Comparative Population Dynamics of Schizothorax wangchiachii (Cyprinidae: Schizothoracinae) in the Middle Reaches of the Yalong River and the Upper Reaches of the Jinsha River, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
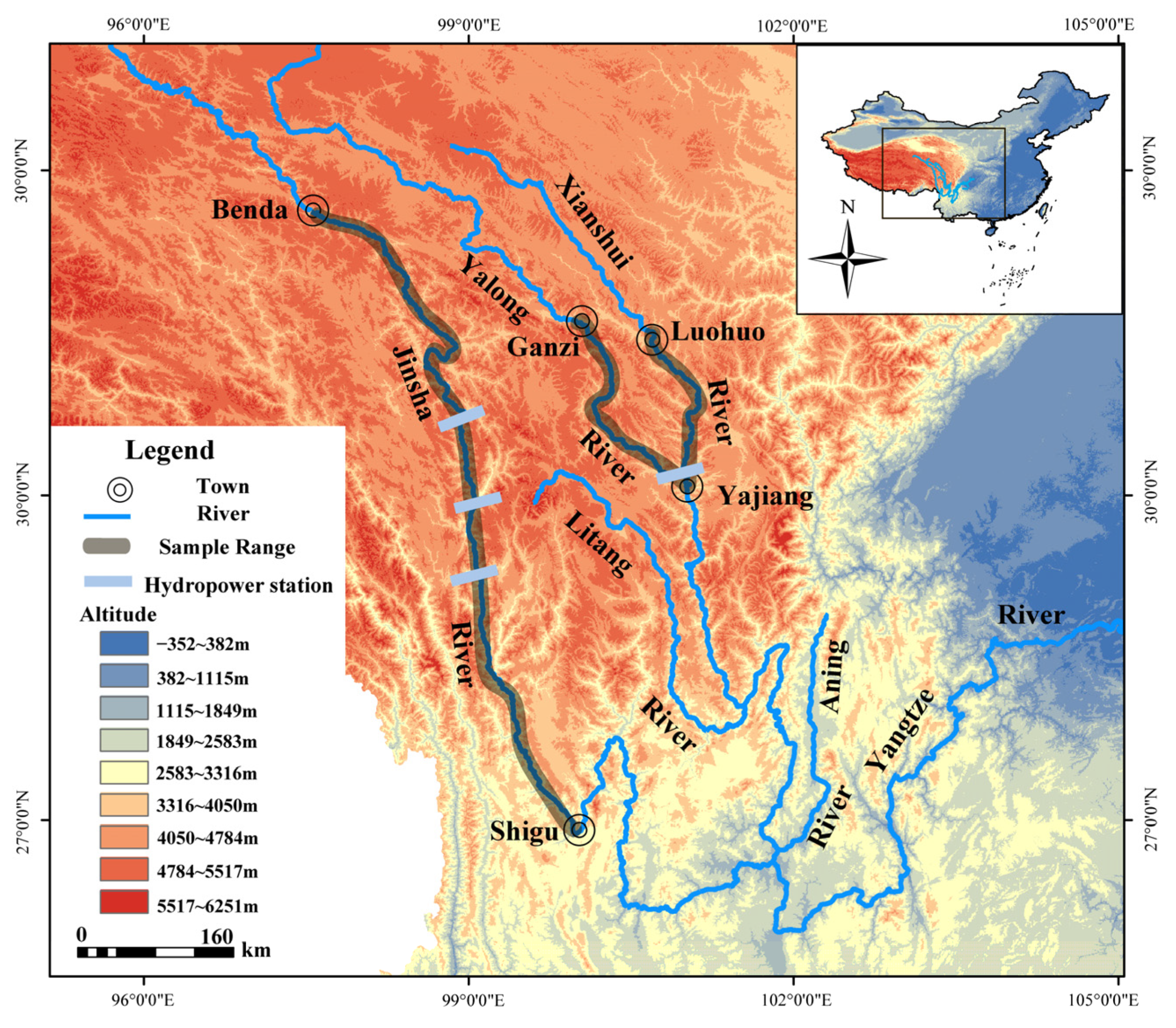
2.1. Sample Collection
2.2. Sample Preparation
2.3. Age Assessment and Verification
2.4. Modeling the Length–Weight Relationship and Growth
2.5. The Ages at First Sexual Maturity
2.6. Estimation of Mortality
2.7. Per-Recruit Analysis
2.8. Biological Reference Points
2.9. Statistical Analysis
3. Results
3.1. Sample Characteristics
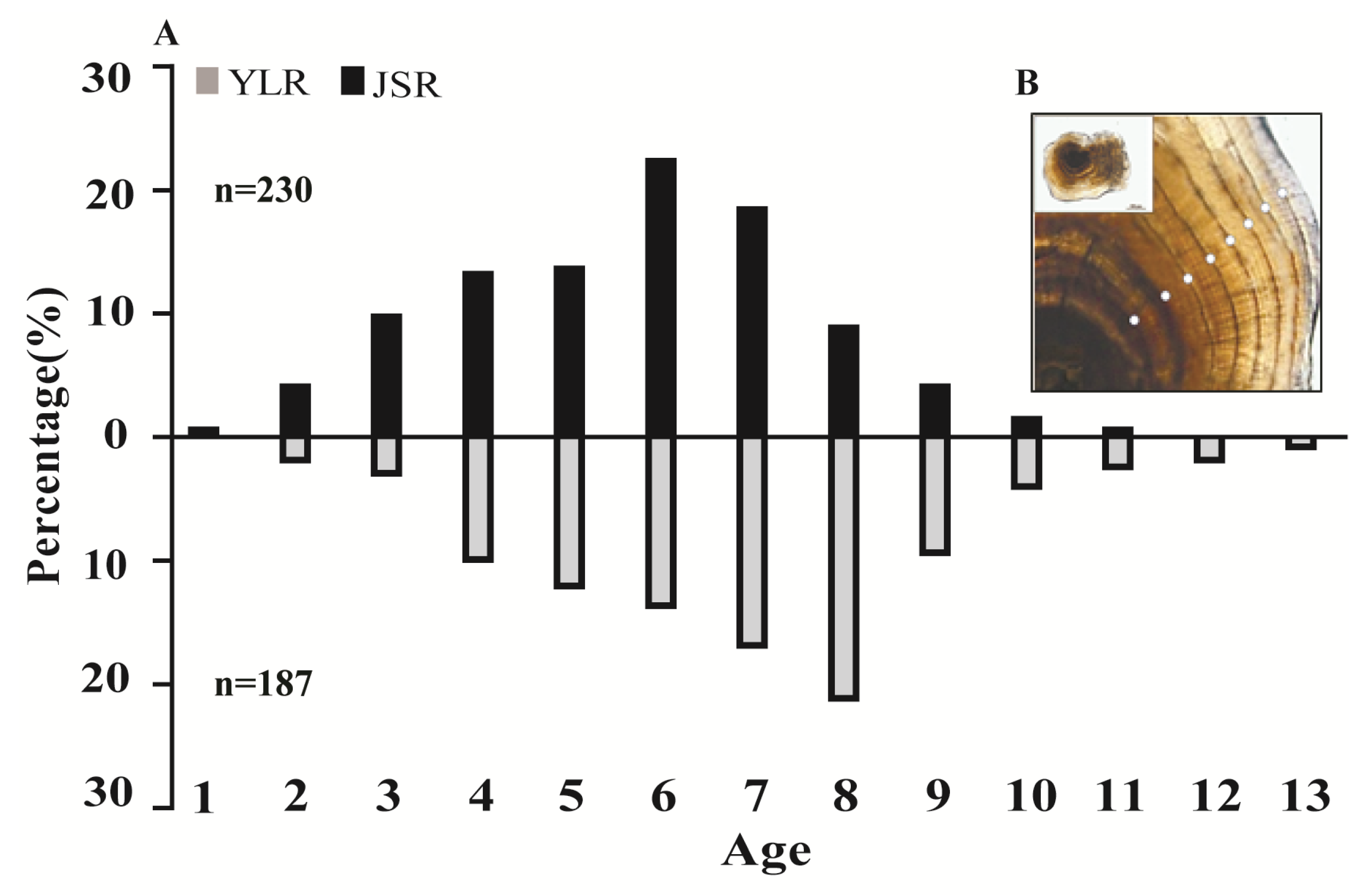
3.2. Age Structure and Verification
3.3. Growth Modeling
3.4. Growth Parameters
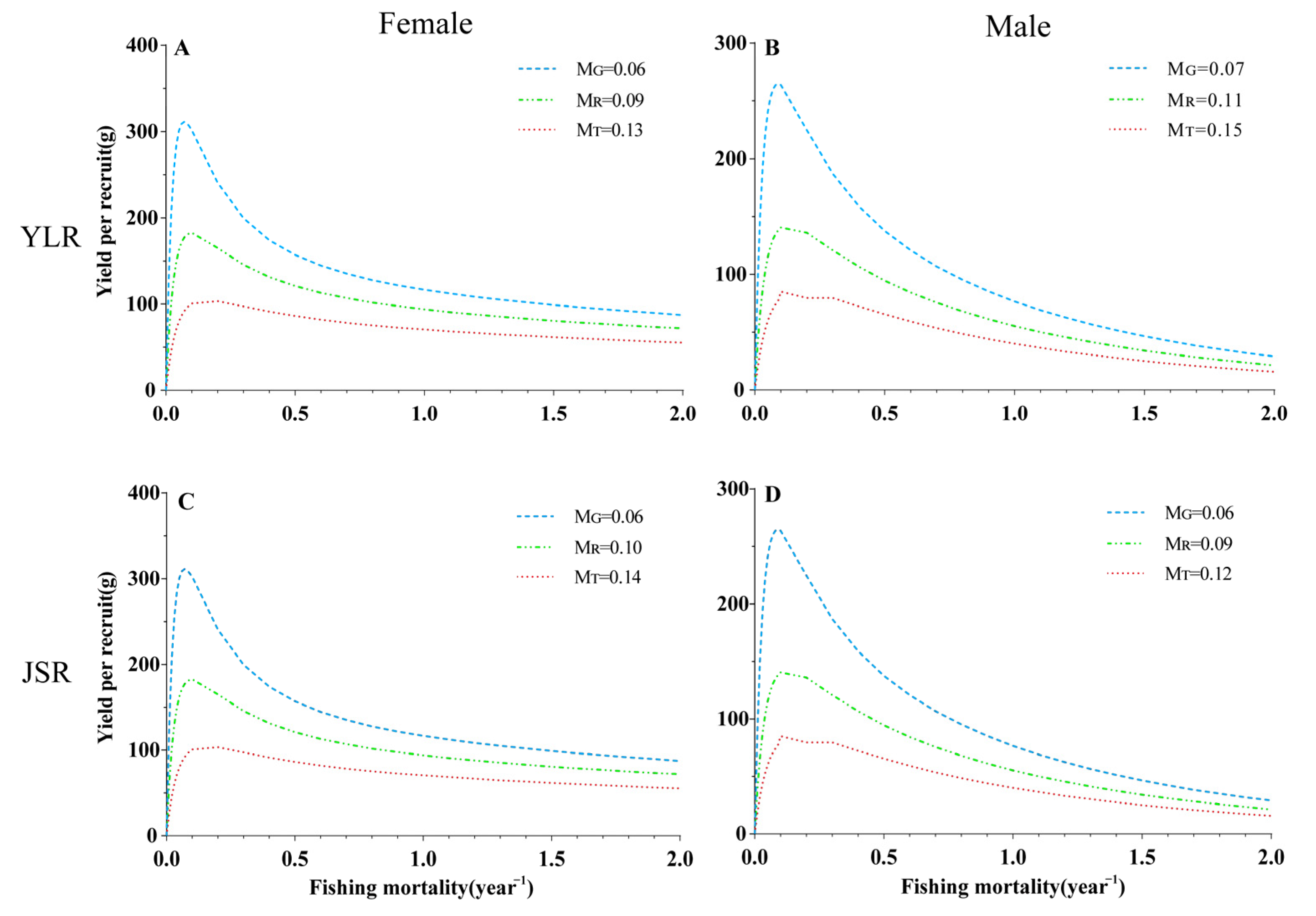
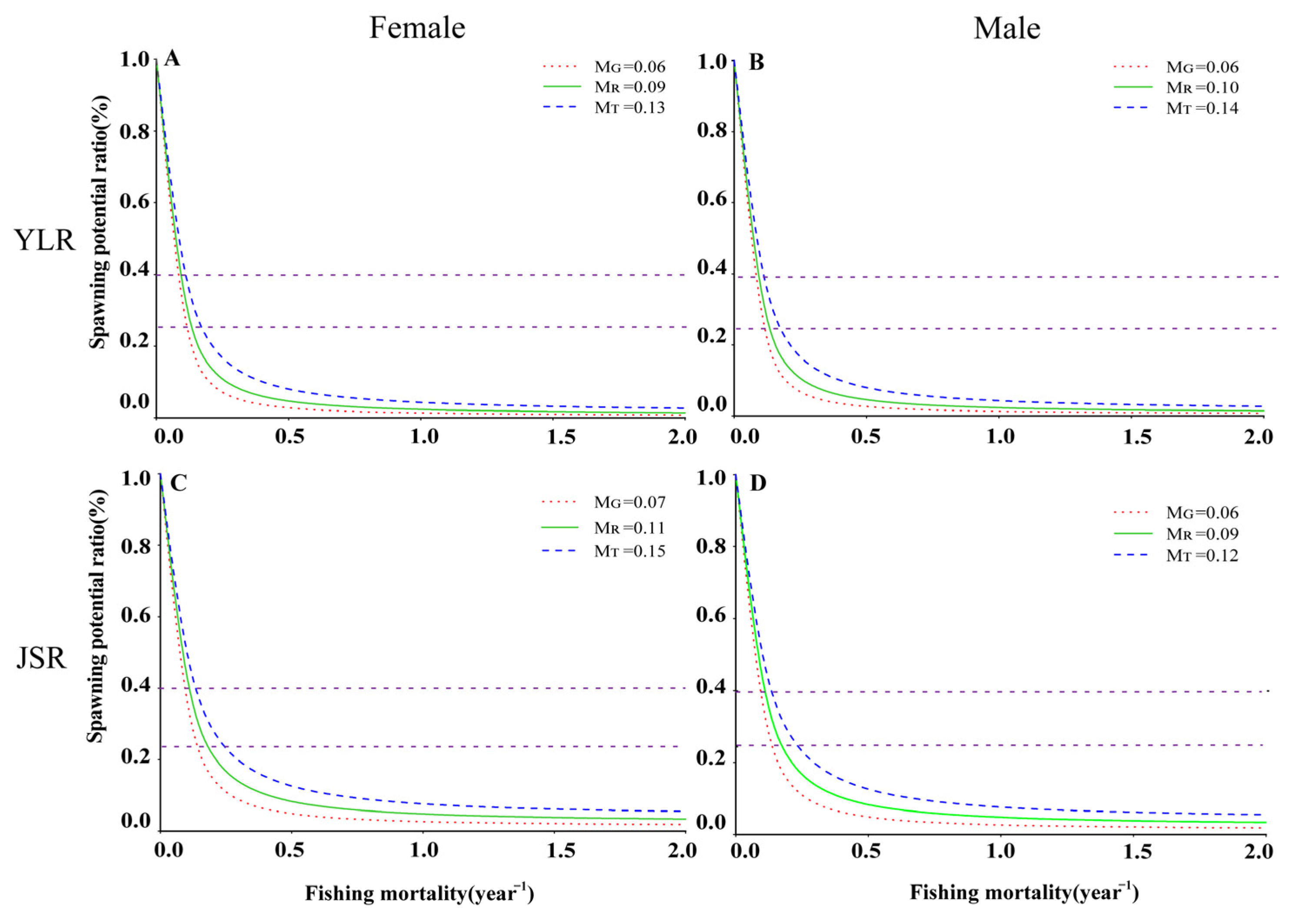
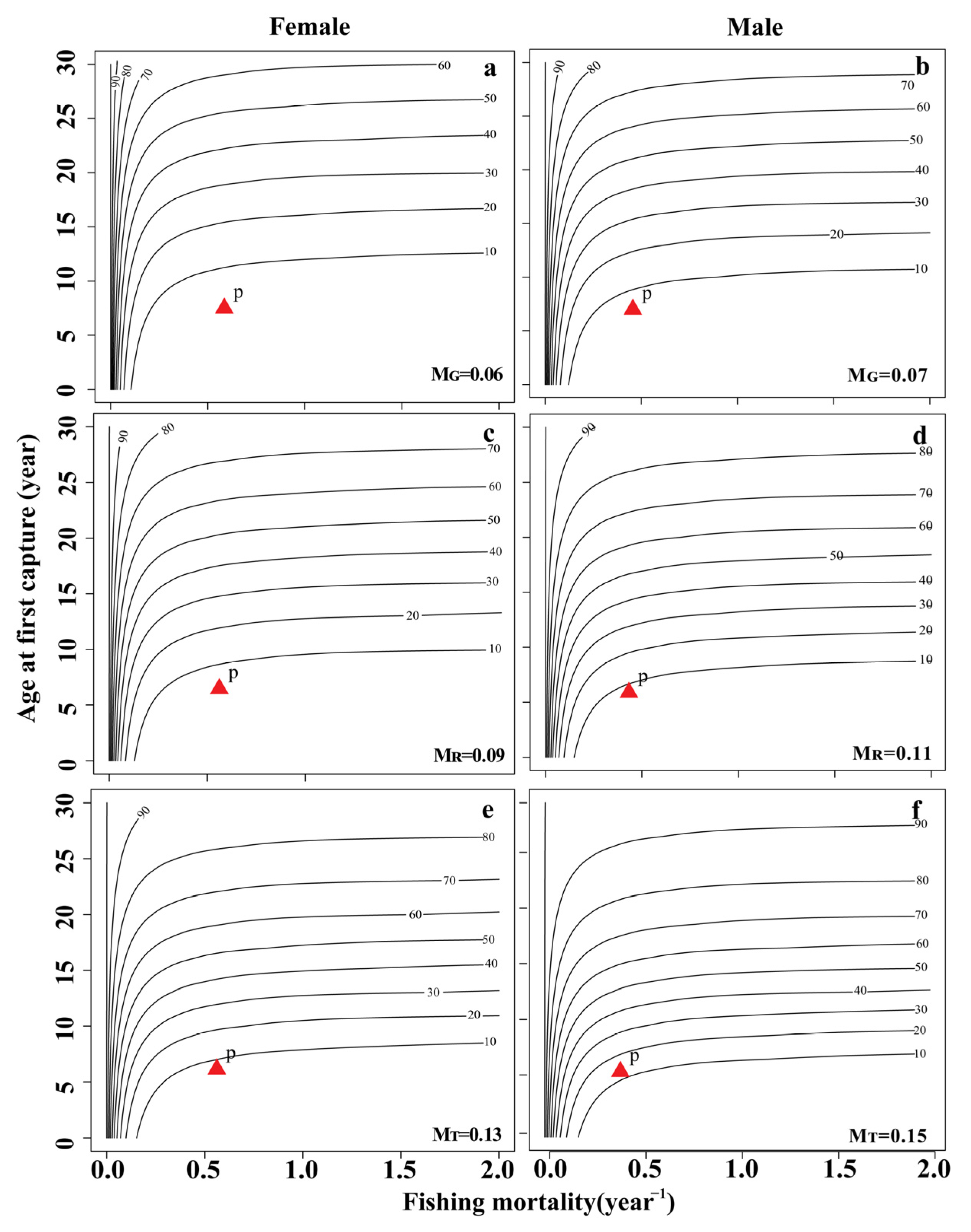
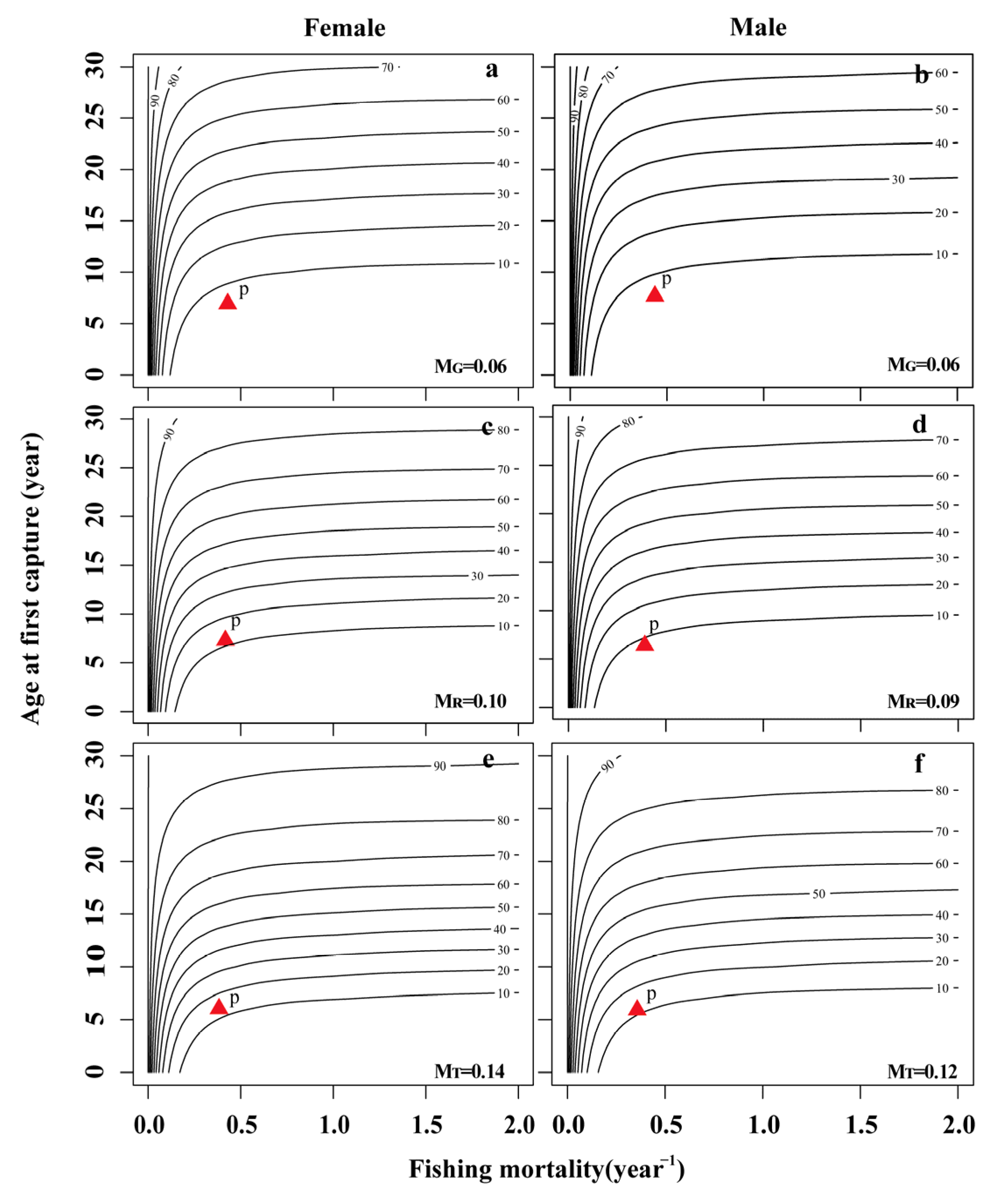
3.5. Per-Recruit Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Q.G.; Tang, H.Y.; Lin, H.; Gong, Y.; Li, X.N.; Yang, Z. Age structure, growth characteristics and population dynamic of Schizothorax chongi in middle and lower Jinsha River. J. Hydrol. 2021, 42, 8. [Google Scholar] [CrossRef]
- Zhou, H.H.; Li, C.; Deng, H.T.; Tian, H.W.; Chen, Y.B.; Gao, X.; Zhu, B.; Chen, D.Q. Research on status and dynamic varietal trends of rare and unique fish stocks in the upper reaches of Yangtze River. Freshw. Fish. 2020, 50, 3–14. [Google Scholar] [CrossRef]
- Stephenson, R.L. Stock complexity in fisheries management: A perspective of emerging issues related to population subunits. Fish. Res. 1999, 43, 247–249. [Google Scholar] [CrossRef]
- Ma, B.S. Study on the Biology and Population Dynamics of Schizothorax o’connori. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2011. [Google Scholar] [CrossRef]
- Dahlke, F.T.; Wohlrab, S.; Butzin, M. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 2020, 369, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Morat, F.; Wicquart, J.; Schiettekatte, N.M.D.; de Sinéty, G.; Bienvenu, J.; Casey, J.M.; Brandl, S.J.; Vii, J.; Carlot, J.; Degregori, S.; et al. Individual back-calculated size-at-age based on otoliths from Pacific coral reef fish species. Sci. Data 2020, 7, 370. [Google Scholar] [CrossRef]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2010; Volume 2, pp. 19–38. [Google Scholar] [CrossRef]
- Yan, W.B.; Zhu, T.B.; Wu, X.B.; Yang, D.G.; Chen, L. An observation of spawning behavior of Schizothorax wangchiachii. Freshw. Fish. 2017, 47, 7. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zhang, J.B.; Qiao, Y.; Wang, L.T.; Hou, Y.Q. Experimental study on aerobic swimming performance and behavior of Schizothorax wangchiachii Fang. J. Hydrol. 2016, 37, 56–62. [Google Scholar] [CrossRef]
- Gao, S.B.; Tang, H.Y.; Qiao, Y.; Yang, Z.; Chen, J.S. Status of fishery resources in the mainstream of the lower reaches of Jinsha River. J. Hydrol. 2013, 34, 44–49. [Google Scholar] [CrossRef]
- Bai, J. Species Diversity and Conservation of Fish and Benthic Fauna in Upper Reaches of the Jinsha River. Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar] [CrossRef]
- Yan, M.J.; Hu, J.C. Artificial domestication and breeding of Schizothorax wangchiachii in Jinsha River. Yunnan Agric. 2021, 10, 56–60. [Google Scholar]
- Zuo, P.X.; Li, G.H.; Leng, Y.; Zhang, J.B.; Miao, X.J.; Wang, Z.F.; Cui, L.L.; Liang, X.; Zhong, W.W.; Yin, X. Embryonic and early larval development of Schizothorax wangchiachii. J. Hydrol. 2015, 36, 77–82. [Google Scholar] [CrossRef]
- Li, G.H.; Jin, F.P.; Zhou, R.; Wu, X.W.; Wu, J.J.; Leng, Y.; Gao, H.T.; Zhang, W.K. Genetic diversity analysis of natural population of Schizothorax wangchiachii based on SNP markers. Acta Hydrobiol. Sin. 2018, 42, 271–276. [Google Scholar] [CrossRef]
- Yang, X. Study on Age, Growth, Feeding Habits and Population Dynamics of Ptychobarbus dipogon in the Yarlung Tsangpo. M.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar] [CrossRef]
- Chen, Y.Y. Fauna of China, Osteichthyes, Cypriniforme; Science Press: Beijing, China, 1998; Volume 2, pp. 291–292. [Google Scholar]
- Ma, Z.L.; Shi, H.F. Cascade exploitation of hydroelectric resources in Jinsha River watershed and its economic development. Res. Develop. Market 2007, 6, 566–568. [Google Scholar]
- Wu, S.Y. Position and role of construction of Jinping I hydropower station in development of hydropower resources in the Yalong River Basin. Sichuan Water Power 2004, 23, 5–6. [Google Scholar]
- Jiang, H.; Xie, S.G.; Zhao, W.Q.; Chang, J.B. Changes of fish assemblages after construction of ertan reservoir in Yalong River. Acta. Hydrobiol. Sin. 2007, 31, 532–539. [Google Scholar]
- Shao, J.; Du, T.; Guo, W.; Ouyang, S.; Wang, K. Water temperature variation and its influencing factors in the upper Jinsha River. J. Yangtze River Sci. Res. Inst. 2022, 39, 17–22. [Google Scholar] [CrossRef]
- Dong, L.J. Spatiotemporal Response of Runoff to Climate Change in Yalong River Basin. Ph.D. Thesis, China Three Gorges University, Yichang, China, 2020. [Google Scholar]
- Tan, N.N.; Ma, X.Q.; Shen, C.Y.; He, S.H.; Cheng, G.M. Characteristics of water vapor content and precipitation in the upstream of Shigu section in Jinsha River Basin. J. Yangtze River Sci. Res. Inst. 2023, 40, 51–59. [Google Scholar]
- Ding, R.H. Ichthyology of Sichuan; Sichuan Science and Technology Press: Chengdu, China, 1994; Volume 1, pp. 365–367. [Google Scholar]
- Yin, M.C. Fish Ecology; China Agricultural Publishing House: Beijing, China, 1995; Volume 1, pp. 21–22. [Google Scholar]
- Shen, Y.F.; Wu, F.; Dai, X.J.; Li, Y.K. Age and growth of oceanic whitetip shark, Carcharhinus longimanus, from the Central and Eastern Pacific. J. Fish. Sci. 2021, 28, 1030–1040. [Google Scholar] [CrossRef]
- Beamish, R.J.; Fournier, D.A. A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- Levenberg, K.Q. A method for the solution of certain non-linear problem in least squares. Quart. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Chen, Y.; Jackson, D.A.; Harvey, H.H. A comparison of von Bertalanffy and polynomial functions in modelling fish growth data. Can. J. Fish. Aquat. Sci. 1992, 49, 1228–1235. [Google Scholar] [CrossRef]
- Bailey, R.S. Fish population dynamics in tropical waters: A manual for use with programmable calculators. Fish. Res. 1986, 4, 171–173. [Google Scholar] [CrossRef]
- Von-Bertalnffy, L. A quantitative theory of organic growth (Inquires on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Ricker, W.E. Growth rates and models. Fish Physiol. Biochem. 1979, 1, 677–743. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations; The Blackburn Press: London, UK, 2010; Volume 1, pp. 190–210. [Google Scholar] [CrossRef]
- Zhu, L.X.; Li, L.F.; Liang, Z.L. Comparison of six statistical approaches in the selection of appropriate fish growth models. Chin. J. Oceanol. Limn. 2009, 27, 457–467. [Google Scholar] [CrossRef]
- Chapman, D.G.; Robson, D.S. The analysis of a catch curve. Biometrics 1960, 16, 354–368. [Google Scholar] [CrossRef]
- Wei, C.J.; Shen, Z.X.; Jia, Y.T.; Li, K.M.; Chen, Y.F. Comparison of aging methods and discrimination analysis on age classification for Gymnocypris eckloni. Chin. J. Ecol. 2015, 34, 2537–2541. [Google Scholar] [CrossRef]
- Xu, S.; Yanagimoto, T.; Song, N.; Cai, S.; Gao, T.; Zhang, X. Population genomics reveals possible genetic evidence for parallel evolution of Sebastiscus marmoratus in the northwestern Pacific Ocean. Open Biol. 2019, 9, 190028. [Google Scholar] [CrossRef]
- Then, A.Y.; Hoenig, J.M.; Hall, N.G.; Hewitt, D. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci. 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Taylor, C.C. Cod growth and temperature. ICES J. Mar. Sci. 1958, 23, 366–370. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Z.; Xie, C.; Liu, C.; Chen, F.; Huang, D.; Li, Z.; Guo, Y.; Chen, Y. An assessment of European bream Abramis brama (Linnaeus, 1758) fishery in the downstream of the Irtysh River in China. J. Freshw. Ecol. 2019, 34, 107–122. [Google Scholar] [CrossRef]
- Huo, B.; Ma, B.S.; Xie, C.X.; Duan, Y.J.; Yang, X.F.; Huang, H.P. Stock assessment and management implications of an endemic fish, Oxygymnocypris stewartii, in the Yarlung Zangbo River in Tibet, China. Zool. Stud. 2015, 54, 53. [Google Scholar] [CrossRef] [PubMed]
- Gulland, J.A.; Boerema, L.K. Scientific advice on catch levels. Fish. Bull. 1973, 71, 325–335. [Google Scholar]
- Goodyear, C. Spawning stock biomass per recruit in fisheries management: Foundation and current use. Can. Spec. Publ. Fish. Aquat. Sci. 1993, 120, 67–81. [Google Scholar]
- Zhu, Q.G.; Yang, Z.; Tang, H.Y.; Fang, H.B.; Hu, L. Biological and ecological characteristics of endemic fishes in the middle and lower reaches of Jinsha River and their conservation measures. Res. Environ. Yangtze Basin 2021, 30, 1594–1602. [Google Scholar] [CrossRef]
- Wu, J.; Wu, M.S. An investigation of fish resources about the Jinshajiang from Shigu to Yibin. J. Southwest Teachers Coll. 1985, 1, 80–87. [Google Scholar] [CrossRef]
- Gong, J.H.; Wang, J.L.; Li, L.; Zhang, C.; Ma, B.; Li, B.; Ji, F. Preliminary study on age and growth of Schizothorax integrilabiatus in the Buqun Lake, Tibet. Freshw. Fish. 2017, 47, 6. [Google Scholar] [CrossRef]
- Basilone, G.; Guisande, C.; Patti, B.; Mazzola, S.; Cuttitta, A.; Bonanno, A.; Kallianiotis, A. Linking habitat conditions and growth in the European anchovy (Engraulis encrasicolus). Fish. Res. 2004, 68, 9–19. [Google Scholar] [CrossRef]
- Wang, M.R.; Yang, S.R.; Liu, F.; Li, M.Z.; Dan, S.G.; Liu, H.Z. Age and growth of Rhinogobio cylindricus günther in the upper reaches of the Yangtze River. Acta Hydrobiol. Sin. 2012, 36, 262–269. [Google Scholar] [CrossRef]
- Burton, M.L.; Potts, J.C.; Page, J.; Poholek, A. Age, growth, mortality and reproductive seasonality of jolthead porgy, Calamus bajonado, from Florida waters. PeerJ 2017, 5, 3774. [Google Scholar] [CrossRef]
- Dulčić, J.; Pallaoro, A.; Matić-Skoko, S.; Dragičević, B.; Tutman, P.; Grgičević, R.; Stagličić, N.; Bukvić, V.; Pavličević, J.; Glamuzina, B.; et al. Age, growth and mortality of common two-banded seabream, Diplodus vulgaris (Geoffroy Saint ilaire, 1817), in the eastern Adriatic Sea (Croatian coast). J. Appl. Ichthyol. 2011, 27, 1254–1258. [Google Scholar] [CrossRef]
- Niu, Y.J.; Ren, D.Q.; Chen, S.A.; Cai, L.; Niu, J.; Xie, C. Growth characteristics of Gymnodiptychus dybowskii Kessler in three tributaries of the Ili River in Xinjiang, China. J. Hydrol. 2015, 36, 59–65. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, Y.; Dong, C.; Tang, H.Y.; Qiao, Y. Fish resource status of the lower reaches of the Heishui River and the measures for their conservation. Res. Environ. Yangtze Basin 2017, 26, 9. [Google Scholar] [CrossRef]
- Dong, L.J.; Dong, X.H.; Zeng, Q. Long-term runoff change trend of Yalong River basin under future climate change scenarios. Clim. Chang. Res. 2019, 15, 596–606. [Google Scholar] [CrossRef]
- Mace, P.M. Relationships between common biological reference points used as thresholds and targets of fisheries management strategies. Can. J. Fish. Aquat. Sci. 1994, 51, 110–122. [Google Scholar] [CrossRef]
- Hutchinson, W.F. The dangers of ignoring stock complexity in fishery management: The case of the North Sea cod. Biol. Lett. 2008, 4, 693. [Google Scholar] [CrossRef]
- Ying, Y.; Chen, Y.; Lin, L.; Gao, T. Risks of ignoring fish population spatial structure in fisheries management. Can. J. Fish. Aquat. Sci. 2011, 68, 2101–2120. [Google Scholar] [CrossRef]
- Yao, S.R.; Gao, X.; Ma, B.S.; Kong, Y. Seasonal dynamics of fish community in mudong section of the three gorges reservoir of the Yangtze River, China. Chin. J. Appl. Environ. Biol. 2010, 16, 555–560. [Google Scholar] [CrossRef]
- Kirchner, C.H. Fisheries regulations based on yield-per-recruit analysis for the linefish silver kob Argyrosomus inodorus in Namibian waters. Fish. Res. 2001, 52, 155–167. [Google Scholar] [CrossRef]
- Zhan, B.Y. Fishery Resources Assessment; China Agriculture Press: Beijing, China, 1995; pp. 100–150. [Google Scholar]
- Yang, Z.; Tang, H.Y.; Gong, Y. Effect of spawning migration on the variations of fish assemblage structures in the lower reaches of the Heishui River, Jinsha River. J. Lake Sci. 2018, 30, 753–762. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Castello, L.; Murphy, B.R.; Xie, S. Potential effects of dam cascade on fish: Lessons from the Yangtze River. Rev. Fish Biol. Fish. 2015, 25, 569–585. [Google Scholar] [CrossRef]


| Groups | Sample Size | Body Length (mm) | Body Weight (g) | ||||
|---|---|---|---|---|---|---|---|
| Female | Male | Total | Mean ± SE | Range | Mean ± SE | Range | |
| YLR | 77 | 84 | 187 (26) | 229.1 ± 60.5 a | 101.0 to 401.0 | 232.6 ± 167.3 a | 20.6 to 1001.2 |
| JSR | 76 | 90 | 230 (64) | 184.0 ± 44.2 b | 88.0 to 334.0 | 131.6 ± 99.15 b | 10.6 to 640.3 |
| Models | |||||
|---|---|---|---|---|---|
| Groups | |||||
| YLR | |||||
| JSR | |||||
| AIC(YLR/JSR) | — | 66.49/80.10 | 77.42/91.05 | 75.72/81.82 | |
| Parameters | YLR | JSR | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| k | 0.0579 year−1 | 0.0683 year−1 | 0.0637 year−1 | 0.0559 year−1 |
| t0 | −0.3318 year−1 | −0.3031 year−1 | −0.2203 year−1 | −0.7094 year−1 |
| 642.9 mm | 594.3 mm | 622.1 mm | 574.1 mm | |
| tc | 6.82 year | 7.10 year | 5.49 year | 5.36 year |
| Z | 0.658 year−1 | 0.453 year−1 | 0.504 year−1 | 0.532 year−1 |
| MG | 0.06 year−1 | 0.07 year−1 | 0.06 year−1 | 0.06 year−1 |
| MR | 0.09 year−1 | 0.11 year−1 | 0.10 year−1 | 0.09 year−1 |
| MT | 0.13 year−1 | 0.15 year−1 | 0.14 year−1 | 0.12 year−1 |
| Fcur(MG) | 0.59 year−1 | 0.38 year−1 | 0.44 year−1 | 0.47 year−1 |
| Fcur(MR) | 0.56 year−1 | 0.34 year−1 | 0.40 year−1 | 0.44 year−1 |
| Fcur(MT) | 0.52 year−1 | 0.30 year−1 | 0.36 year−1 | 0.41 year−1 |
| tr | 1 year | 1 year | 1 year | 1 year |
| a | 0.0127 | 0.0143 | 0.0343 | 0.0297 |
| b | 3.0744 | 3.0485 | 2.7800 | 2.8362 |
| T50 | 7.09 | 5.41 | 6.09 | 5.19 |
| Group | M (year−1) | Fcur (year−1) | F25% (year−1) | F40% (year−1) | Fmax (year−1) | YPRcur (g) | YPR25% (g) | YPR40% (g) | YPRmax (g) | SPRcur (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| YLR | Female | |||||||||
| 0.06 | 0.59 | 0.09 | 0.05 | 0.07 | 288.47 | 198.25 | 156.13 | 290.12 | 2.92 | |
| 0.09 | 0.56 | 0.12 | 0.07 | 0.10 | 168.92 | 140.97 | 117.90 | 1+ 69.13 | 6.66 | |
| 0.13 | 0.52 | 0.17 | 0.09 | 0.15 | 165.85 | 91.73 | 82.02 | 96.39 | 11.25 | |
| Male | ||||||||||
| 0.07 | 0.38 | 0.10 | 0.06 | 0.09 | 125.30 | 157.44 | 121.37 | 207.80 | 5.91 | |
| 0.11 | 0.34 | 0.14 | 0.08 | 0.13 | 133.86 | 98.85 | 81.43 | 110.63 | 11.24 | |
| 0.15 | 0.30 | 0.21 | 0.11 | 0.17 | 146.13 | 63.52 | 55.23 | 65.93 | 18.57 | |
| JSR | Female | |||||||||
| 0.06 | 0.44 | 0.06 | 0.04 | 0.06 | 107.85 | 234.85 | 221.95 | 234.85 | 2.38 | |
| 0.10 | 0.40 | 0.09 | 0.06 | 0.09 | 87.88 | 134.82 | 128.99 | 134.82 | 4.94 | |
| 0.14 | 0.36 | 0.12 | 0.07 | 0.12 | 76.75 | 99.62 | 94.20 | 99.62 | 7.59 | |
| Male | ||||||||||
| 0.06 | 0.47 | 0.08 | 0.05 | 0.07 | 85.11 | 193.21 | 189.74 | 197.76 | 1.85 | |
| 0.09 | 0.44 | 0.11 | 0.06 | 0.10 | 64.47 | 100.26 | 93.48 | 100.38 | 3.91 | |
| 0.12 | 0.41 | 0.13 | 0.08 | 0.14 | 53.66 | 67.98 | 63.74 | 67.99 | 6.50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Gao, K.; Chen, H.; Yang, D.; Pu, Y.; Zheng, L.; Jiao, Y.; Xiong, J.; Chen, Q.; Lai, B.; et al. Comparative Population Dynamics of Schizothorax wangchiachii (Cyprinidae: Schizothoracinae) in the Middle Reaches of the Yalong River and the Upper Reaches of the Jinsha River, China. Animals 2023, 13, 2209. https://doi.org/10.3390/ani13132209
He Z, Gao K, Chen H, Yang D, Pu Y, Zheng L, Jiao Y, Xiong J, Chen Q, Lai B, et al. Comparative Population Dynamics of Schizothorax wangchiachii (Cyprinidae: Schizothoracinae) in the Middle Reaches of the Yalong River and the Upper Reaches of the Jinsha River, China. Animals. 2023; 13(13):2209. https://doi.org/10.3390/ani13132209
Chicago/Turabian StyleHe, Zhi, Kuo Gao, Hongjun Chen, Deying Yang, Yong Pu, Li Zheng, Yuanyuan Jiao, Jinxin Xiong, Qiqi Chen, Bolin Lai, and et al. 2023. "Comparative Population Dynamics of Schizothorax wangchiachii (Cyprinidae: Schizothoracinae) in the Middle Reaches of the Yalong River and the Upper Reaches of the Jinsha River, China" Animals 13, no. 13: 2209. https://doi.org/10.3390/ani13132209
APA StyleHe, Z., Gao, K., Chen, H., Yang, D., Pu, Y., Zheng, L., Jiao, Y., Xiong, J., Chen, Q., Lai, B., Zhang, M., Tang, Z., & Yan, T. (2023). Comparative Population Dynamics of Schizothorax wangchiachii (Cyprinidae: Schizothoracinae) in the Middle Reaches of the Yalong River and the Upper Reaches of the Jinsha River, China. Animals, 13(13), 2209. https://doi.org/10.3390/ani13132209







