Thoroughbred Racehorses in Hong Kong Require Vitamin D Supplementation to Mitigate the Risk of Low Vitamin D Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Serum Samples
2.3. Vitamin D Analyses
2.4. Statistical Analysis
3. Results
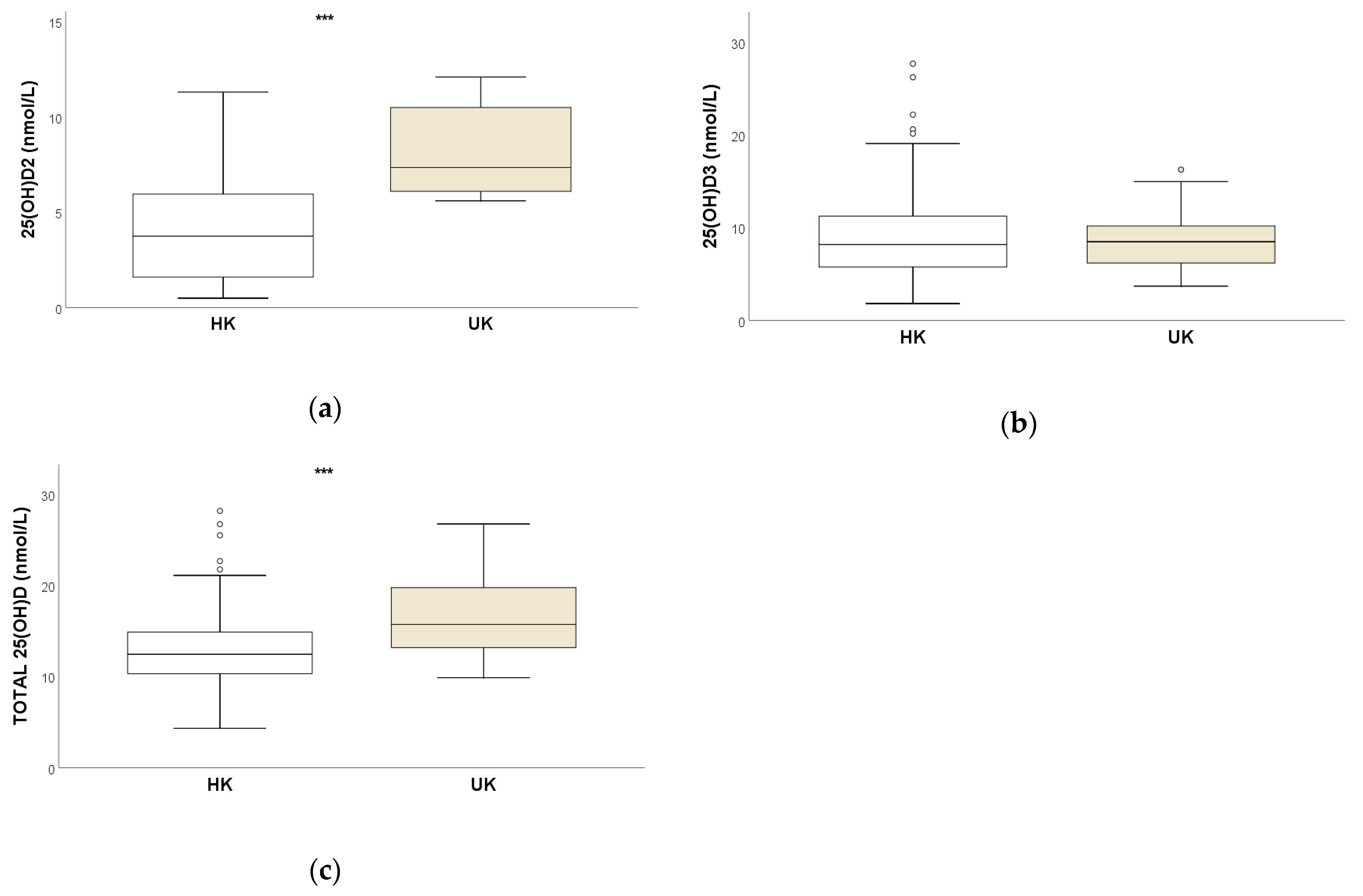
3.1. Intergroup Comparisons
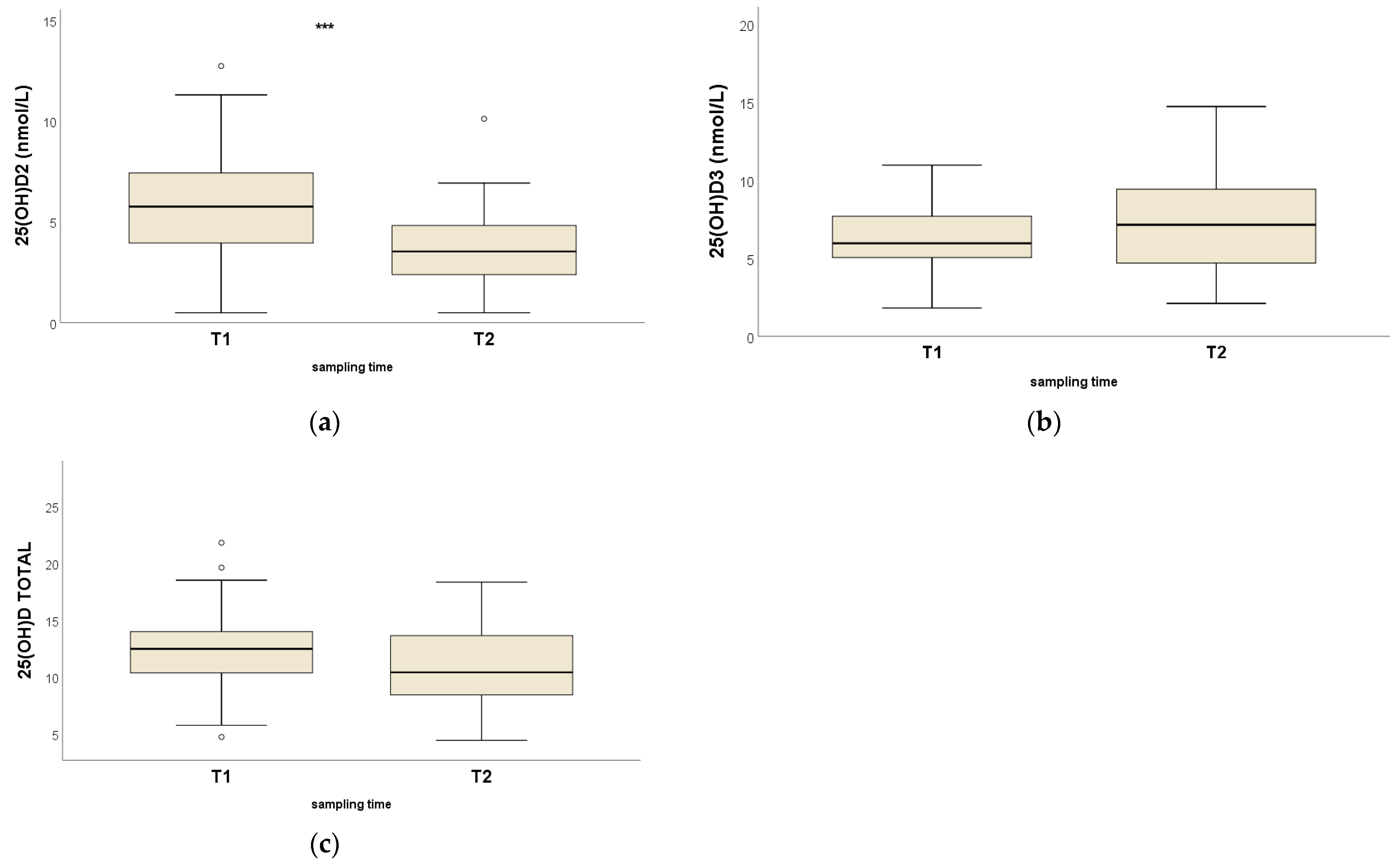
3.2. Intragroup Comparisons
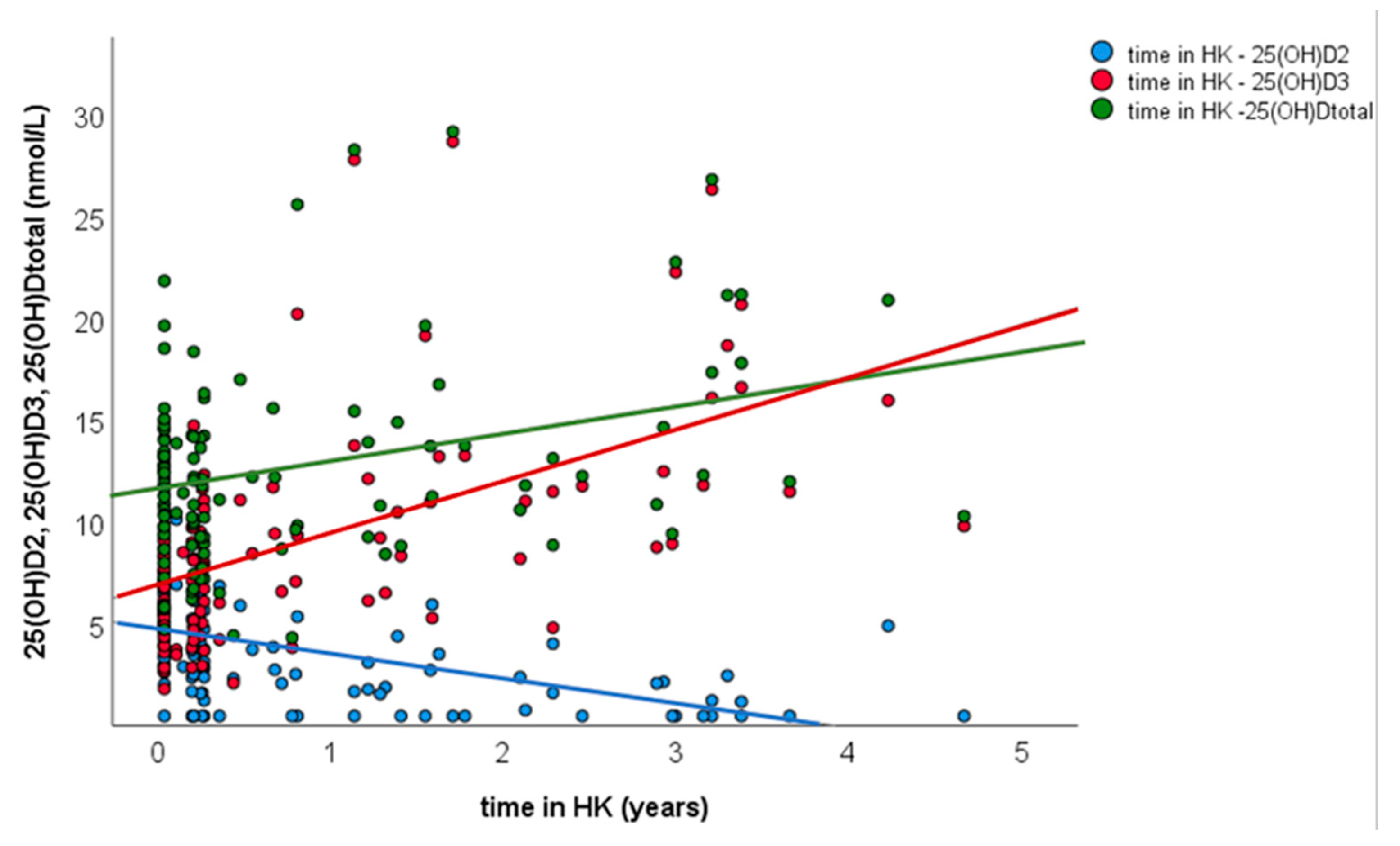
3.3. Univariate and Multivariate Linear Regression
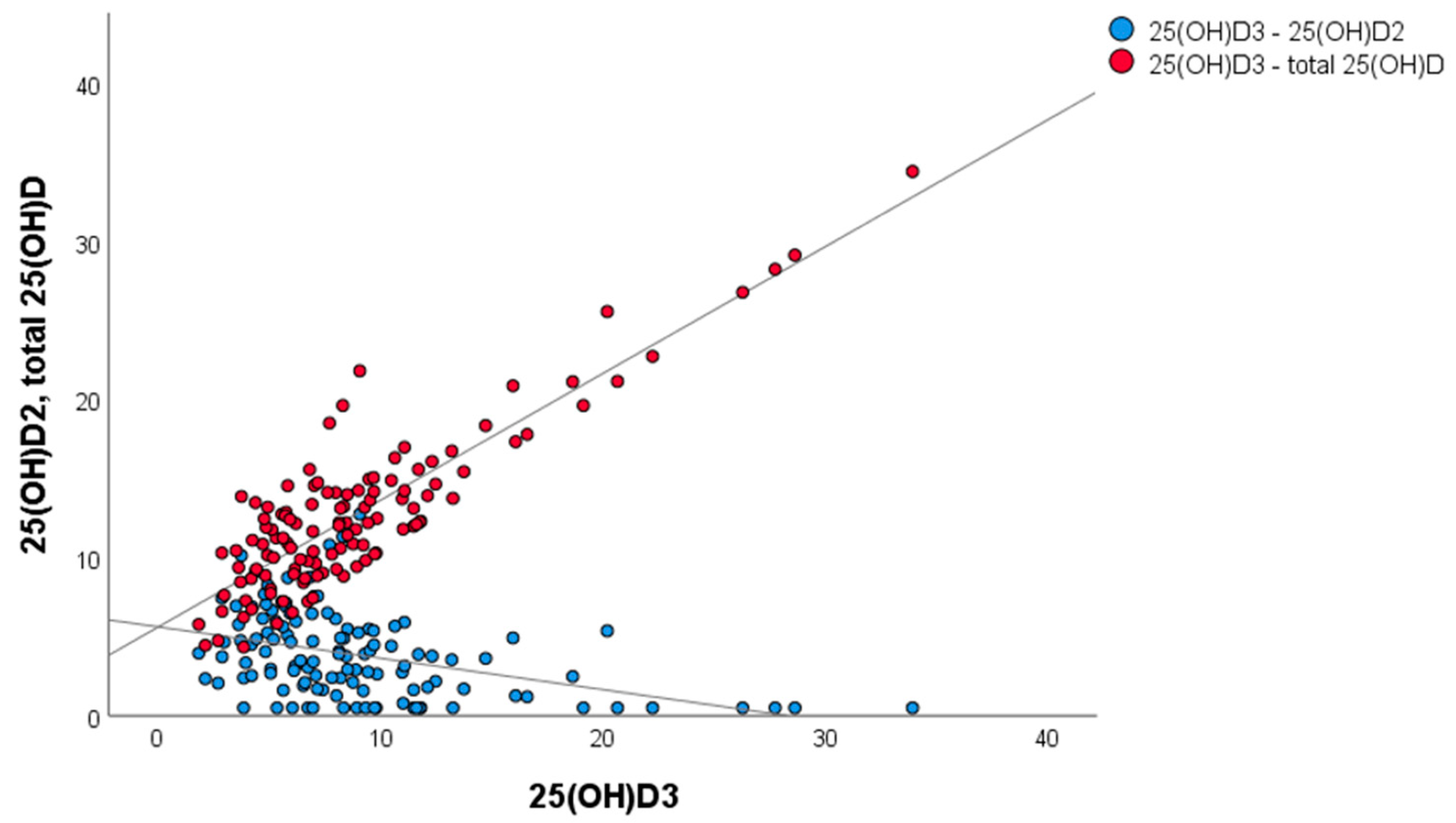
3.4. Correlation of 25OHD2, 25OHD3 and Total 25OHD
4. Discussion
4.1. Vitamin D Status of UK and HK Thoroughbred Racehorses
4.2. In Racehorses, Serum 25OHD3 Is Probably Derived Solely from Dietary D3 Supplementation
4.3. Vitamin D2 or D3?
4.4. The Role of Vitamin D in Equine Musculoskeletal Health and Athletic Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hymøller, L.; Jensen, S.K. We Know Next to Nothing About Vitamin D in Horses! J. Equine Vet. Sci. 2015, 35, 785–792. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [PubMed]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The Nonskeletal Effects of Vitamin D: An Endocrine Society Scientific Statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Jäpelt, R.B.; Didion, T.; Smedsgaard, J.; Jakobsen, J. Seasonal variation of provitamin D2 and vitamin D2 in perennial ryegrass (Lolium perenne L.). J. Agric. Food Chem. 2011, 59, 10907–10912. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef]
- Hurst, E.A.; Homer, N.Z.; Mellanby, R.J. Vitamin D Metabolism and Profiling in Veterinary Species. Metabolites 2020, 10, 371. [Google Scholar] [CrossRef]
- Toribio, R.E. Disorders of calcium and phosphate metabolism in horses. Vet. Clin. N. Am. Equine Pract. 2011, 27, 129–147. [Google Scholar] [CrossRef]
- Azarpeykan, S.; Dittmer, K.; Gee, E.; Marshall, J.; Wallace, J.; Elder, P.; Acke, E.; Thompson, K. Influence of blanketing and season on vitamin D and parathyroid hormone, calcium, phosphorus, and magnesium concentrations in horses in New Zealand. Domest. Anim. Endocrinol. 2016, 56, 75–84. [Google Scholar] [CrossRef]
- Dosi, M.C.; McGorum, B.C.; Kirton, R.D.; Cillán-García, E.; Mellanby, R.J.; Keen, J.A.; Hurst, E.A.; Morgan, R.A. The effect of season, management and endocrinopathies on vitamin D status in horses. Equine Vet. J. 2023, 55, 672–680. [Google Scholar] [CrossRef]
- Puangthong, C.; Sukhong, P.; Saengnual, P.; Srikuea, R.; Chanda, M. A single bout of high-intensity exercise modulates the expression of vitamin D receptor and vitamin D-metabolising enzymes in horse skeletal muscle. Equine Vet. J. 2021, 53, 796–805. [Google Scholar] [CrossRef]
- Azarpeykan, S.; Gee, E.K.; Thompson, K.G.; Dittmer, K.E. Undetectable vitamin D(3) in equine skin irradiated with ultraviolet light. J. Equine Sci. 2022, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Dede, S.; Deger, Y.; Yoruk, I. Investigation of the Effects of Carrying Heavy Load on Prooxidation/ Antioxidant Status and Vitamin D3 in Healthy Horses. Asian J. Anim. Vet. Adv. 2009, 4, 41–46. [Google Scholar] [CrossRef]
- Karagianni, A.E.; Kurian, D.; Cillán-Garcia, E.; Eaton, S.L.; Wishart, T.M.; Pirie, R.S. Training associated alterations in equine respiratory immunity using a multiomics comparative approach. Sci. Rep. 2022, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Bedenice, D.H.-t.A.; Rozanski, E.; Mazan, M. The relationship between serum 25-hydroxyvitamin D levels and inflammatory airway disease in horses. In Proceedings of the ACVIM Congress Proceedings, Indianapolis, IN, USA, 3–6 June 2015. [Google Scholar]
- NRC (National Research Council). Nutrient Requirements of Horses, 6th revised ed.; The National Academies Press: Washington DC, USA, 2007. [Google Scholar]
- Breidenbach, A.; Schlumbohm, C.; Harmeyer, J. Peculiarities of vitamin D and of the calcium and phosphate homeostatic system in horses. Vet. Res. 1998, 29, 173–186. [Google Scholar]
- Azarpeykan, S.; Dittmer, K.E.; Gee, E.K.; Marshall, J.C.; Elder, P.; Acke, E.; Thompson, K.G. Circadian rhythm of calciotropic hormones, serum calcium, phosphorus and magnesium during the shortest and longest days of the year in horses in New Zealand. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1058–1066. [Google Scholar] [CrossRef]
- Mäenpää, P.H.; Koskinen, T.; Koskinen, E. Serum profiles of vitamins A, E and D in mares and foals during different seasons. J. Anim. Sci. 1988, 66, 1418–1423. [Google Scholar] [CrossRef]
- Pozza, M.E.; Kaewsakhorn, T.; Trinarong, C.; Inpanbutr, N.; Toribio, R.E. Serum vitamin D, calcium, and phosphorus concentrations in ponies, horses and foals from the United States and Thailand. Vet. J. 2014, 199, 451–456. [Google Scholar] [CrossRef]
- Hymøller, L.; Jensen, S.K. 25-Hydroxycholecalciferol status in plasma is linearly correlated to daily summer pasture time in cattle at 56° N. Br. J. Nutr. 2012, 108, 666–671. [Google Scholar] [CrossRef]
- Rourke, K.; Coe, S.; Kohn, C.; Rosol, T.; Mendoza, F.; Toribio, R. Cloning, comparative sequence analysis and mRNA expression of calcium-transporting genes in horses. Gen. Comp. Endocrinol. 2010, 167, 6–10. [Google Scholar] [CrossRef]
- Azarpeykan, S.; Dittmer, K.E.; Marshall, J.C.; Perera, K.C.; Gee, E.K.; Acke, E.; Thompson, K.G. Evaluation and comparison of vitamin D responsive gene expression in ovine, canine and equine kidney. PLoS ONE 2016, 11, e0162598. [Google Scholar] [CrossRef]
- Kohler, M.; Leiber, F.; Willems, H.; Merbold, L.; Liesegang, A. Influence of altitude on vitamin D and bone metabolism of lactating sheep and goats. J. Anim. Sci. 2013, 91, 5259–5268. [Google Scholar] [CrossRef]
- Kovács, S.; Wilkens, M.R.; Liesegang, A. Influence of UVB exposure on the vitamin D status and calcium homoeostasis of growing sheep and goats. J. Anim. Physiol. Anim. Nutr. 2015, 99 (Suppl. S1), 1–12. [Google Scholar] [CrossRef]
- Hymøller, L.; Jensen, S.K. Vitamin D(3) synthesis in the entire skin surface of dairy cows despite hair coverage. J. Dairy Sci. 2010, 93, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, O.; Brustad, M.; Aksnes, L.; Lund, E. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem. Photobiol. 2005, 81, 1287–1290. [Google Scholar] [CrossRef]
- Hammami, M.M.; Abuhdeeb, K.; Hammami, S.; Yusuf, A. Vitamin-D2 treatment-associated decrease in 25(OH)D3 level is a reciprocal phenomenon: A randomized controlled trial. BMC Endocr. Disord. 2019, 19, 8. [Google Scholar] [CrossRef]
- Hammami, M.M.; Yusuf, A. Differential effects of vitamin D2 and D3 supplements on 25-hydroxyvitamin D level are dose, sex, and time dependent: A randomized controlled trial. BMC Endocr. Disord. 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Vieth, R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am. J. Clin. Nutr. 2006, 84, 694–697. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.; Bucca, G.; Penson, S.; Chope, G.; Hypponen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- El Shorafa, W.M. Effect of vitamin D and sunlight on growth and bone development of young ponies. J. Anim. Sci. 1979, 48, 882–886. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Harmeyer, J.; Schlumbohm, C. Effects of pharmacological doses of Vitamin D3 on mineral balance and profiles of plasma Vitamin D3 metabolites in horses. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.D.; Page, E.H. Acute vitamin D3 toxicosis in horses: Case reports and experimental studies of the comparative toxicity of vitamins D2 and D3. J. Am. Vet. Med. Assoc. 1983, 182, 1358–1369. [Google Scholar] [PubMed]
- de la Puente Yagüe, M.; Yurrita, L.C.; Cabañas, M.J.C.; Cenzual, M.A.C. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef]
- Bartoszewska, M.; Kamboj, M.; Patel, D.R. Vitamin D, Muscle Function, and Exercise Performance. Pediatr. Clin. N. Am. 2010, 57, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Rodman, J.S.; Baker, T. Changes in the kinetics of muscle contraction in vitamin D-depleted rats. Kidney Int. 1978, 13, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef]
- Zhang, L.; Quan, M.; Cao, Z.-B. Effect of vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: A meta-analysis. PLoS ONE 2019, 14, e0215826. [Google Scholar] [CrossRef]
- Yoon, S.; Kwon, O.; Kim, J. Vitamin D in athletes: Focus on physical performance and musculoskeletal injuries. Phys. Act. Nutr. 2021, 25, 20–25. [Google Scholar] [CrossRef]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport 2014, 17, 8–12. [Google Scholar] [CrossRef]
- Mendes, M.M.; Botelho, P.B.; Ribeiro, H. Vitamin D and musculoskeletal health: Outstanding aspects to be considered in the light of current evidence. Endocr. Connect. 2022, 11, e210596. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Riggs, C.M.; Cogger, N.; Wright, J.; Al-Alawneh, J.I. Noncatastrophic and catastrophic fractures in racing Thoroughbreds at the Hong Kong Jockey Club. Equine Vet. J. 2019, 51, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.A.; Bilezikian, J.P. Stress Fractures: Concepts and Therapeutics. J. Clin. Endocrinol. Metab. 2016, 102, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Riggs, C.M.; Boyde, A. Effect of exercise on bone density in distal regions of the equine third metacarpal bone in 2-year-old thoroughbreds. Equine Vet. J. 1999, 30, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Loughridge, A.B.; Hess, A.M.; Parkin, T.D.; Kawcak, C.E. Qualitative assessment of bone density at the distal articulating surface of the third metacarpal in Thoroughbred racehorses with and without condylar fracture. Equine Vet. J. 2017, 49, 172–177. [Google Scholar] [CrossRef]
| HK | UK | |||||
|---|---|---|---|---|---|---|
| nmol/L | 25(OH)D2 | 25(OH)D3 | 25(OH)Dtot | 25(OH)D2 | 25(OH)D3 | 25(OH)Dtot |
| Median | 3.4 A | 8.0 | 12.5 A | 7.4 A | 8.5 | 15.8 A |
| Mean ± SD | 4.0 ± 2.97 | 9.4 ± 5.61 | 13.4 ± 5.01 | 8.1 ± 2.20 | 8.6 ± 3.22 | 16.7 ± 3.51 |
| Range | <0.5–12.7 | 1.8–28.6 | 4.3–29.1 | 5.6–12.1 | 3.7–16.3 | 9.9–26.8 |
| Q1–Q3 | 1.6–6.0 | 5.7–11.5 | 10.3–15.0 | 6.1–10.6 | 6.2–10.2 | 13.2–19.7 |
| First Sample | Second Sample | ||
|---|---|---|---|
| 25(OH)D2 A | Mean ± SD | 5.9 ± 2.6 | 3.6 ± 2.1 |
| Median (range) | 5.8 (0.5–12.8) | 3.5 (0.5–10.1) | |
| Q1–Q3 | 4.0–7.4 | 2.4–4.8 | |
| 25(OH)D3 | Mean ± SD | 6.4 ± 2.1 | 7.8 ± 5.2 |
| Median (range) | 6.0 (11.0–1.8) | 7.2 (2.1–33.9) | |
| Q1–Q3 | 5.1–7.7 | 4.7–9.4 | |
| 25(OH)Dtot | Mean ± SD | 12.3 ± 3.5 | 11.4 ± 4.9 |
| Median (range) | 12.5 | 10.4 (4.4–34.4) | |
| Q1–Q3 | 10.4–14.0 | 8.5–13.6 |
| Variables Retained | Regression Coefficient (β) | Standard Error |
|---|---|---|
| Ln25(OH)D3 | 0.985 | 0.024 |
| LnDuration in HK | −0.330 | 0.056 |
| intercept | 1.220 | 0.107 |
| p value | <0.001 | |
| Pearson’s coefficient | 0.827 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosi, M.C.M.; Riggs, C.M.; May, J.; Lee, A.; Cillan-Garcia, E.; Pagan, J.; McGorum, B.C. Thoroughbred Racehorses in Hong Kong Require Vitamin D Supplementation to Mitigate the Risk of Low Vitamin D Status. Animals 2023, 13, 2145. https://doi.org/10.3390/ani13132145
Dosi MCM, Riggs CM, May J, Lee A, Cillan-Garcia E, Pagan J, McGorum BC. Thoroughbred Racehorses in Hong Kong Require Vitamin D Supplementation to Mitigate the Risk of Low Vitamin D Status. Animals. 2023; 13(13):2145. https://doi.org/10.3390/ani13132145
Chicago/Turabian StyleDosi, Miranda C. M., Chris M. Riggs, Jessica May, Adele Lee, Eugenio Cillan-Garcia, Joe Pagan, and Bruce C. McGorum. 2023. "Thoroughbred Racehorses in Hong Kong Require Vitamin D Supplementation to Mitigate the Risk of Low Vitamin D Status" Animals 13, no. 13: 2145. https://doi.org/10.3390/ani13132145
APA StyleDosi, M. C. M., Riggs, C. M., May, J., Lee, A., Cillan-Garcia, E., Pagan, J., & McGorum, B. C. (2023). Thoroughbred Racehorses in Hong Kong Require Vitamin D Supplementation to Mitigate the Risk of Low Vitamin D Status. Animals, 13(13), 2145. https://doi.org/10.3390/ani13132145







