Analytical Validation and Assessment of Baseline Fecal Glucocorticoid Metabolites in Northern Sea Otters (Enhydra lutris kenyoni) in Human Care
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sea Otter Subjects: Exhibit Description and Husbandry
2.2. Sample Collection and Processing
2.3. Assay Methods—Enzyme-Linked Immunosorbent Assay (EIA)
2.4. Baseline and Peak Calculations
2.5. Statistical Analysis
3. Results
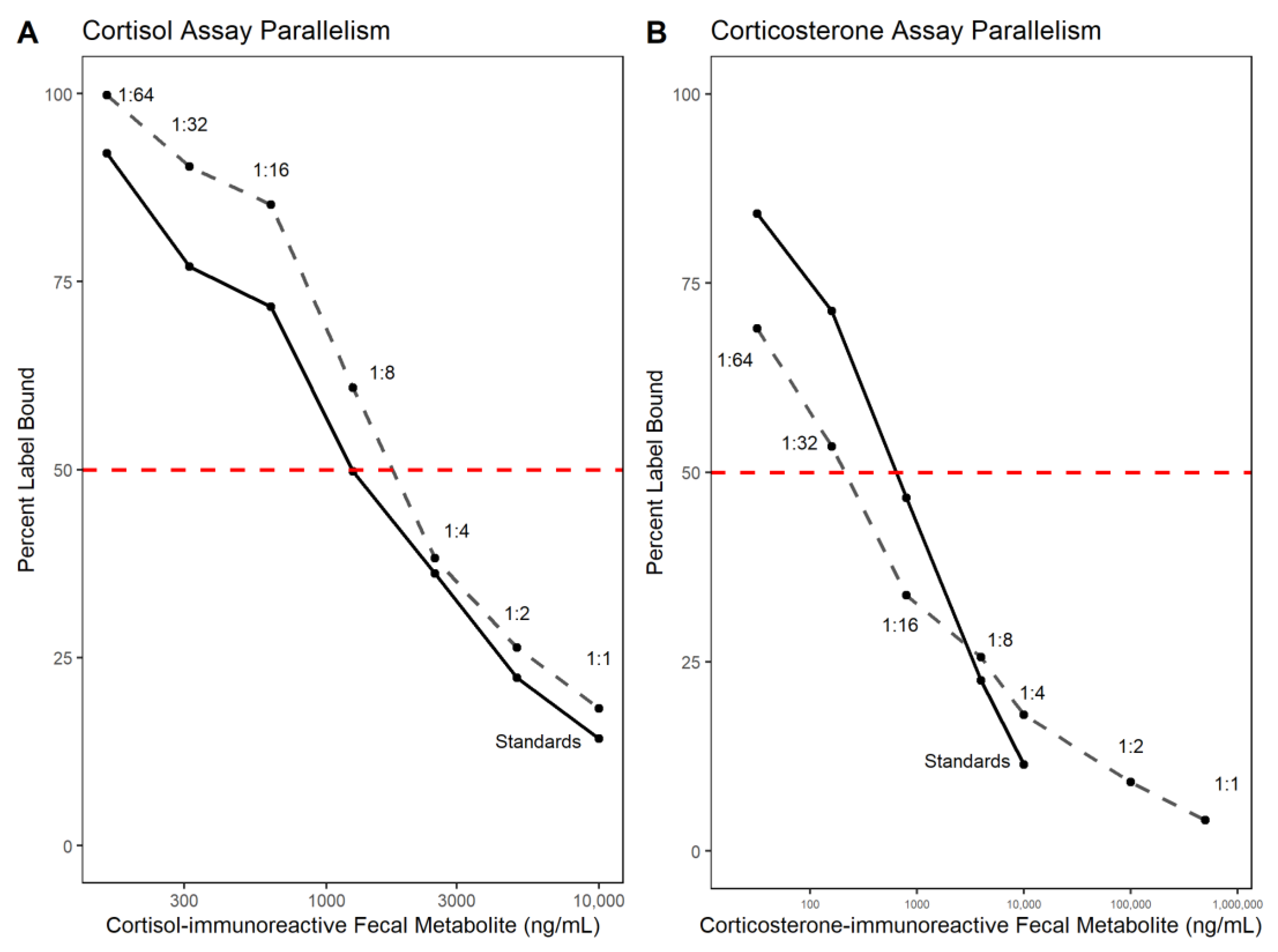
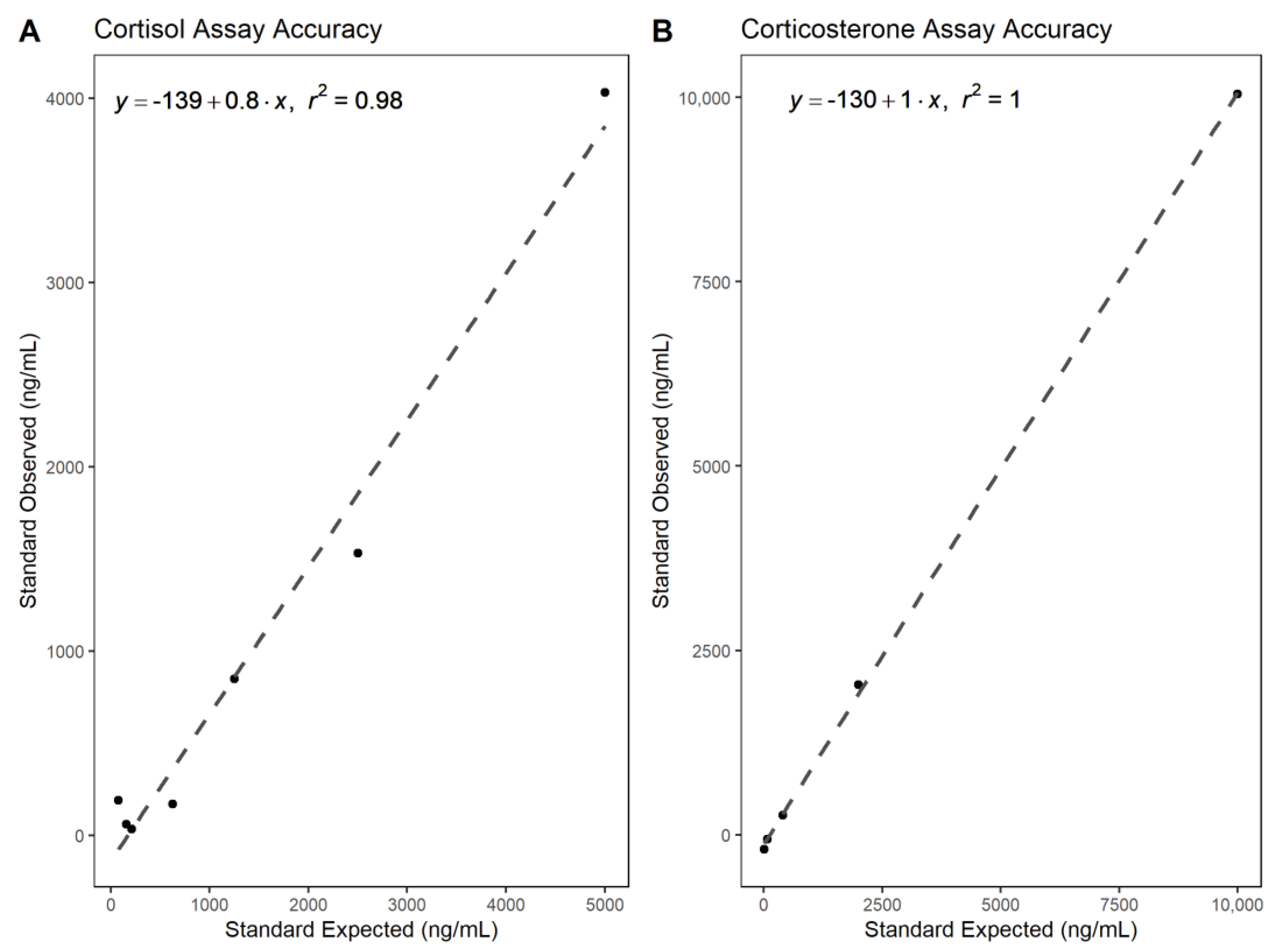
3.1. Analytical Validation—Parallelism and Accuracy
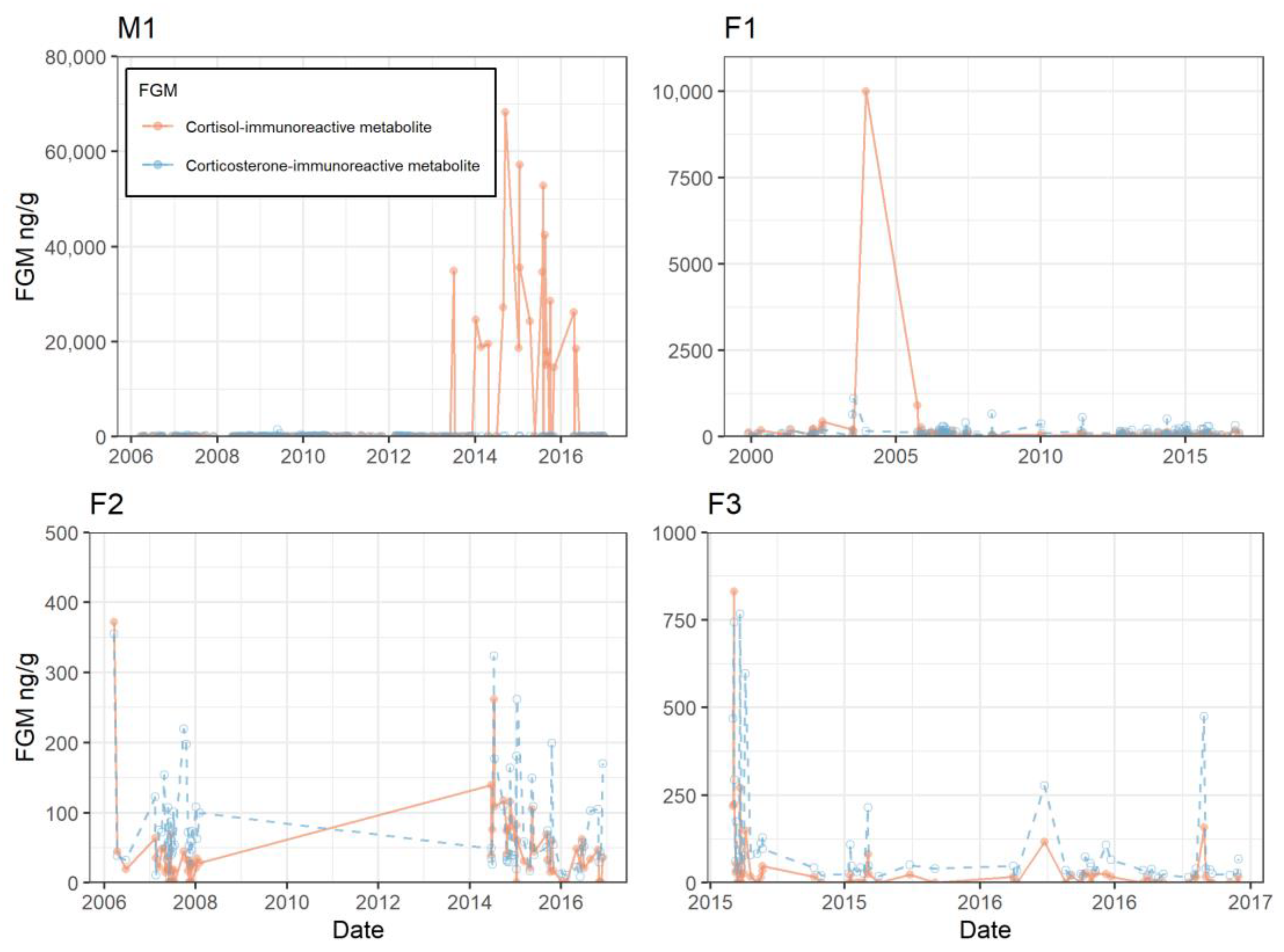
3.2. Individual Longitudinal Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belting, T. AZA Regional Studbook—Sea Otter (Enhydra lutris); Association of Zoos and Aquariums: Silver Spring, MD, USA, 2019. [Google Scholar]
- Casson, C.J.; Murray, M.; Johnson, A.; Belting, T. Sea Otter (Enhydra Lutris) Care Manual 2018. Available online: https://assets.speakcdn.com/assets/2332/sea_otter_care_manual_2019.pdf (accessed on 1 June 2017).
- Busch, D.S.; Hayward, L.S. Stress in a Conservation Context: A Discussion of Glucocorticoid Actions and How Levels Change with Conservation-Relevant Variables. Biol. Conserv. 2009, 142, 2844–2853. [Google Scholar] [CrossRef]
- Dierauf, L.; Gulland, F.M. CRC Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Larson, S.; Anderson, R. Fecal Hormones Measured within Giant Pacific Octopuses Enteroctopus dofleini. J. Aquat. Anim. Health 2010, 22, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.O. Vertebrate Endocrinology, 3rd ed.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Dickens, M.J.; Romero, L.M. A Consensus Endocrine Profile for Chronically Stressed Wild Animals Does Not Exist. Gen. Comp. Endocrinol. 2013, 191, 177–189. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [Green Version]
- Cockrem, J.F. Individual Variation in Glucocorticoid Stress Responses in Animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef]
- Romero, L.M. Physiological Stress in Ecology: Lessons from Biomedical Research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Reeder, D.M.; Kramer, K.M. Stress in Free-Ranging Mammals: Integrating Physiology, Ecology, and Natural History. J. Mammal. 2005, 86, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, B.; Fletcher, Q.E.; Boonstra, R.; Sheriff, M.J. Measures of Physiological Stress: A Transparent or Opaque Window into the Status, Management and Conservation of Species? Conserv. Physiol. 2014, 2, cou023. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring Stress in Wildlife: Techniques for Quantifying Glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Palme, R. Non-Invasive Measurement of Glucocorticoids: Advances and Problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Harper, J.M.; Austad, S.N. Fecal Glucocorticoids: A Noninvasive Method of Measuring Adrenal Activity in Wild and Captive Rodents. Physiol. Biochem. Zool. 2000, 73, 12–22. [Google Scholar] [CrossRef]
- Larson, S.; Casson, C.J.; Wasser, S. Noninvasive Reproductive Steroid Hormone Estimates from Fecal Samples of Captive Female Sea Otters (Enhydra lutris). Gen. Comp. Endocrinol. 2003, 134, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.; Belting, T.; Rifenbury, K.; Fisher, G.; Boutelle, S.M. Preliminary Findings of Fecal Gonadal Hormone Concentrations in Six Captive Sea Otters (Enhydra lutris) after Deslorelin Implantation: Sea Otter Fecal Hormones after Deslorelin. Zoo Biol. 2013, 32, 307–315. [Google Scholar] [CrossRef]
- Wasser, S.K.; Hunt, K.E.; Brown, J.L.; Cooper, K.; Crockett, C.M.; Bechert, U.; Millspaugh, J.J.; Larson, S.; Monfort, S.L. A Generalized Fecal Glucocorticoid Assay for Use in a Diverse Array of Nondomestic Mammalian and Avian Species. Gen. Comp. Endocrinol. 2000, 120, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.; Monson, D.; Ballachey, B.; Jameson, R.; Wasser, S.K. Stress-Related Hormones and Genetic Diversity in Sea Otters (Enhydra lutris). Mar. Mammal Sci. 2009, 25, 351–372. [Google Scholar] [CrossRef]
- Murray, M.J.; Young, M.A.; Santymire, R.M. Use of the ACTH Challenge Test to Identify the Predominant Glucocorticoid in the Southern Sea Otter (Enhydra lutris Nereis). Conserv. Physiol. 2020, 8, coz116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, L.B.; Mukobi, A.; Gruber, L.; Reed, C.; DeLibero, J.; Jackson, S.; Neill, S.; Walz, J.; Sines, C.; VanBeek, B.; et al. Longitudinal Analysis of Variability in Fecal Glucocorticoid Metabolite Concentrations in Three Orangutans (Pongo pygmaeus Pygmaeus and Pongo pygmaeus Abelii) before, during, and after Transition from a Regular Habitat Environment to Temporary Housing in Indoor Holding Facilities. Animals 2022, 12, 3303. [Google Scholar] [CrossRef]
- Khan, A.S.; Brown, J.L.; Kumar, V.; Umapathy, G.; Baskaran, N. Measures of Adrenal and Gonadal Hormones in Relation to Biological and Management Factors among Captive Red Pandas in Indian Zoos. Animals 2023, 13, 1298. [Google Scholar] [CrossRef]
- Glaeser, S.S.; Edwards, K.L.; Wielebnowski, N.; Brown, J.L. Effects of Physiological Changes and Social Life Events on Adrenal Glucocorticoid Activity in Female Zoo-Housed Asian Elephants (Elephas maximus). PLoS ONE 2020, 15, e0241910. [Google Scholar] [CrossRef]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and How Should We Use Environmental Enrichment to Tackle Stereotypic Behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef] [Green Version]
- Bodkin, J.L.; Mulcahy, D.; Lensink, C.J. Age-Specific Reproduction in Female Sea Otters (Enhydra lutris) from South-Central Alaska: Analysis of Reproductive Tracts. Can. J. Zool. 1993, 71, 1811–1815. [Google Scholar] [CrossRef]
- Hempstead, C.; Larson, S. Sea Otter (Enhydra lutris) Diet Diversity in Zoos and Aquariums. Aquat. Mamm. 2019, 45, 374–379. [Google Scholar] [CrossRef]
- Riedman, M.L.; Estes, J.A. The Sea Otter (Enhydra lutris): Behavior, Ecology, and Natural History. Biol. Rep. 1990, 90, 1–126. [Google Scholar]
- Olsen, A.Y.; Smith, A.; Larson, S. A Temporal Analysis of Water Quality Variability at the Seattle Aquarium in Elliott Bay, Puget Sound, WA. In Water Challenges of an Urbanizing World; Glavan, M., Ed.; InTech: London, UK, 2018; ISBN 978-953-51-3893-8. [Google Scholar]
- Millspaugh, J.J.; Washburn, B.E. Use of Fecal Glucocorticoid Metabolite Measures in Conservation Biology Research: Considerations for Application and Interpretation. Gen. Comp. Endocrinol. 2004, 138, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid Extraction: Get the Best out of Faecal Samples. Wien. Tierärztliche Mon. 2013, 100, 238–246. [Google Scholar]
- Brown, J.L.; Bellem, A.C.; Fouraker, M.; Wildt, D.E.; Roth, T.L. Comparative Analysis of Gonadal and Adrenal Activity in the Black and White Rhinoceros in North America by Noninvasive Endocrine Monitoring. Zoo Biol. 2001, 20, 463–486. [Google Scholar] [CrossRef]
- Hogan, L.A.; Lisle, A.T.; Johnston, S.D.; Robertson, H. Non-Invasive Assessment of Stress in Captive Numbats, Myrmecobius fasciatus (Mammalia: Marsupialia), Using Faecal Cortisol Measurement. Gen. Comp. Endocrinol. 2012, 179, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.L.; Roth, T.L. Annual Faecal Glucocorticoid Metabolite Concentrations in Pregnant and Pseudopregnant Polar Bears (Ursus maritimus) in North American Zoos. J. Zoo Aquar. Res. 2017, 6, 6–11. [Google Scholar] [CrossRef]
- Dudek, N.K.; Switzer, A.D.; Costello, E.K.; Murray, M.J.; Tomoleoni, J.A.; Staedler, M.M.; Tinker, M.T.; Relman, D.A. Characterizing the Oral and Distal Gut Microbiota of the Threatened Southern Sea Otter (Enhydra lutris nereis) to Enhance Conservation Practice. Conserv. Sci Pr. 2022, 4, e12640. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 1 June 2017).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Müller, K. Here: A Simpler Way to Find Your Files. R Package Version 1.0.1. 2020. Available online: https://CRAN.R-project.org/package=here (accessed on 1 June 2017).
- Xu, S.; Chem, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use Ggbreak to Effectively Utilize Plotting Space to Deal with Large Datasets and Outliers. Front. Genet. 2021, 12, 2122. [Google Scholar] [CrossRef]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for “ggplot2”. R Package Version 1.1.1. 2020. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 1 June 2017).
- Kolbe, T.; Palme, R.; Tichy, A.; Rülicke, T. Lifetime Dependent Variation of Stress Hormone Metabolites in Feces of Two Laboratory Mouse Strains. PLoS ONE 2015, 10, e0136112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCaluwe, H.B.; Wielebnowski, N.C.; Howard, J.; Pelican, K.M.; Ottinger, M.A. Characterization of Multiple Pathways Modulating Aggression in the Male Clouded Leopard (Neofelis nebulosa): Aggression in the Male Clouded Leopard. Zoo Biol. 2016, 35, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, K.W. The Sea Otter in the Eastern Pacific Ocean; US Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1969. [Google Scholar]
- Wasser, S.K.; Bevis, K.; King, G.; Hanson, E. Noninvasive Physiological Measures of Disturbance in the Northern Spotted Owl. Conserv. Biol. 1997, 11, 1019–1022. [Google Scholar] [CrossRef]
- Apfelbeck, B.; Helm, B.; Illera, J.C.; Mortega, K.G.; Smiddy, P.; Evans, N.P. Baseline and Stress-Induced Levels of Corticosterone in Male and Female Afrotropical and European Temperate Stonechats during Breeding. BMC Evol. Biol. 2017, 17, 114. [Google Scholar] [CrossRef] [Green Version]
- Chinn, S.M.; Monson, D.H.; Tinker, M.T.; Staedler, M.M.; Crocker, D.E. Lactation and Resource Limitation Affect Stress Responses, Thyroid Hormones, Immune Function, and Antioxidant Capacity of Sea Otters (Enhydra lutris). Ecol. Evol. 2018, 8, 8433–8447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, L.M.; Remage-Healey, L. Daily and Seasonal Variation in Response to Stress in Captive Starlings (Sturnus vulgaris): Corticosterone. Gen. Comp. Endocrinol. 2000, 119, 52–59. [Google Scholar] [CrossRef]
- Boonstra, R. Reality as the Leading Cause of Stress: Rethinking the Impact of Chronic Stress in Nature. Funct. Ecol. 2013, 27, 11–23. [Google Scholar] [CrossRef]
- Larson, S.E.; Bodkin, J.L. The Conservation of Sea Otters: A Prelude. In Sea Otter Conservation; Academic Press: Cambridge, MA, USA, 2015; pp. 1–17. [Google Scholar]

| Animal ID | Sex | Age Sampled (Years) | N Fecal Samples |
|---|---|---|---|
| M1 | M | 9–17 (2008–2016) | 233 |
| F1 | F | 2–19 (1999–2016) | 122 |
| F2 | F | 4–14 (2006–2016) | 72 |
| F3 | F | 1–2 (2015–2016) | 60 |
| Animal ID | Fecal Cortisol- Immunoreactive Metabolite Baseline (ng/g) | Fecal Corticosterone- Immunoreactive Metabolite Baseline (ng/g) | Fecal Cortisol- Immunoreactive Metabolite Peaks | Fecal Corticosterone- Immunoreactive Metabolite Peaks | Both FGM Peaks | Peak Note | Note Detail |
|---|---|---|---|---|---|---|---|
| M1 | 38.3 | 59.7 | 33 | 26 | 11 | 1 | Deslorelin implants and associated vet exam |
| F1 | 83.7 | 102 | 4 | 16 | 0 | NA | NA |
| F2 | 32.8 | 54.4 | 8 | 13 | 5 | 1 | Vet exam |
| F3 | 20.2 | 52.3 | 7 | 9 | 7 | 7 | Arrival at SA, vet exam, normal behavior, shifted exhibit (2), and enrichment (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olsen, A.Y.; Smith, A.; Hempstead, C.; Larson, S.E. Analytical Validation and Assessment of Baseline Fecal Glucocorticoid Metabolites in Northern Sea Otters (Enhydra lutris kenyoni) in Human Care. Animals 2023, 13, 2175. https://doi.org/10.3390/ani13132175
Olsen AY, Smith A, Hempstead C, Larson SE. Analytical Validation and Assessment of Baseline Fecal Glucocorticoid Metabolites in Northern Sea Otters (Enhydra lutris kenyoni) in Human Care. Animals. 2023; 13(13):2175. https://doi.org/10.3390/ani13132175
Chicago/Turabian StyleOlsen, Amy Y., Angela Smith, Caroline Hempstead, and Shawn E. Larson. 2023. "Analytical Validation and Assessment of Baseline Fecal Glucocorticoid Metabolites in Northern Sea Otters (Enhydra lutris kenyoni) in Human Care" Animals 13, no. 13: 2175. https://doi.org/10.3390/ani13132175
APA StyleOlsen, A. Y., Smith, A., Hempstead, C., & Larson, S. E. (2023). Analytical Validation and Assessment of Baseline Fecal Glucocorticoid Metabolites in Northern Sea Otters (Enhydra lutris kenyoni) in Human Care. Animals, 13(13), 2175. https://doi.org/10.3390/ani13132175





