1. Introduction
Over the last decade, a continuous increase in poultry production costs has been recognized due to the elevated prices of feed ingredients. Accordingly, alternative low-input feeding approaches based on agro-industrial byproducts are fundamental to lessening poultry nutrition expenditures [
1,
2,
3]. At the same time, animal products are currently expected to meet consumers’ nutritional needs and protect them from metabolic health problems. Nowadays, the improvement of diet composition becomes a key factor to improve the health status and welfare of animals [
4], as well as enhancing productivity and performance in livestock [
5,
6]. Antioxidants and antimicrobial food additives from a synthetic origin are commonly used in the food industry to postpone the natural degradation and peroxidation of foods. The consumption of these additives has been linked to the incidence of carcinogenesis, and their influence on human health due to long-term consumption is still unknown [
3]. Thus, seeking natural alternatives to these components is essential without inducing any detrimental health influences. Agro-industrial byproducts are a cheap source of antioxidant components, and the consumption of animal products, for instance, meat and eggs, rich in these beneficial nutraceuticals can strengthen humans’ health and immunity [
2,
3,
7]. Moreover, the application of agro-industrial byproducts in poultry nutrition could represent a prospect to reduce environmental pollution and permit the sustainability of their beneficial ingredients in the food chain [
8]. The use of agro-industrial byproducts may efficiently lessen the cost of waste processing and management [
7], which is considered an interesting area in the livestock industry.
Oranges are well-known fruits, belonging to the genus
Citrus, with a production of more than 76 million tons worldwide in 2020, and the annual orange production in Egypt increased from 3.01 Mt in 2019 to 3.16 Mt in 2020 (FAOSTAT, 2022;
https://www.fao.org/faostat/en/#data, accessed on 10 February 2023). Dried orange pulp (DOP) is an industrial byproduct produced after the juice extraction from orange fruits and drying of the remains. Orange pulp (OP) is a mixture of peel, seeds, and pulp that contains naturally active components, such as phenolic acids and flavonoids [
9,
10,
11]. Moreover, orange peel is a good source of dietary fiber, pectin, phenolic acids, and flavonoids [
10,
11]. Dietary fiber is a vital source to avoid the incidence of cardiovascular diseases, diabetes, cancer, and gastrointestinal disorders [
12]. Flavonoids and vitamin C present in DOP were reported to have beneficial antioxidant properties [
13,
14], antimicrobial actions, immune-stimulating activities [
15,
16,
17], and chelating actions [
13,
14].
Currently, DOP is considered a cheap feedstuff in the diets of dairy cattle and fattening lambs [
18], and it was reported to enhance growth performance and lessen production costs, whereas its application in poultry diets is limited [
16,
19,
20,
21]. Citrus pulp and its flavonoids have previously been applied in diets for broiler chickens, with different findings concerning the growth performance, but with positive impacts on the antioxidant properties of the meat. Dietary inclusion of dried citrus pulp (DCP) at up to 3% had a non-influence on the performance of broiler chickens [
16,
19]. It was shown that DCP [
19] or
Citrus sinensis peel extract [
16] can decrease the abdominal fat percentage and augment gut microbiota in broiler chickens [
20]. However, with higher inclusion levels, Mourao et al. [
21] showed that dietary incorporation of DCP at a level of 5% to 10% decreased body gain, while increasing feed intake and reducing the feed conversion ratio (FCR) in broiler chickens. Recently, Zoidis et al. [
22] observed that dietary supplementation DOP at 50 g/kg along with organic Se improved the meat oxidative stability and nutritional value without inducing any detrimental effects on the performance of broiler chickens. Nazok et al. [
23] reported that the inclusion of DCP up to 12% in the diets of laying hens had no negative influence on the performance and egg quality of laying hens. Moreover, Goliomytis et al. [
24] showed that dietary incorporation of DOP at 90 g per kg of feed improved the oxidative stability of eggs in laying hens, while its supplementation was associated with adverse effects on the performance and egg quality of laying hens.
To our knowledge, very limited research is published on the effect of DOP on laying performance and egg yolk oxidative stability, and no data exist regarding the effects of graded dietary inclusion of DOP on egg quality traits, egg shelf life, yolk fatty acid (FA) composition, or reproduction morphology in laying hens. Therefore, the current study aimed to demonstrate the effects of dietary inclusion of DOP on the laying performance, egg quality, antioxidant capacity, egg shelf life, yolk fatty acid composition, and reproductive structure of laying hens. We hypothesized that the dietary inclusion of DOP could be effective in enhancing egg quality traits and antioxidant capacity without adversely impacting the performance of laying hens.
4. Discussion
To our knowledge, this is the first report on the effects of graded dietary inclusion levels of DOP on the laying rate, egg quality, reproduction morphology, oxidative stability, and egg yolk FA profile in laying hens during the early phase of egg production. The dietary inclusion of DOP at a level of 7% and 10% improved feed intake, egg production, egg mass, and FCR compared to the control and DOP
5% groups. Our results agreed with the findings of Yang and Chung [
36] and Nazok et al. [
23]. Karunajeewa [
37] observed that egg production was not affected by the inclusion of 5% citrus pulp in laying hens’ diets. Florou-Paneri et al. [
38] found that the inclusion of DCP at a level of 6% in the laying quails’ diet had no negative impact on egg production. Nazok et al. [
23] reported the inclusion of DCP at 12–16% in the diets of laying hens caused a significant reduction in the egg production, egg mass, FCR, and final body weight of laying hens. They admitted this decrease in the laying rate was due to the increase in dietary CF content. Ojabo et al. [
39] recorded adverse effects on FI, FCR, and body weight in the pullets-fed diet containing 10% orange peel pulp. Goliomytis et al. [
24] reported a reduction in FI, laying rate, and FCR in laying hens fed 9% DOP, and they suggested that the reduced values of FI may be attributed to the low palatability of DOP, rather than to the dietary CF content. Based on previous research, the current findings can indicate that dietary inclusion of DOP up to 10% did not adversely affect the palatability of the diets. However, in the above-mentioned studies, they did not report the citrus species from which citrus pulp was obtained and whether seeds were involved in it. The involvement of seeds in citrus pulp or the various citrus species may be accountable for the difference in the level of anti-nutritional constituents, such as tannins and pectin, which may consequently clarify inconsistencies among studies.
In the current study, the CF contents were 2.93%, 3.45%, 3.49%, and 3.60% in the control, DOP
5%, DOP
7%, and DOP
10% diets, respectively. The addition of moderate levels of CF in the diet of laying hens improves nutrient digestibility and laying performance [
2]. Overall, the present results give further provision for the use of DOP at up to 10% in the diet of laying hens (35 g CF/kg of diet) without negatively affecting their laying performance during the early phase of production. Chickens generally adapt to CF-rich diets by enlarging their digestive tract volume [
40,
41], and consequently augmenting their FI and growth performance [
2,
41]. This is perhaps one of the causes underlying the better laying rate of hens fed diets having DOP up to 100 g/kg, since this improvement was associated with an increase in FI. Inadequate nutrient intake did not permit laying hens to prompt their genetic potential for egg production [
24]. In support of this, the current study verified the amelioratory activity of DOP on ovarian follicles of laying hens, particularly the number and weight of follicles. Nevertheless, the DOP mechanism of action is not yet determined; we proposed that DOP may augment follicular growth and development, possibly because of its valuable dietary constituents, including flavonoid and phenolic compounds. To our knowledge, there is currently no more research reported about the effects of DOP on the morphology of the reproductive tract to compare with the results recorded herein.
Dietary inclusion of DOP linearly increased the egg weight, shell weight%, shell thickness, and yolk color score, particularly in the eggs obtained from the DOP
7% and DOP
10% groups. Nazok et al. [
23] reported that the utilization of DCP at up to 16% did not affect the eggshell thickness, the eggshell index, or the yolk color score. Moreover, Goliomytis et al. [
24] recorded deterioration in egg quality traits, such as egg weight, shell thickness and strength, and yolk color, in eggs obtained from laying hens fed 9% DOP. Our findings partially agreed with Nazok et al. [
23], who observed a higher egg weight and egg mass from hens fed 8, 12, and 16% citrus pulp. The reduction in FI observed in the study by Goliomytis et al. [
24] not only affected the laying rate and FCR, but also caused some of the egg quality traits to deteriorate. In the current study, the improved FI in the DOP-fed groups, and thus sufficient nutrient supply, led to an increase in egg production, egg mass, egg weight, and egg yolk weight. Our findings agreed with Florou-Paneri et al. [
38], who found that the inclusion of DCP at up to 6% in the laying quails’ diet had no adverse effect on egg weight. The fact that these authors did not report any improvement in FI or egg quality traits due to citrus pulp utilization supports the assumption that the enhancement of some egg quality traits in the current trial may be attributed to the better FI of the DOP-fed groups. In agreement with the present study, Oyewole et al. [
42] reported a heavier egg weight in laying hens fed 10% to 40% dried sweet orange peel. Furthermore, Ahmed et al. [
43] recorded improved FCR, egg mass, and egg weight with the addition of 5% and 10% dried orange peel in the diet of laying hens.
For the egg industry and consumer preferences, yolk color is an essential feature of egg quality. Consumers prefer golden, orange, and pigmented egg yolk [
44]. The orange pulp contains a considerable content of carotenoids and xanthophyll [
45], which may be responsible for better yolk color in the DOP-fed groups. However, we did not analyze the total carotenoid content of DOP in this study to confirm this assumption. Angalet et al. [
46] found additional orange yolk color with an increase in the dietary levels of citrus sludge from 2.5% to 20% in hens. Chowdhury et al. [
47] observed higher egg yolk color in hens fed 4% orange skin. The improvement in the eggshell% and thickness in the current study, particularly in DOP
10%, may be due to the adequate dietary Ca intake, which is beneficial for eggshell formation as a result of the increased FI of the DOP
10% laying hens. The study by Goliomytis et al. [
24] observed deterioration in the shell thickness and shell strength due to DOP inclusion at a level of 90 g/kg of the diet. These authors attributed this effect to inadequate dietary Ca intake because of reduced FI.
One of the main aims of the current trial was to determine the egg yolk oxidative stability of laying hens fed DOP. Our findings showed that the dietary inclusion of DOP for 8 weeks was efficient in enhancing the antioxidant activities (GPx) and lowering the MDA contents of both fresh and stored eggs for 40 days, and subsequently extended its shelf life. It was assumed that the exerted antioxidant activities of DOP on the egg yolk can be attributed to its hesperidin and naringin contents [
24]. Hesperidin and naringin presented in DOP can diminish radical chain reactions, in the egg yolk lipid portion, by donation of hydrogen atoms to free radicals simulating the antioxidant properties of α-tocopherol [
24,
48]. The positive effects of dietary hesperidin and naringin [
24,
49] or DOP [
24] on egg yolk antioxidant activities, oxidative stability, and shelf life have been recorded [
24,
49]. In line with this, the current findings showed that the egg yolk cholesterol level was positively correlated with the serum MDA level, but negatively correlated with both the serum and yolk GPx levels. Dietary orange peel and grapefruit peel were reported to effectively decrease the oxidation process in the meat of broiler chickens following storage [
20]. Recently, Zoidis et al. [
22] observed that dietary supplementation of DOP at 50 g/kg, along with organic Se, improved meat oxidative stability and nutritive value. Flavonoids and vitamin C present in DOP have beneficial antioxidant properties [
13,
14] and antimicrobial [
15,
16,
17] and chelating actions [
13,
14]. We can declare that orange pulp waste management can help to diminish egg MDA levels, augment the antioxidant status of eggs, and subsequently extend egg shelf life. At the same time, more research is necessary to measure carotenoid contents in the egg yolks from laying hens fed diets containing DOP to support the assumption of the present study.
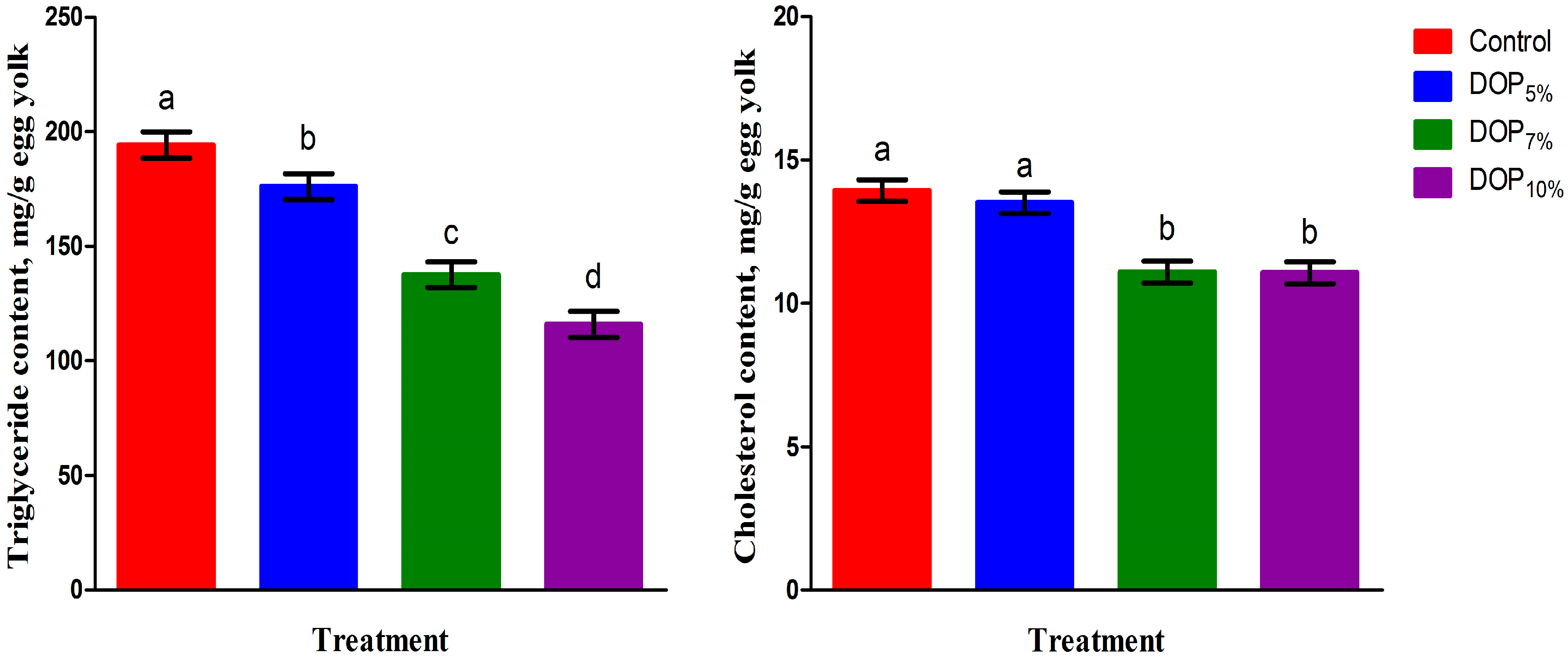
The inclusion of DOP in the diets of laying hens induced positive effects on blood cholesterol, triglyceride, LDL, and HDL contents, as well as egg yolk cholesterol and triglyceride levels. These findings are consistent with Abbasi et al. [
19], who observed that the utilization of dried sweet orange pulp in the diets of broiler chickens at up to 2% reduced blood triglycerides, total cholesterol, and LDL cholesterol. Nazok et al. [
23] found that the use of dietary DCP in the diets of laying hens at more than 16% increased blood serum HDL, while it decreased blood cholesterol, LDL, and triglyceride contents. The decrease in cholesterol levels may be attributed to the diminishing of HMG-CoA reductase activity, which is the main enzyme in cholesterol biosynthesis [
19,
50]. The inhibition of this enzyme enhances LDL receptors and improves blood HDL levels [
50,
51]. Likewise, some suggestions proposed that hesperidin works as a cholesterol-lowering factor by reducing the hepatic activity of HMG-CoA reductase [
50]. Moreover, the elevated level of soluble fiber may induce an adverse influence on lipid digestion by stimulating the synthesis of bile salts and fiber complex [
52]. This complex can lessen cholesterol levels in both the serum and egg yolk of hens [
23]. Additionally, citrus fruits were reported to contain an abundant quantity of pectin, which appears both in the edible and inedible portions of fruit [
53]. Numerous mechanisms have been intended for the cholesterol-lowering effect of pectin. Pectin was reported to reduce pancreatic enzyme activity, which in sequence could increase fecal lipid excretion [
54]. Nevertheless, additional evidence is required to fully clarify the potential mechanism of the cholesterol-lowering effect of DOP.
Blood AST is a useful marker of hepatic function and health. An elevated blood AST level is a reflexive reaction of an organism to hepatic inflammation and injury [
55]. It has been observed that the utilization of citrus maxima peel in the diet of mice with CC14-induced hepatic damage significantly reduced both serum and hepatic AST levels [
56]. The serum level of AST was decreased in the DOP-supplemented groups compared to the control, while there was no change in the serum ALT concentration. These results suggested that the hepatic function was not adversely affected by the dietary inclusion of DOP at up to 10% in laying hens. The current findings agreed with Nazok et al. [
23], Abbasi et al. [
19], and Lu et al. [
57]. Blood proteins are components of the immune response, in which antibodies are composed. In this study, serum total proteins, albumin, and globulin were significantly increased in the DOP-fed hens, which implied a better health status of laying hens in the DOP groups. Higher plasma albumin content and the albumin to globulin ratio were recorded in the dried citrus pulp-fed rabbits [
57,
58], revealing the same trend in the current trial.
Dietary modification by DOP inclusion significantly augmented the egg yolk FA profile due to the improvement in the health-promoting n-3 FA and PUFA contents and a reduction in SFA content. An average increase in the egg yolk n-3 PUFA content by 33.16%, 53.1%, and 88.27% was determined among the DOP-fed groups and the control groups. Consequently, the inclusion of laying hens’ diets with DOP not only enhanced the antioxidant properties of the fresh and stored egg yolks, but also boosted their nutritional value through enrichment with beneficial n-3 FA and PUFA. The noticeable increase in the n-3 and n-6 PUFA contents may be attributed to the increased PUFA content of the DOP diets. At the same time, the improved PUFA content in the egg yolk of laying hens fed with DOP may be attributed to the protective effect of the DOP phenolic and flavonoids on PUFA from oxidation. This was supported by the observed positive relationship among yolk PUFA, n-6 FA, and n-3 FA contents, as well as a negative correlation between SFA and PUFA (also n-6 and n-3 FA). Furthermore, yolk n-3 FA correlated positively with both the serum and yolk GPx contents, but negatively with the serum MDA concentration. An increase in PUFA content and a decrease in both MUFA and SFA in meat has been recorded in ostriches fed with DCP at 20% of the diet [
59]. Moreover, Zoidis et al. [
22] observed an increase in the n-3 FA and MUFA content, whereas the SFA content slightly decreased in the breast meat of broiler chickens fed with DOP at 50 g/kg of the feed. Unfortunately, no data exist on the effect of dietary inclusion of DOP in layers on the FA composition of the egg yolk to compare with the findings reported herein.