Bacteria in Normal Canine Milk Analyzed by Blood Agar Medium
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Milk Sampling Procedure
2.4. Inoculation of Blood Agar Plates
2.5. MALDI-TOF and 16S rDNA Sequence
2.6. Statistical Analyses
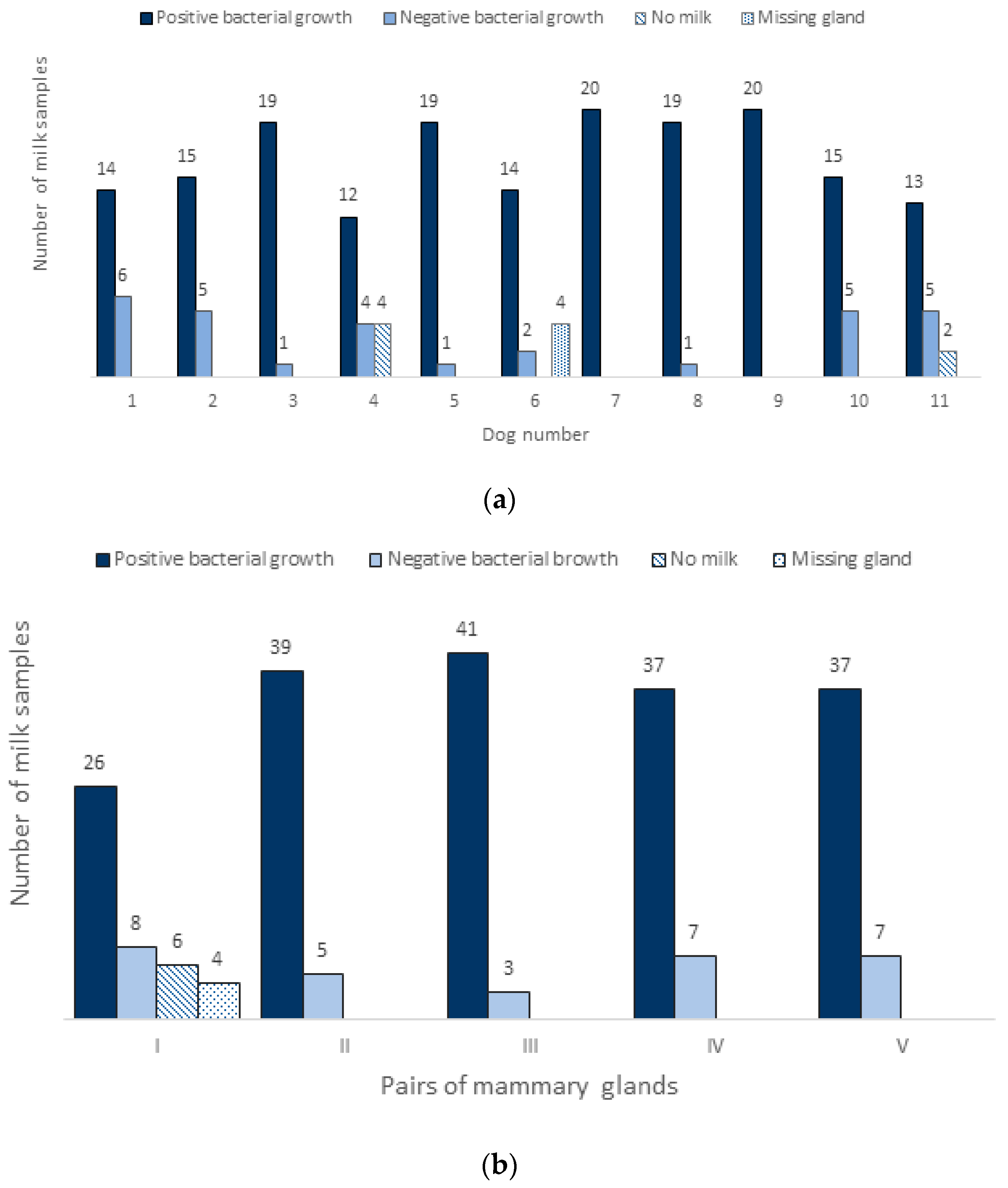
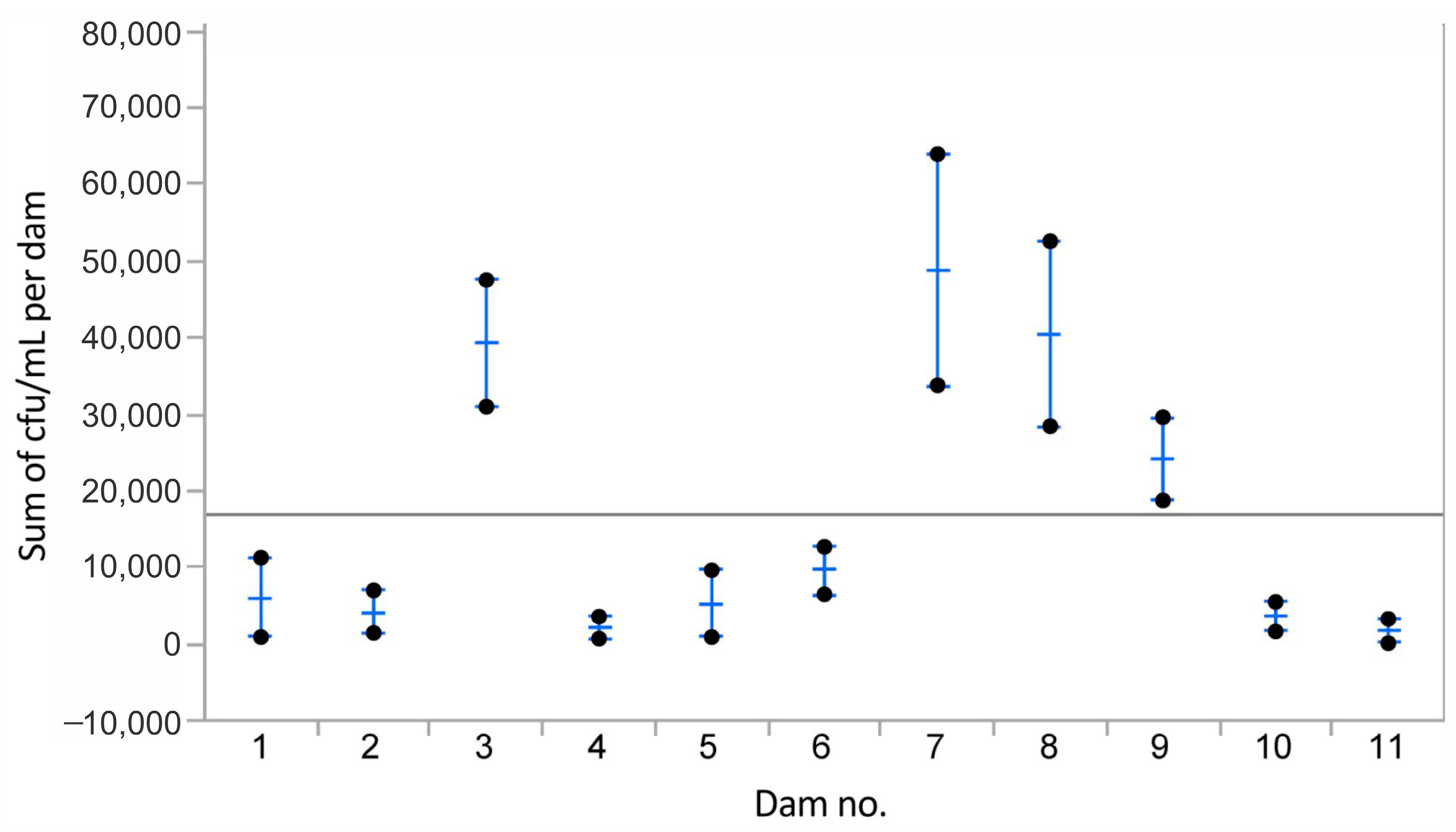
3. Results
3.1. Bacteria in Normal Milk
3.2. Bacterial Species in Normal Canine Milk
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kielland, C.; Rootwelt, V.; Reksen, O.; Framstad, T. The association between immunoglobulin G in sow colostrum and piglet plasma. J. Anim. Sci. 2015, 93, 4453–4462. [Google Scholar] [CrossRef]
- Balan, P.; Sik-Han, K.; Moughan, P.J. Impact of oral immunoglobulins on animal health—A review. Anim. Sci. J. 2019, 90, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; McGuire, M.A. Got bacteria? The astounding, yet not-so-surprising, microbiome of human milk. Curr. Opin. Biotechnol. 2017, 44, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Tolle, A. The microflora of the udder. In Factors influencing the bacteriological quality of raw milk. Bull. Int. Dairy Fed. 1980, 120, 4. [Google Scholar]
- Wang, X.; Lu, H.; Feng, Z.; Cao, J.; Fang, C.; Xu, X.; Zhao, L.; Shen, J. Development of Human Breast Milk Microbiota-Associated Mice as a Method to Identify Breast Milk Bacteria Capable of Colonizing Gut. Front. Microbiol. 2017, 8, 1242. [Google Scholar] [CrossRef] [PubMed]
- Taponen, S.; McGuinness, D.; Hiitiö, H.; Simojoki, H.; Zadoks, R.; Pyörälä, S. Bovine milk microbiome: A more complex issue than expected. Vet. Res. 2019, 50, 44. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Heikkila, M.P.; Saris, P.E. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J. Appl. Microbiol. 2003, 95, 471–478. [Google Scholar] [CrossRef]
- Kuhn, G.; Pohl, S.; Hingst, V. Elevation of the bacteriological content of milk of clinically unaffected lactating bitches of a canine research stock. Berl. Munch. Tierarztl. Wochenschr. 1991, 104, 130–133. [Google Scholar] [PubMed]
- Wiebe, V.J.; Howard, J.P. Pharmacologic advances in canine and feline reproduction. Top. Companion Anim. Med. 2009, 24, 71–99. [Google Scholar] [CrossRef] [PubMed]
- Walser, K.; Henschelchen, O. Contribution to the etiology of acute mastitis in the bitch. Berl. Munch. Tierarztl. Wochenschr. 1983, 96, 195–197. [Google Scholar] [PubMed]
- Schäfer-Somi, S.; Spergser, J.; Breitenfellner, J.; Aurich, J.E. Bacteriological status of canine milk and septicaemia in neonatal puppies—A retrospective study. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Sager, M.; Remmers, C. Perinatal mortality in dogs. Clinical, bacteriological and pathological studies. Tierarztl. Prax. 1990, 18, 415–419. [Google Scholar] [PubMed]
- Tonnessen, R.; Borge, K.S.; Nodtvedt, A.; Indrebo, A. Canine perinatal mortality: A cohort study of 224 breeds. Theriogenology 2012, 77, 1788–1801. [Google Scholar] [CrossRef]
- Ann, G.M. Perinatal and Late Neonatal Mortality in the Dog; University of Sydney: Camperdown, Australia, 2001. [Google Scholar]
- Zakosek Pipan, M.; Svara, T.; Zdovc, I.; Papic, B.; Avbersek, J.; Kusar, D.; Mrkun, J. Staphylococcus pseudintermedius septicemia in puppies after elective cesarean section: Confirmed transmission via dam’s milk. BMC Vet. Res. 2019, 15, 41. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Dingle, T.C.; Butler-Wu, S.M. MALDI-TOF mass spectrometry for microorganism identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef]
- Martín, R.; Langa, S.; Reviriego, C.; Jimínez, E.; Marín, M.L.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003, 143, 754–758. [Google Scholar] [CrossRef]
- Martín, R.; Heilig, H.G.; Zoetendal, E.G.; Jiménez, E.; Fernández, L.; Smidt, H.; Rodríguez, J.M. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res. Microbiol. 2007, 158, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Delgado, S.; Fernández, L.; García, N.; Albújar, M.; Gómez, A.; Rodríguez, J.M. Assessment of the bacterial diversity of human colostrum and screening of staphylococcal and enterococcal populations for potential virulence factors. Res. Microbiol. 2008, 159, 595–601. [Google Scholar] [CrossRef] [PubMed]
- West, P.A.; Hewitt, J.H.; Murphy, O.M. Influence of methods of collection and storage on the bacteriology of human milk. J. Appl. Bacteriol. 1979, 46, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, H.N.; Mavrogianni, V.S.; Fragkou, I.A.; Orfanou, D.C.; Gougoulis, D.A.; Tzivara, A.; Gouletsou, P.G.; Athanasiou, L.; Boscos, C.M.; Fthenakis, G.C. Experimental staphylococcal mastitis in bitches: Clinical, bacteriological, cytological, haematological and pathological features. Vet. Microbiol. 2007, 124, 95–106. [Google Scholar] [CrossRef]
- Rodríguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef]
- Perez, P.F.; Dore, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics 2007, 119, e724–e732. [Google Scholar] [CrossRef]
- Martín, R.; Langa, S.; Reviriego, C.; Jiménez, E.; Marín, M.L.; Olivares, M.; Boza, J.; Jiménez, J.; Fernández, L.; Xaus, J. The commensal microflora of human milk: New perspectives for food bacteriotherapy and probiotics. Trends Food Sci. Technol. 2004, 15, 121–127. [Google Scholar] [CrossRef]
- Kordy, K.; Gaufin, T.; Mwangi, M.; Li, F.; Cerini, C.; Lee, D.J.; Adisetiyo, H.; Woodward, C.; Pannaraj, P.S.; Tobin, N.H.; et al. Contributions to human breast milk microbiome and enteromammary transfer of Bifidobacterium breve. PLoS ONE 2020, 15, e0219633. [Google Scholar] [CrossRef]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Bond, R.; Loeffler, A. What’s happened to Staphylococcus intermedius? Taxonomic revision and emergence of multi-drug resistance. J. Small Anim. Pract. 2012, 53, 147–154. [Google Scholar] [CrossRef]
- Lee, J.-D.; Lee, Y.-K.; Jung, J.-Y.; Son, C.-H.; Shin, S.-S.; Oh, K.-S.; Hur, T.-Y.; Suh, G.-H. Isolation and antimicrobial susceptibility of microorganisms from milk samples of Jindo dogs (Canis familiaris var. jindo). Korean J. Vet. Res. 2011, 51, 29–35. [Google Scholar] [CrossRef]
- Corrò, M.; Skarin, J.; Börjesson, S.; Rota, A. Occurrence and characterization of methicillin-resistant Staphylococcus pseudintermedius in successive parturitions of bitches and their puppies in two kennels in Italy. BMC Vet. Res. 2018, 14, 308. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, K.; Anyika, K.; Ochai, S.; Gyendeng, J.; Olaolu, O. Determination of bacterial load in dog milk with its associated risk factors in Jos, Plateau State. World J. Pharm. Med. Res 2017, 3, 130–134. [Google Scholar]
- Vasiu, I.; Meroni, G.; Dąbrowski, R.; Martino, P.A.; Tvarijonaviciute, A.; Bochniarz, M.; Pop, R.A.; Brudaşcă, F.G.; Fiţ, N.I. Aerobic isolates from gestational and non-gestational lactating bitches (Canis lupus familiaris). Animals 2021, 11, 3259. [Google Scholar] [CrossRef]
- Ettinger, S.J.; Feldman, E.C.; Côté, E. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat; Saunders: Montreal, QC, Canada, 2017; Volume 8. [Google Scholar]
- Allaker, R.; Jensen, L.; Lloyd, D.; Lamport, A. Colonization of neonatal puppies by staphylococci. Br. Vet. J. 1992, 148, 523–528. [Google Scholar] [CrossRef]
- Pipan, M.Z.; Kajdič, L.; Kalin, A.; Plavec, T.; Zdovc, I. Do newborn puppies have their own microbiota at birth? Influence of type of birth on newborn puppy microbiota. Theriogenology 2020, 152, 18–28. [Google Scholar] [CrossRef]
- Ben Braiek, O.; Smaoui, S. Enterococci: Between emerging pathogens and potential probiotics. BioMed Res. Int. 2019, 2019, 5938210. [Google Scholar] [CrossRef]
- Biasucci, G.; Rubini, M.; Riboni, S.; Morelli, L.; Bessi, E.; Retetangos, C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010, 86, 13–15. [Google Scholar] [CrossRef]
- Chastant, S.; Mila, H. Passive immune transfer in puppies. Anim. Reprod. Sci. 2019, 207, 162–170. [Google Scholar] [CrossRef]
- Banchi, P.; Colitti, B.; Del Carro, A.; Corrò, M.; Bertero, A.; Ala, U.; Del Carro, A.; Van Soom, A.; Bertolotti, L.; Rota, A. Challenging the Hypothesis of in Utero Microbiota Acquisition in Healthy Canine and Feline Pregnancies at Term: Preliminary Data. Vet. Sci. 2023, 10, 331. [Google Scholar] [CrossRef]
- Del Carro, A.; Corrò, M.; Bertero, A.; Colitti, B.; Banchi, P.; Bertolotti, L.; Rota, A. The evolution of dam-litter microbial flora from birth to 60 days of age. BMC Vet. Res. 2022, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Corrò, M.; Drigo, M.; Rota, A. Antimicrobial resistance in bacteria from breeding dogs housed in kennels with differing neonatal mortality and use of antibiotics. Theriogenology 2012, 78, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Species | Dam 1 | Dam 2 | Dam 3 | Dam 4 | Dam 5 | Dam 6 | Dam 7 | Dam 8 | Dam 9 | Dam 10 | Dam 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus pseudintermedius | + | + | + | + | + | + | + | + | + | + | + |
| Enterococcus spp. | + | + | + | + | + | + | + | + | + | + | + |
| Clostridium spp. | + | + | + | + | + | + | + | + | + | ||
| Coagulase negative staphylococci | + | + | + | + | + | + | + | ||||
| Streptococcus spp. | + | + | + | + | + | ||||||
| Streptococcus canis | + | + | |||||||||
| Bacillus spp. | + | ||||||||||
| Pasteurella spp. | + | ||||||||||
| Escherichia coli | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolasinac, S.S.; Moe, L.; Rootwelt, V.; Sørum, H. Bacteria in Normal Canine Milk Analyzed by Blood Agar Medium. Animals 2023, 13, 2206. https://doi.org/10.3390/ani13132206
Kolasinac SS, Moe L, Rootwelt V, Sørum H. Bacteria in Normal Canine Milk Analyzed by Blood Agar Medium. Animals. 2023; 13(13):2206. https://doi.org/10.3390/ani13132206
Chicago/Turabian StyleKolasinac, Sabina Sibcic, Lars Moe, Vibeke Rootwelt, and Henning Sørum. 2023. "Bacteria in Normal Canine Milk Analyzed by Blood Agar Medium" Animals 13, no. 13: 2206. https://doi.org/10.3390/ani13132206
APA StyleKolasinac, S. S., Moe, L., Rootwelt, V., & Sørum, H. (2023). Bacteria in Normal Canine Milk Analyzed by Blood Agar Medium. Animals, 13(13), 2206. https://doi.org/10.3390/ani13132206





