Canine Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Atopic Dermatitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Canine ASCs (cASCs)
2.2. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.3. Reverse Transcription Polymerase Chain Reaction (RT-PCR) for Phenotypical Characterization of cASCs
2.4. Osteogenic Differentiation: Alizarin Red Staining
2.5. Chondrogenic Differentiation: Alcian Blue Staining
2.6. Adipogenic Differentiation: Oil Red O Staining
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) for Analysis of Differentiation
2.8. Isolation of cASC-EVs
2.9. Quantification of cASC-EVs
2.10. Bead-Based Flow Cytometric Analysis of Exosomal Surface Markers
2.11. In Vivo Efficacy Study
2.11.1. Animals and Study Design
2.11.2. Clinical Observation of AD
2.11.3. Total Serum IgE Measurement
2.11.4. Quantitative Real-Time PCR Analysis
2.11.5. Histopathologic Evaluation
2.11.6. Immunohistochemistry
2.12. In Vivo Toxicity Studies
2.12.1. Single-Dose Toxicity Study
2.12.2. Twenty-Eight-Day Repeat-Dose Toxicity Study
2.13. microRNA Profiling
2.14. Statistical Analysis
3. Results
3.1. Characterization of Canine Adipose-Tissue-Derived Mesenchymal Stem Cells (cASC)
3.2. cASCs Have a Differentiation Phenotype for Osteogenesis, Adipogenesis, and Chondrogenesis
3.3. Isolation and Characterization of the cASC-Derived Extracellular Vesicles
3.4. Effect of EVs on Improvement of Biostir-Induced Atopic Skin Lesions in Mice
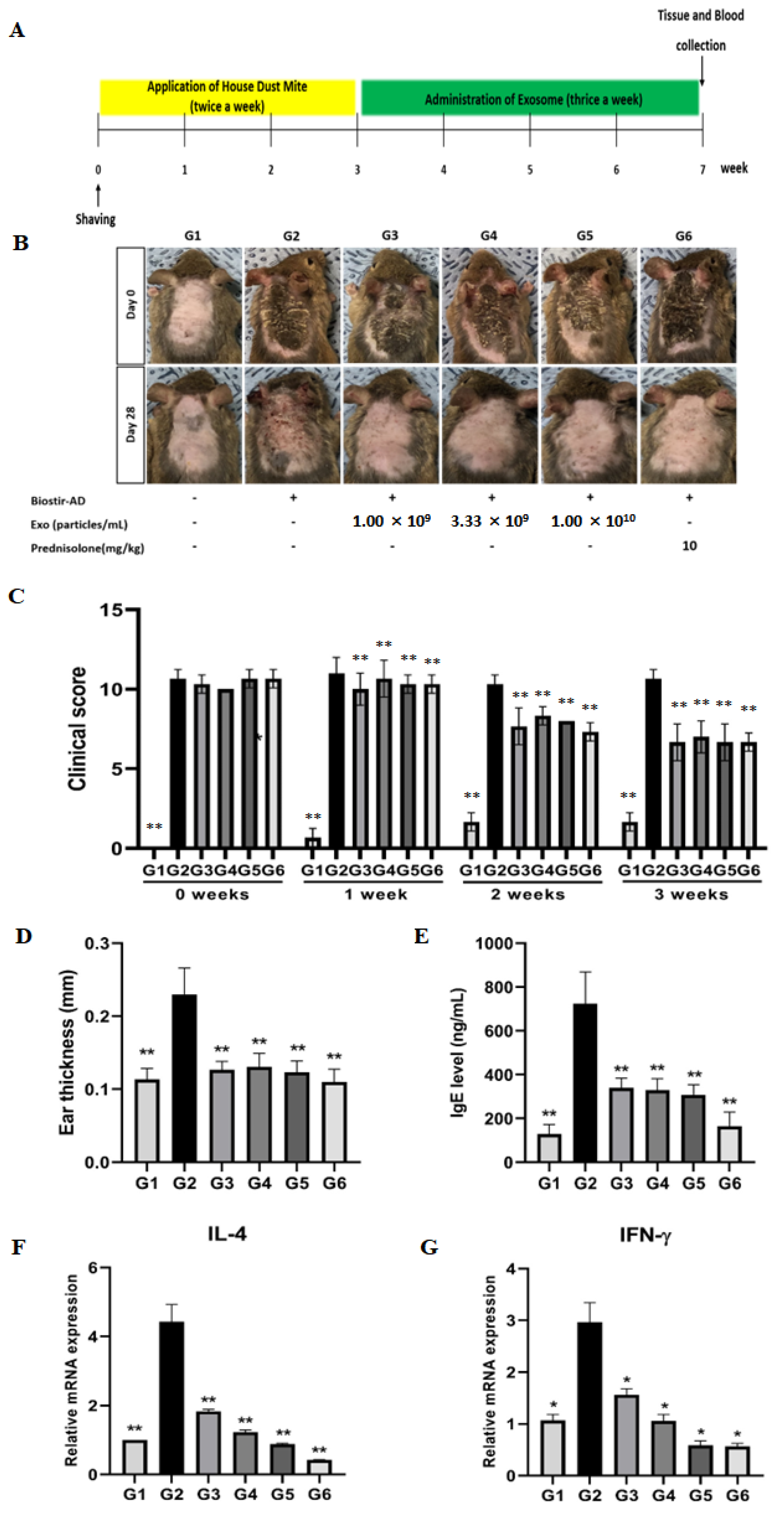
3.5. cASC-EVs Ameliorate the Signs of AD In Vivo
3.6. In Vivo Toxicity Studies
3.6.1. Single-Dose Toxicity Study
3.6.2. Twenty-Eight-Day Repeat-Dose Toxicity Study
3.7. MicroRNA Profiling of cASC-Derived EVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16. [Google Scholar] [CrossRef]
- Gedon, N.K.Y.; Mueller, R.S. Atopic dermatitis in cats and dogs: A difficult disease for animals and owners. Clin. Transl. Allergy 2018, 8, 41. [Google Scholar] [CrossRef]
- Pucheu-Haston, C.M. Atopic dermatitis in the domestic dog. Clin. Dermatol. 2016, 34, 299–303. [Google Scholar] [CrossRef]
- Jassies-van der Lee, A.; Rutten, V.; Spiering, R.; van Kooten, P.; Willemse, T.; Broere, F. The immunostimulatory effect of CpG oligodeoxynucleotides on peripheral blood mononuclear cells of healthy dogs and dogs with atopic dermatitis. Vet. J. 2014, 200, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; DeBoer, D.J.; Favrot, C.; Jackson, H.A.; Mueller, R.S.; Nuttall, T.; Prelaud, P.; International Task Force on Canine Atopic Dermatitis. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet. Dermatol. 2010, 21, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Little, P.R.; King, V.L.; Davis, K.R.; Cosgrove, S.B.; Stegemann, M.R. A blinded, randomized clinical trial comparing the efficacy and safety of oclacitinib and ciclosporin for the control of atopic dermatitis in client-owned dogs. Vet. Dermatol. 2015, 26, 23-e8. [Google Scholar] [CrossRef] [PubMed]
- Anjos-Afonso, F.; Siapati, E.K.; Bonnet, D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J. Cell Sci. 2004, 117, 5655–5664. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.W.; Kang, K.S.; Koo, H.C.; Park, J.R.; Choi, E.W.; Park, Y.H. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008, 17, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, S.; Yanagawa, B.; Tanaka, K.; Miyahara, Y.; Obata, H.; Kataoka, M.; Kodama, M.; Ishibashi-Ueda, H.; Kangawa, K.; Kitamura, S.; et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J. Mol. Cell. Cardiol. 2007, 42, 88–97. [Google Scholar] [CrossRef]
- Kunter, U.; Rong, S.; Djuric, Z.; Boor, P.; Muller-Newen, G.; Yu, D.; Floege, J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J. Am. Soc. Nephrol. 2006, 17, 2202–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batten, P.; Sarathchandra, P.; Antoniw, J.W.; Tay, S.S.; Lowdell, M.W.; Taylor, P.M.; Yacoub, M.H. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: Relevance to tissue engineering human heart valves. Tissue Eng. 2006, 12, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringden, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Ringden, O.; Uzunel, M.; Rasmusson, I.; Remberger, M.; Sundberg, B.; Lonnies, H.; Marschall, H.U.; Dlugosz, A.; Szakos, A.; Hassan, Z.; et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006, 81, 1390–1397. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065. [Google Scholar] [CrossRef]
- Mocchi, M.; Dotti, S.; Bue, M.D.; Villa, R.; Bari, E.; Perteghella, S.; Torre, M.L.; Grolli, S. Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier? Cells 2020, 9, 1453. [Google Scholar] [CrossRef]
- Eom, Y.W.; Lee, J.E.; Yang, M.S.; Jang, I.K.; Kim, H.E.; Lee, D.H.; Kim, Y.J.; Park, W.J.; Kong, J.H.; Shim, K.Y.; et al. Rapid isolation of adipose tissue-derived stem cells by the storage of lipoaspirates. Yonsei Med. J. 2011, 52, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Zoller, N.; Schreiner, S.; Petry, L.; Hoffmann, S.; Steinhorst, K.; Kleemann, J.; Jager, M.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Collagen I Promotes Adipocytogenesis in Adipose-Derived Stem Cells In Vitro. Cells 2019, 8, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enciso, N.; Avedillo, L.; Fermin, M.L.; Fragio, C.; Tejero, C. Cutaneous wound healing: Canine allogeneic ASC therapy. Stem Cell Res. Ther. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yoon, T.H.; Na, J.; Yi, S.J.; Jin, Y.; Kim, M.; Oh, T.H.; Chung, T.W. Mesenchymal Stem Cells and Extracellular Vesicles Derived from Canine Adipose Tissue Ameliorates Inflammation, Skin Barrier Function and Pruritus by Reducing JAK/STAT Signaling in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 4868. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W.; et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Kuca-Warnawin, E.; Janicka, I.; Bonek, K.; Kontny, E. Modulatory Impact of Adipose-Derived Mesenchymal Stem Cells of Ankylosing Spondylitis Patients on T Helper Cell Differentiation. Cells 2021, 10, 280. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.J.; Lew, B.L.; Lee, K.H.; Hong, S.P.; Jang, Y.H.; Park, K.Y.; Seo, S.J.; Bae, J.M.; Choi, E.H.; et al. Consensus Guidelines for the Treatment of Atopic Dermatitis in Korea (Part I): General Management and Topical Treatment. Ann. Dermatol. 2015, 27, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef]
- Jee, M.K.; Im, Y.B.; Choi, J.I.; Kang, S.K. Compensation of cATSCs-derived TGFbeta1 and IL10 expressions was effectively modulated atopic dermatitis. Cell. Death Dis. 2013, 4, e497. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, H.; Mahon, B.P. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy 2011, 66, 523–531. [Google Scholar] [CrossRef]
- Hu, P.; Yang, Q.; Wang, Q.; Shi, C.; Wang, D.; Armato, U.; Pra, I.D.; Chiarini, A. Mesenchymal stromal cells-exosomes: A promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burns Trauma. 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Ownby, D.R. Allergies and Asthma: Do Atopic Disorders Result from Inadequate Immune Homeostasis arising from Infant Gut Dysbiosis? Expert Rev. Clin. Immunol. 2016, 12, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Marofi, F.; Vahedi, G.; Biglari, A.; Esmaeilzadeh, A.; Athari, S.S. Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer. Front. Immunol. 2017, 8, 1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.H.; Chen, Y.A.; Ke, C.C.; Liu, R.S. Mesenchymal Stem Cell-Derived Extracellular Vesicle: A Promising Alternative Therapy for Osteoporosis. Int. J. Mol. Sci. 2021, 22, 12750. [Google Scholar] [CrossRef]
- Hong, S.; Kim, E.Y.; Lim, S.E.; Kim, J.H.; Sohn, Y.; Jung, H.S. Dendrobium nobile Lindley Administration Attenuates Atopic Dermatitis-like Lesions by Modulating Immune Cells. Int. J. Mol. Sci. 2022, 23, 4470. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Yi, Y.W.; Lee, J.H.; Kim, S.Y.; Pack, C.G.; Ha, D.H.; Park, S.R.; Youn, J.; Cho, B.S. Advances in Analysis of Biodistribution of Exosomes by Molecular Imaging. Int. J. Mol. Sci. 2020, 21, 665. [Google Scholar] [CrossRef] [Green Version]
- Munir, J.; Yoon, J.K.; Ryu, S. Therapeutic miRNA-Enriched Extracellular Vesicles: Current Approaches and Future Prospects. Cells 2020, 9, 2271. [Google Scholar] [CrossRef]
- Koeppen, K.; Nymon, A.; Barnaby, R.; Bashor, L.; Li, Z.; Hampton, T.H.; Liefeld, A.E.; Kolling, F.W.; LaCroix, I.S.; Gerber, S.A.; et al. Let-7b-5p in vesicles secreted by human airway cells reduces biofilm formation and increases antibiotic sensitivity of P. aeruginosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2105370118. [Google Scholar] [CrossRef]
- Castro-Leyva, V.; Arenas-Huertero, F.; Espejel-Nunez, A.; Giono Cerezo, S.; Flores-Pliego, A.; Espino, Y.S.S.; Reyes-Munoz, E.; Vadillo-Ortega, F.; Borboa-Olivares, H.; Camacho-Arroyo, I.; et al. miR-21 differentially regulates IL-1beta and IL-10 expression in human decidual cells infected with streptococcus B. Reprod. Biol. 2022, 22, 100604. [Google Scholar] [CrossRef] [PubMed]
- Tangtanatakul, P.; Klinchanhom, S.; Sodsai, P.; Sutichet, T.; Promjeen, C.; Avihingsanon, Y.; Hirankarn, N. Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac. J. Allergy Immunol. 2019, 37, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.G.; Wang, W.H.; Dai, Y.; Wang, S.J.; Chu, Y.X.; Li, J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS ONE 2013, 8, e56709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheedy, F.J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, B.S.; Kim, S.-B.; Kim, S.; Rhee, B.; Yoon, J.; Lee, J.W. Canine Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Atopic Dermatitis. Animals 2023, 13, 2215. https://doi.org/10.3390/ani13132215
Cho BS, Kim S-B, Kim S, Rhee B, Yoon J, Lee JW. Canine Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Atopic Dermatitis. Animals. 2023; 13(13):2215. https://doi.org/10.3390/ani13132215
Chicago/Turabian StyleCho, Byong Seung, Sung-Bae Kim, Sokho Kim, Beomseok Rhee, Jungho Yoon, and Jae Won Lee. 2023. "Canine Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Atopic Dermatitis" Animals 13, no. 13: 2215. https://doi.org/10.3390/ani13132215
APA StyleCho, B. S., Kim, S.-B., Kim, S., Rhee, B., Yoon, J., & Lee, J. W. (2023). Canine Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Atopic Dermatitis. Animals, 13(13), 2215. https://doi.org/10.3390/ani13132215




