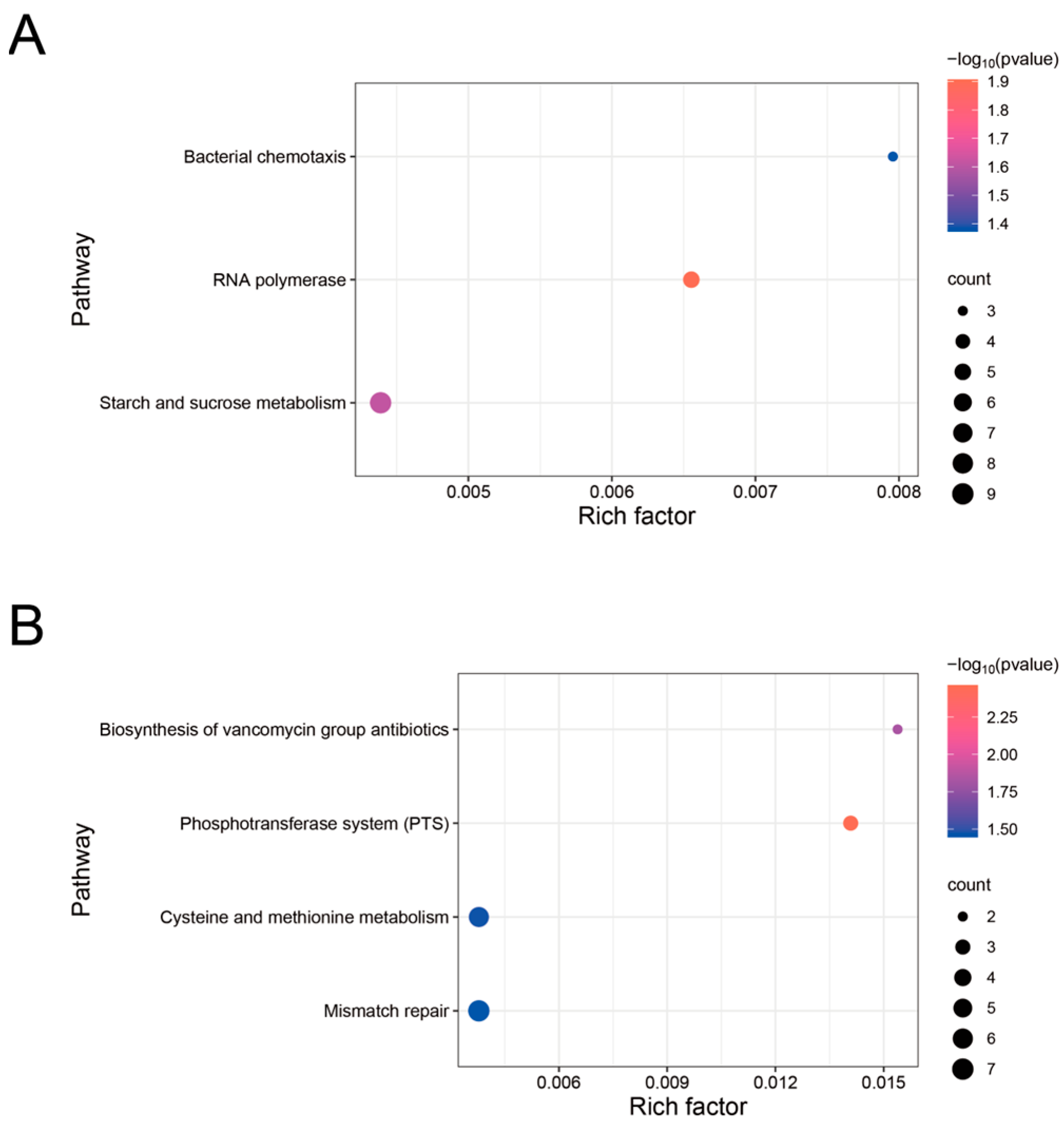
3.4. Analysis and Enrichment of Differentially Expressed Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M. Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. Biomed. Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X. Gut microbiota: Effect of pubertal status. BMC Microbiol. 2020, 20, 334. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Small intestinal bacterial overgrowth (SIBO) as a potential cause of impaired spermatogenesis. Gut 2020, 69, 2058–2059. [Google Scholar] [CrossRef]
- Colldén, H.; Landin, A.; Wallenius, V.; Elebring, E.; Fändriks, L.; Nilsson, M.E.; Ryberg, H.; Poutanen, M.; Sjögren, K.; Vandenput, L.; et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1182–E1192. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Pulkkinen, M.O.; Hämäläinen, E.K.; Korpela, J.T. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J. Steroid Biochem. 1984, 20, 217–229. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmakh, M.; Stukenborg, J.B.; Reda, A.; Anuar, F.; Strand, M.L.; Hedin, L.; Pettersson, S.; Söder, O. The gut microbiota and developmental programming of the testis in mice. PLoS ONE 2014, 9, e103809. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, Y.; Yang, X.; Zhao, L.; Wen, S.; Liu, Y.; Tang, L. Association between Polycystic Ovary Syndrome and Gut Microbiota. PLoS ONE 2016, 11, e0153196. [Google Scholar] [CrossRef]
- Han, Y.; Zhan, J.; Xu, Y.; Zhang, F.; Yuan, Z.; Weng, Q. Proliferation and apoptosis processes in the seasonal testicular development of the wild Daurian ground squirrel (Citellus dauricus Brandt, 1844). Reprod. Fertil. Dev. 2017, 29, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Kumar, V. Photoperiodic regulation of seasonal reproduction in higher vertebrates. Indian J. Exp. Biol. 2014, 52, 413–419. [Google Scholar]
- Anand, S.; Losee-Olson, S.; Turek, F.W.; Horton, T.H. Differential regulation of luteinizing hormone and follicle-stimulating hormone in male siberian hamsters by exposure to females and photoperiod. Endocrinology 2002, 143, 2178–2188. [Google Scholar] [CrossRef]
- Bartness, T.J.; Goldman, B.D. Mammalian pineal melatonin: A clock for all seasons. Experientia 1989, 45, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Okimura, K.; Yoshimura, T. Light and Hormones in Seasonal Regulation of Reproduction and Mood. Endocrinology 2020, 161, bqaa130. [Google Scholar] [CrossRef] [PubMed]
- Meethal, S.V.; Liu, T.; Chan, H.W.; Ginsburg, E.; Wilson, A.C.; Gray, D.N.; Bowen, R.L.; Vonderhaar, B.K.; Atwood, C.S. Identification of a regulatory loop for the synthesis of neurosteroids: A steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors. J. Neurochem. 2009, 110, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Vadakkadath Meethal, S.; Atwood, C.S. Alzheimer’s disease: The impact of age-related changes in reproductive hormones. Cell. Mol. Life Sci. CMLS 2005, 62, 257–270. [Google Scholar] [CrossRef]
- Dardente, H.; Simonneaux, V. GnRH and the photoperiodic control of seasonal reproduction: Delegating the task to kisspeptin and RFRP-3. J. Neuroendocrinol. 2022, 34, e13124. [Google Scholar] [CrossRef]
- Dardente, H.; Migaud, M. Thyroid hormone and hypothalamic stem cells in seasonal functions. Vitam. Horm. 2021, 116, 91–131. [Google Scholar] [CrossRef]
- Kriegsfeld, L.J.; Ubuka, T.; Bentley, G.E.; Tsutsui, K. Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Front. Neuroendocrinol. 2015, 37, 65–75. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Han, W.; Wang, J.; Zhang, H.; Sheng, X.; Yuan, Z.; Weng, Q.; Han, Y. Seasonal changes of androgen receptor, estrogen receptors and aromatase expression in the hippocampus of the wild male ground squirrels (Citellus dauricus Brandt). Gen. Comp. Endocrinol. 2017, 249, 93–100. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Zhang, S.; Sheng, X.; Han, X.; Weng, Q.; Yuan, Z. Seasonal expression of androgen receptor, aromatase, and estrogen receptor alpha and beta in the testis of the wild ground squirrel (Citellus dauricus Brandt). Eur. J. Histochem. 2015, 59, 2456. [Google Scholar] [CrossRef]
- Fan, S.; Lu, W.; Zhang, H.; Yuan, Z.; Han, Y.; Weng, Q. Seasonal Change in Adiponectin Associated with Ovarian Morphology and Function in Wild Ground Squirrels (Citellus dauricus Brandt). Int. J. Mol. Sci. 2022, 23, 14698. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, Y.; Yu, W.; Xie, W.; Zhang, Z.; Wang, J.; Zhang, H.; Han, Y.; Weng, Q. Seasonal expressions of oxytocin and oxytocin receptor in the epididymides in the wild ground squirrels (Citellus dauricus Brandt). Gen. Comp. Endocrinol. 2020, 289, 113391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Zhang, J.; Wang, L.; Li, Q.; Sheng, X.; Han, Y.; Yuan, Z.; Weng, Q. Testicular expression of NGF, TrkA and p75 during seasonal spermatogenesis of the wild ground squirrel (Citellus dauricus Brandt). Eur. J. Histochem. 2015, 59, 2522. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Wang, Z.; Sheng, X.; Zhang, H.; Han, Y.; Yuan, Z.; Weng, Q. Seasonal expressions of luteinising hormone receptor, follicle-stimulating hormone receptor and prolactin receptor in the epididymis of the male wild ground squirrel (Spermophilus dauricus). Reprod. Fertil. Dev. 2019, 31, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Jiao, Y.; Zhang, L.; Zhang, H.; Sheng, X.; Han, Y.; Yuan, Z.; Weng, Q. Seasonal changes of androgen receptor, estrogen receptors and aromatase expression in the medial preoptic area of the wild male ground squirrels (Citellus dauricus Brandt). Eur. J. Histochem. 2016, 60, 2621. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, X.; Zhang, X.; Zhu, J.; Chen, Y.; Gao, F.; Zhang, H.; Han, Y.; Weng, Q.; Yuan, Z. Seasonal expression of extracellular signal regulated kinases in the colon of wild ground squirrels (Spermophilus dauricus). Mol. Biol. Rep. 2022, 49, 2209–2215. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Song, F.; Wei, H.; Gao, F.; Zhang, H.; Han, Y.; Weng, Q.; Yuan, Z. Seasonal expressions of GPR41 and GPR43 in the colon of the wild ground squirrels (Spermophilus dauricus). Eur. J. Histochem. 2022, 66, 3351. [Google Scholar] [CrossRef]
- Yang, X.; Yao, Y.; Zhang, X.; Zhong, J.; Gao, F.; Zhang, H.; Han, Y.; Weng, Q.; Yuan, Z. Seasonal Changes in the Distinct Taxonomy and Function of the Gut Microbiota in the Wild Ground Squirrel (Spermophilus dauricus). Animals 2021, 11, 2685. [Google Scholar] [CrossRef]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2016, 469, 967–977. [Google Scholar] [CrossRef]
- Truong, D.T.; Franzosa, E.A.; Tickle, T.L.; Scholz, M.; Weingart, G.; Pasolli, E.; Tett, A.; Huttenhower, C.; Segata, N. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 2015, 12, 902–903. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience 2012, 1, 2047–2217X-1-18. [Google Scholar] [CrossRef]
- Zhu, W.; Lomsadze, A.; Borodovsky, M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010, 38, e132. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Chen, T.; Liu, Y.-X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Davis, A.J.; Kay, S. Writing statistical methods for ecologists. Ecosphere 2023, 14, e4539. [Google Scholar] [CrossRef]
- Stevenson, T.J.; Duddleston, K.N.; Buck, C.L. Effects of season and host physiological state on the diversity, density, and activity of the arctic ground squirrel cecal microbiota. Appl. Environ. Microbiol. 2014, 80, 5611–5622. [Google Scholar] [CrossRef] [PubMed]
- Maurice, C.F.; Knowles, S.C.; Ladau, J.; Pollard, K.S.; Fenton, A.; Pedersen, A.B.; Turnbaugh, P.J. Marked seasonal variation in the wild mouse gut microbiota. ISME J. 2015, 9, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Boutin, S.; Humphries, M.M.; Dantzer, B.; Gorrell, J.C.; Coltman, D.W.; McAdam, A.G.; Wu, M. Seasonal, spatial, and maternal effects on gut microbiome in wild red squirrels. Microbiome 2017, 5, 163. [Google Scholar] [CrossRef]
- Wan, X.; Li, J.; Cheng, Z.; Ao, M.; Tian, R.; Mclaughlin, R.W.; Zheng, J.; Wang, D. The intestinal microbiome of an Indo-Pacific humpback dolphin (Sousa chinensis) stranded near the Pearl River Estuary, China. Integr. Zool. 2021, 16, 287–299. [Google Scholar] [CrossRef]
- Li, H.; Xia, W.; Liu, X.; Wang, X.; Liu, G.; Chen, H.; Zhu, L.; Li, D. Food provisioning results in functional, but not compositional, convergence of the gut microbiomes of two wild Rhinopithecus species: Evidence of functional redundancy in the gut microbiome. Sci. Total Environ. 2023, 858, 159957. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Yi, X. Alteration of Gut Microbiota of a Food-Storing Hibernator, Siberian Chipmunk Tamias sibiricus. Microb. Ecol. 2022, 84, 603–612. [Google Scholar] [CrossRef]
- Shor, E.K.; Brown, S.P.; Freeman, D.A. A novel role for the pineal gland: Regulating seasonal shifts in the gut microbiota of Siberian hamsters. J. Pineal Res. 2020, 69, e12696. [Google Scholar] [CrossRef]
- Tan, X.; Han, H.; Niu, P. Investigation on the food pattern of the Spermophilus dauricus in Bashang area of Chengde City. Chin. J. Hyg. Insectic. Equip. 2017, 23, 538–540. (In Chinese) [Google Scholar] [CrossRef]
- Fan, C.; Zhang, L.; Jia, S.; Tang, X.; Fu, H.; Li, W.; Liu, C.; Zhang, H.; Cheng, Q.; Zhang, Y. Seasonal variations in the composition and functional profiles of gut microbiota reflect dietary changes in plateau pikas. Integr. Zool. 2022, 17, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Grigor’eva, I.N. Gallstone Disease, Obesity and the Firmicutes/Bacteroidetes Ratio as a Possible Biomarker of Gut Dysbiosis. J. Pers. Med. 2021, 11, 13. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020, 1461, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, X.; Yang, H.; Tu, T.; Zhang, J.; Luo, H.; Huang, H.; Su, X. PUL-Mediated Plant Cell Wall Polysaccharide Utilization in the Gut Bacteroidetes. Int. J. Mol. Sci. 2021, 22, 3077. [Google Scholar] [CrossRef]
- Turpin, W.; Humblot, C.; Thomas, M.; Guyot, J.-P. Lactobacilli as multifaceted probiotics with poorly disclosed molecular mechanisms. Int. J. Food Microbiol. 2010, 143, 87–102. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Joung, J.Y.; Lim, W.; Seo, Y.J.; Ham, J.; Oh, N.S.; Kim, S.H. A Synbiotic Combination of Lactobacillus gasseri 505 and Cudrania tricuspidata Leaf Extract Prevents Stress-Induced Testicular Dysfunction in Mice. Front. Endocrinol. 2022, 13, 835033. [Google Scholar] [CrossRef] [PubMed]
- Mahiddine, F.Y.; You, I.; Park, H.; Kim, M.J. Management of dog sperm parameters and gut microbiota composition with Lactobacillus rhamnosus supplementation. Vet. Res. Commun. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Z. Exploring the role of gut microbiome in male reproduction. Andrology 2022, 10, 441–450. [Google Scholar] [CrossRef]
- Vílchez, M.C.; Santangeli, S.; Maradonna, F.; Gioacchini, G.; Verdenelli, C.; Gallego, V.; Peñaranda, D.S.; Tveiten, H.; Pérez, L.; Carnevali, O.; et al. Effect of the probiotic Lactobacillus rhamnosus on the expression of genes involved in European eel spermatogenesis. Theriogenology 2015, 84, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sheng, X.; Hu, X.; Li, X.; Xu, H.; Zhang, M.; Li, B.; Xu, M.; Weng, Q.; Zhang, Z.; et al. Seasonal changes in spermatogenesis and immunolocalization of cytochrome P450 17alpha-hydroxylase/c17-20 lyase and cytochrome P450 aromatase in the wild male ground squirrel (Citellus dauricus Brandt). J. Reprod. Dev. 2010, 56, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhang, H.; Zhang, W.; Song, M.; Zhang, M.; Li, B.; Weng, Q.; Watanabe, G.; Taya, K. Seasonal changes in spermatogenesis and immunolocalization of inhibin/activin subunits in the wild male ground squirrel (Citellus dauricus Brandt). J. Reprod. Dev. 2008, 54, 460–464. [Google Scholar] [CrossRef][Green Version]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Deutscher, J.; Francke, C.; Postma, P.W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 939–1031. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Mu, C.; Wang, H.; Huang, Z.; Yu, K.; Zoetendal, E.G.; Zhu, W. Stimulation of Gastric Transit Function Driven by Hydrolyzed Casein Increases Small Intestinal Carbohydrate Availability and Its Microbial Metabolism. Mol. Nutr. Food Res. 2020, 64, e2000250. [Google Scholar] [CrossRef]
- Hayashi, A.; Mikami, Y.; Miyamoto, K.; Kamada, N.; Sato, T.; Mizuno, S.; Naganuma, M.; Teratani, T.; Aoki, R.; Fukuda, S.; et al. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus murinus in Mice. Cell Rep. 2017, 20, 1513–1524. [Google Scholar] [CrossRef]
- Song, F.; Xu, Y.; Peng, P.; Li, H.; Zheng, R.; Zhang, H.; Han, Y.; Weng, Q.; Yuan, Z. Seasonal Changes in the Structure and Function of Gut Microbiota in the Muskrat (Ondatra zibethicus). Metabolites 2023, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.; Ma, S.; Zhang, Y.; Yang, X.; Zhang, H.; Han, Y.; Liu, Y.; Gao, F.; Yuan, Z. Seasonal Variation in Gut Microbiota of the Wild Daurian Ground Squirrel (Spermophilus dauricus): Metagenomic Insights into Seasonal Breeding. Animals 2023, 13, 2235. https://doi.org/10.3390/ani13132235
Song F, Ma S, Zhang Y, Yang X, Zhang H, Han Y, Liu Y, Gao F, Yuan Z. Seasonal Variation in Gut Microbiota of the Wild Daurian Ground Squirrel (Spermophilus dauricus): Metagenomic Insights into Seasonal Breeding. Animals. 2023; 13(13):2235. https://doi.org/10.3390/ani13132235
Chicago/Turabian StyleSong, Fengcheng, Shubao Ma, Yujiao Zhang, Xiaoying Yang, Haolin Zhang, Yingying Han, Yuning Liu, Fuli Gao, and Zhengrong Yuan. 2023. "Seasonal Variation in Gut Microbiota of the Wild Daurian Ground Squirrel (Spermophilus dauricus): Metagenomic Insights into Seasonal Breeding" Animals 13, no. 13: 2235. https://doi.org/10.3390/ani13132235
APA StyleSong, F., Ma, S., Zhang, Y., Yang, X., Zhang, H., Han, Y., Liu, Y., Gao, F., & Yuan, Z. (2023). Seasonal Variation in Gut Microbiota of the Wild Daurian Ground Squirrel (Spermophilus dauricus): Metagenomic Insights into Seasonal Breeding. Animals, 13(13), 2235. https://doi.org/10.3390/ani13132235







