Surveillance and Risk Analysis for Bovine Babesiosis in England and Wales to Inform Disease Distribution
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Area and Participant Recruitment
2.3. Blood Smear Examination
2.4. Molecular Detection of Blood-Borne Pathogens
2.5. Statistical Analysis
3. Results
3.1. Detection of B. divergens and A. phagocytophilum in Cattle
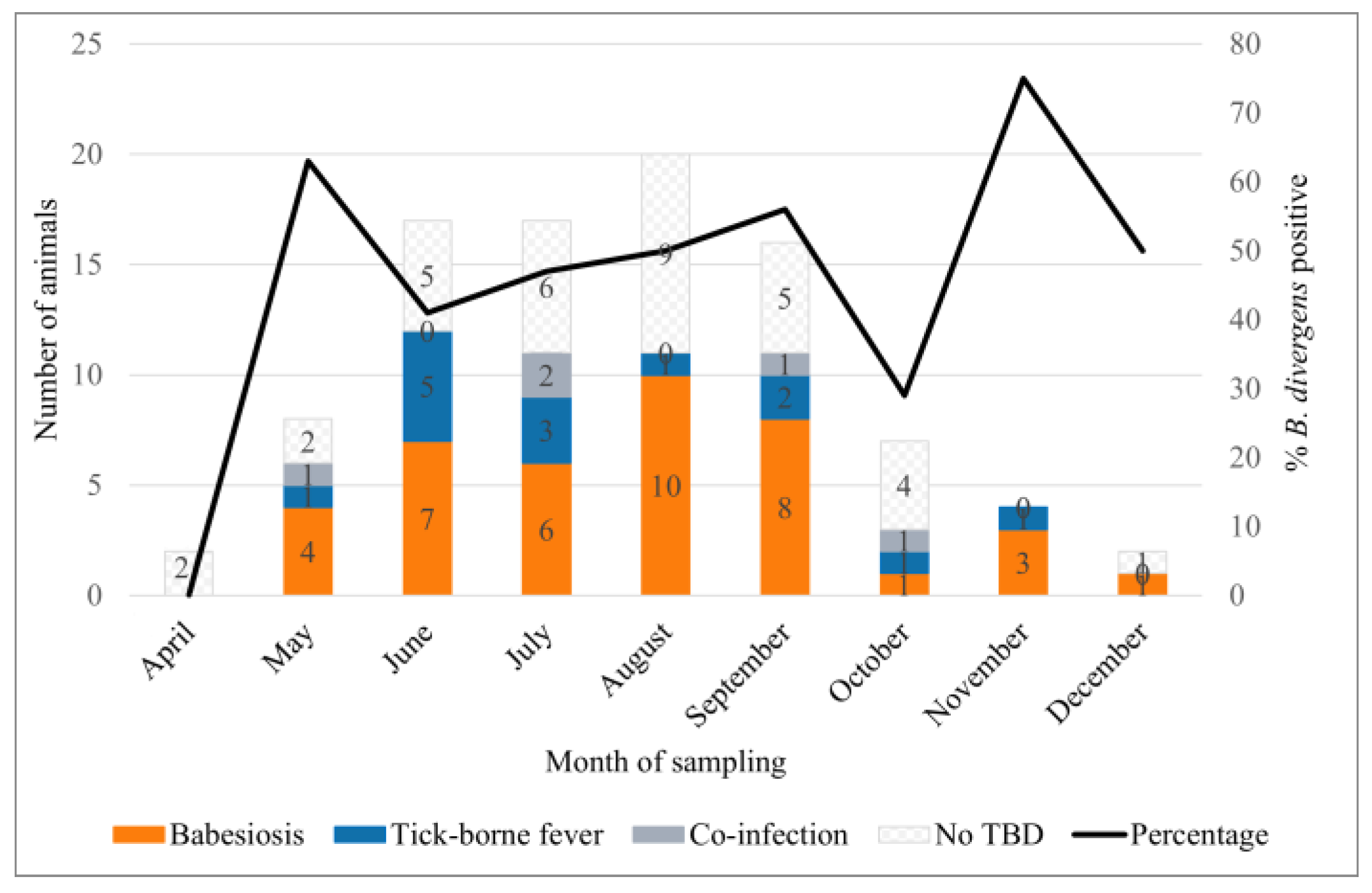
3.2. Seasonal Distribution
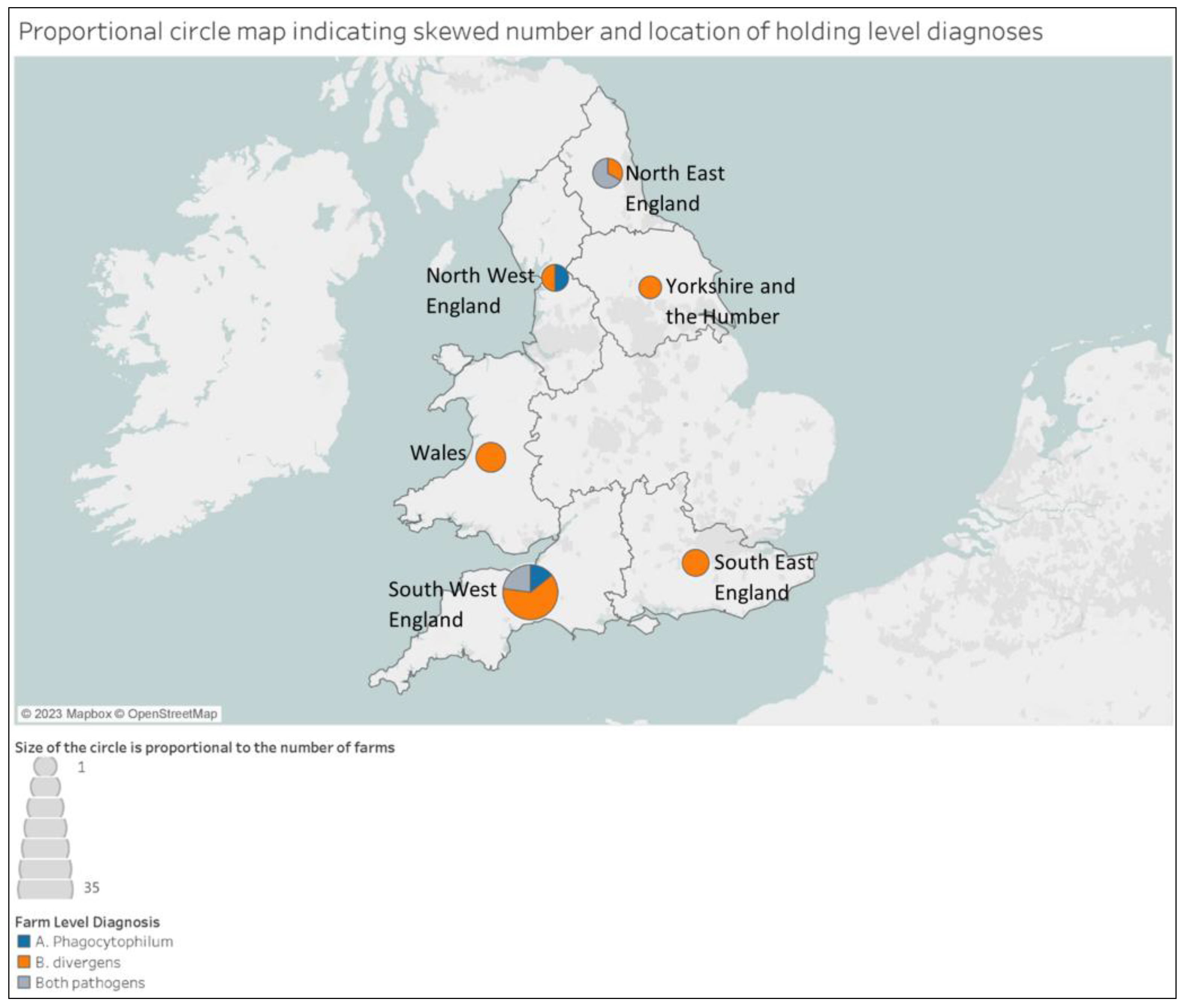
3.3. Geographical Distribution
3.4. Risk Factor Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donnelly, J. Epidemiology of Babesia infection in cattle. Proc. R. Soc. Med. 1973, 66, 774–775. [Google Scholar] [PubMed]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin. Microbiol. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zintl, A.; McGrath, G.; O’Grady, L.; Fanning, J.; Downing, K.; Roche, D.; Casey, M.; Gray, J.S. Changing incidence of bovine babesiosis in Ireland. Ir. Vet. J. 2014, 67, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centre for Food Security and Public Health (CFSPH). Bovine Babesiosis. Available online: https://www.cfsph.iastate.edu/Factsheets/pdfs/bovine_babesiosis.pdf (accessed on 8 March 2022).
- Woldehiwet, Z. Immune evasion and immunosuppression by Anaplasma phagocytophilum, the causative agent of tick-borne fever of ruminants and human granulocytic anaplasmosis. Vet. J. 2008, 175, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Woldehiwet, Z. Anaplasma phagocytophilum in ruminants in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 446–460. [Google Scholar] [CrossRef]
- Andersson, M.O.; Víchová, B.; Tolf, C.; Krzyzanowska, S.; Waldenström, J.; Karlsson, M.E. Co-infection with Babesia divergens and Anaplasma phagocytophilum in cattle (Bos Taurus), Sweden. Ticks Tick Borne Dis. 2017, 8, 933–935. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Meli, M.L.; Dreher, U.M.; Gönczi, E.; Deplazes, P.; Braun, U.; Engels, M.; Schüpbach, J.; Jörger, K.; Thoma, R.; et al. Concurrent infections with vector-borne pathogens associated with fatal haemolytic anaemia in a cattle herd in Switzerland. J. Clin. Microbiol. 2004, 42, 3775–3780. [Google Scholar] [CrossRef] [Green Version]
- McFadzean, H.; Johnson, N.; Phipps, L.P.; Hobbs, R.L. High morbidity associated with an outbreak of tick-borne disease in a dairy herd, Cornwall. Vet. Rec. Case Rep. 2021, 9, e171. [Google Scholar] [CrossRef]
- Entrican, J.H.; Williams, H.; Cook, I.A.; Lancaster, W.M.; Clark, J.C.; Joyner, C.P.; Lewis, D. Babesiosis in man: A case from Scotland. BMJ 1979, 2, 474. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.Y.; MacDonald, C.; Keenan, A.; Xu, K.; Bain, B.J.; Chiodini, P.L. Severe babesiosis due to Babesia divergens acquired in the United Kingdom. Am. J. Hematol. 2021, 96, 889–890. [Google Scholar] [CrossRef]
- Perret, J.L.; Guigoz, E.; Rais, O.; Gern, L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 2000, 86, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Perret, J.L.; Rais, O.; Gern, L. Influence of climate on the proportion of Ixodes ricinus nymphs and adults questing in a tick population. J. Med. Entomol. 2004, 41, 361–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaenson, T.G.; Lindgren, E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis. 2011, 2, 44–49. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPPC). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; 2021/17/PR; IPPC Secretariat: Geneva, Switzerland, 2021; Available online: https://www.ipcc.ch/site/assets/uploads/2021/08/IPCC_WGI-AR6-Press-Release_en.pdf (accessed on 7 March 2021).
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- Allan, B.F.; Keesing, F.; Ostfeld, R.S. Effect of Forest Fragmentation on Lyme Disease Risk. Conserv. Biol. 2003, 17, 267–272. [Google Scholar] [CrossRef] [Green Version]
- United Nations (UN). 68% of the World Population Projected to Live in Urban Areas by 2050, Says UN. Available online: https://www.un.org/development/desa/en/news/population/2018-revision-of-world-urbanization-prospects.html (accessed on 9 March 2022).
- Medlock, J.M.; Vaux, A.G.C.; Hansford, K.M.; Pietzsche, M.E.; Gillingham, E.L. Ticks in the ecotone: The impact of agri-environment field margins on the presence and intensity of Ixodes ricinus ticks (Acari: Ixodidae) in farmland in southern England. Med. Vet. Entomol. 2020, 34, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.T.; Jaenson, D.G.E.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Cull, B.; Pietzsch, M.E.; Hansford, K.M.; Gillingham, E.L.; Medlock, J.M. Surveillance of British ticks: An overview of species records, host associations, and new records of Ixodes ricinus distribution. Ticks Tick Borne Dis. 2018, 9, 605–614. [Google Scholar] [CrossRef]
- Cairns, V.; Wallenhorst, C.; Rietbrock, S.; Martinez, C. Incidence of Lyme disease in the UK: A population-based cohort study. BMJ Open 2019, 9, e025916. [Google Scholar] [CrossRef] [Green Version]
- Černý, J.; Lynn, G.; Hrnková, J.; Golovchenko, M.; Rudenko, N.; Grubhoffer, L. Management options for Ixodes ricinus-associated pathogens: A review of prevention strategies. Int. J. Environ. Res. Public Health 2020, 17, 1830. [Google Scholar] [CrossRef] [Green Version]
- Folly, A.J.; Dorey-Robinson, D.; Hernández-Triana, L.M.; Phipps, L.P.; Johnson, N. Emerging threats to animals in the United Kingdom by arthropod-borne diseases. Front. Vet. Sci. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Kingdom. Department for Environment, Food and Rural Affairs (Defra). Farming is Changing, Last Revised July 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1003924/farming-changing.pdf (accessed on 23 August 2022).
- Lihou, K.; Vineer, H.R.; Wall, R. Distribution and prevalence of ticks and tick-borne disease on sheep and cattle farms in Great Britain. Parasites Vectors 2020, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; Król, N.; Obiegala, A. Prevention and control of tick-borne anaplasmosis, cowdriosis and babesiosis in the cattle industry. In Pests and Vector-Borne Diseases in the Livestock Industry; Garros, C., Bouyer, J., Takken, R., Smallegange, R.C., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 1998; pp. 175–194. ISBN 978-90-8686-315-0. [Google Scholar]
- Armstrong, P.M.; Katavolos, P.; Caporale, D.A.; Smith, R.P.; Telford, S.R. 3rd. Diversity of Babesia infecting deer ticks (Ixodes dammini). Am. J. Trop. Med. 1998, 58, 739–742. [Google Scholar] [CrossRef]
- Courtney, J.W.; Kostelnik, L.M.; Zeidner, N.S.; Massung, R.F. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 2004, 42, 3164–3168. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.; Phipps, L.P.; McFadzean, H.; Barlow, A.B. An outbreak of bovine babesiosis in February, 2019, triggered by above average winter temperatures in southern England and co-infection with Babesia divergens and Anaplasma phagocytophilum. Parasites Vectors 2020, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- British Cattle Movement Service (BCMS). CTS Online. Available online: https://secure.services.defra.gov.uk/wps/portal/ctso (accessed on 5 January 2022).
- L’Hostis, M.; Chauvin, A.; Valentin, A.; Marchand, A.; Gorenflot, A. Large scale survey of bovine babesiosis due to Babesia divergens in France. Vet. Rec. 1995, 136, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, e593232. [Google Scholar] [CrossRef]
- Qviller, L.; Grøva, L.; Viljugrein, H.; Klingen, I.; Mysterud, A. Temporal pattern of questing tick Ixodes ricinus density at differing elevations in the coastal region of western Norway. Parasites Vectors 2014, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, P.A. Climate change impacts on tick and tick-borne infections. Biologia 2021, 77, 1503–1512. [Google Scholar] [CrossRef]
- Gray, A.G. An Investigation of Endemic and Emerging Tick-Borne Protozoa and Rickettsia in Scottish Livestock. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2017. Available online: https://theses.gla.ac.uk/8750/ (accessed on 25 April 2022).
- Brocklesby, D.W.; Sellwood, S.A. Babesia major in Britain: Tick transmitted infections in splenectomised calves. Res. Vet. Sci. 1973, 14, 47–52. [Google Scholar] [CrossRef]
- Phipps, L.P.; Hansford, K.M.; Hernández-Triana, L.M.; Golding, M.; McGinley, L.; Folly, A.J.; Vaux, A.G.C.; de Marco, M.F.; Carter, D.P.; Medlock, J.M.; et al. Detection of Borrelia and Babesia species in Haemaphysalis punctata ticks sampled in Southern England. Ticks Tick Borne Dis. 2022, 13, e101902. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.F.; Brocklesby, D.W. The isolation of a large Babesia species and other blood parasites from British cattle. Vet. Rec. 1971, 88, 260. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Marco, M.; Brugman, V.A.; Hernández-Triana, L.M.; Thorne, L.; Phipps, L.P.; Nikolova, N.I.; Fooks, A.R.; Johnson, N. Detection of Theileria orientalis in mosquito blood meals in the United Kingdom. Vet. Parasitol. 2016, 229, 31–36. [Google Scholar] [CrossRef]
- Sun, M.; Guan, G.; Liu, Z.; Wang, J.; Wang, D.; Wang, S.; Ma, C.; Cheng, S.; Yin, H.; Luo, J. Molecular survey and genetic diversity of Babesia spp. and Theileria spp. in cattle in Gansu Province, China. Acta Parasitol. 2020, 65, 422–429. [Google Scholar] [CrossRef]
- de Marco, M.dM.F.; Hernández-Triana, L.M.; Phipps, L.P.; Hansford, K.; Mitchell, E.S.; Cull, B.; Sainsbury, C.S.; Fooks, A.R.; Medlock, J.M.; Johnson, N. Emergence of Babesia canis in southern England. Parasites Vectors 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Bastos, R.G.; Sun, Y.; Hua, G.; Guan, G.; Zhao, J.; Suarez, C.E. Babesiosis as a potential threat for bovine production in China. Parasites Vectors 2021, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Hansford, K.M.; Phipps, L.P.; Cull, B.; Pietzsch, M.E.; Medlock, J.M. Rhipicephalus sanguineus importation into the UK: Surveillance, risk, public health awareness and One Health response. Vet. Rec. 2017, 180, 119. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Phipps, L.P.; Hansford, K.M.; Folly, A.J.; Fooks, A.R.; Medlock, J.M.; Mansfield, K.M. One Health approach to tick and tick-borne disease surveillance in the United Kingdom. Int. J. Environ. Res. Public Health 2022, 19, 5833. [Google Scholar] [CrossRef]
- Uchtmann, N.; Herrmann, J.A.; Hahn, E.C.; Beasley, V.R. Barriers to, efforts in, and optimization of integrated one health surveillance: A review and synthesis. EcoHealth 2015, 12, 368–384. [Google Scholar] [CrossRef]
- Ayling, R.D.; Bisgaard-Frantzen, S.; Adler, A.; Blowey, R.W.; Barlow, A.M.; Millar, M.F.; van der Burgt, G.M. Detection of ‘Candidatus Mycoplasma haemobos’, Mycoplasma wenyonii and Anaplasma phagocytophilum from cattle in England. Vet. Rec. 2012, 170, 543. [Google Scholar] [CrossRef]
- Meli, M.L.; Willi, B.; Dreher, U.M.; Cattori, V.; Knubben-Schweizer, G.; Nuss, K.; Braun, U.; Lutz, H.; Hofmann-Lehmann, R. Identification, molecular characterization, and occurrence of two bovine hemoplasma species in Swiss cattle and development of real-time TaqMan quantitative PCR assays for diagnosis of bovine hemoplasma infections. J. Clin. Microbiol. 2010, 10, 3563–3568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerram, L.; Willshire, J. Babesiosis in the UK and approach to treatment. Livestock 2019, 24, 18–24. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 978-92-5-107920-1. [Google Scholar]
- Taylor, S.M.; Kenny, J.; Strain, A. The distribution of Babesia divergens infection within the cattle population of Northern Ireland. Br. Vet. J. 1982, 138, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Saif, L.J. Bovine immunology: Implications for dairy cattle. Front. Immunol. 2021, 12, 643206. [Google Scholar] [CrossRef]
- Gray, J.; Kahl, O.; Zintl, A. What do we still need to know about Ixodes ricinus? Ticks Tick Borne Dis. 2021, 12, 101682. [Google Scholar] [CrossRef]
- Tack, W.; Madder, M.; Baeten, L.; Vanhellemont, M.; Gruwez, R.; Verheyen, K. Local habitat and landscape affect Ixodes ricinus tick abundances in forests on poor, sandy soils. For. Ecol. Manag. 2012, 265, 30–36. [Google Scholar] [CrossRef] [Green Version]
- McFadzean, H.; Strugnell, B.; Collins, C.; Jones, A.; Phipps, L.P.; Johnson, N. Bovine babesiosis in Northumberland. Vet. Rec. 2021, 189, 207–220. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total Animals | Babesia-Positive (%) | Estimate | Standard Error | p Value | Odds Ratio (OR) | 95% Confidence Interval (CI) |
|---|---|---|---|---|---|---|---|
| Region (n = 95) | |||||||
| South West England | 76 | 34 (44.7) | - | - | - | - | - |
| Rest of England and Wales | 19 | 11 (57.9) | 0.54 | 0.556 | 0.33 | 1.71 | 0.58–5.09 |
| Farm type (n = 94) | |||||||
| Dairy | 37 | 13 (35.1) | - | - | - | - | - |
| Beef | 57 | 32 (56.1) | 1.07 | 0.576 | 0.06 | 2.91 | 0.94–8.99 |
| Number on holding (n = 95) | |||||||
| 0–99 | 23 | 13 (56.5) | - | - | - | - | - |
| 100–199 | 24 | 14 (58.3) | 0.06 | 0.625 | 0.92 | 1.06 | 0.31–3.61 |
| 200–299 | 17 | 8 (47.1) | −0.42 | 0.691 | 0.54 | 0.66 | 0.17–2.54 |
| 300–399 | 15 | 4 (26.7) | −1.35 | 0.794 | 0.09 | 0.26 | 0.05–1.23 |
| >400 | 16 | 6 (37.5) | −0.83 | 0.723 | 0.25 | 0.44 | 0.11–1.81 |
| Variable | Total Animals | Babesia-Positive (%) | Estimate | Standard Error | p Value | Odds Ratio (OR) | 95% Confidence Interval (CI) |
|---|---|---|---|---|---|---|---|
| Age (n = 82) | |||||||
| Age (<2 years) | 14 | 6 (42.8) | - | - | - | - | - |
| Age (2–5 years) | 27 | 15 (55.5) | 0.53 | 0.722 | 0.46 | 1.70 | 0.41–7.01 |
| Age (>5 years) | 41 | 21 (51.2) | 0.31 | 0.680 | 0.65 | 1.36 | 0.36–5.17 |
| Sex (n = 93) | |||||||
| Female | 81 | 42 (51.9) | - | - | - | - | - |
| Male | 12 | 3 (25) | −1.17 | 0.708 | 0.10 | 0.31 | 0.08–1.24 |
| Breed (n = 89) | |||||||
| Pedigree | 58 | 29 (50) | - | - | - | - | - |
| Crossbreed | 31 | 15 (48.4) | −0.07 | 0.467 | 0.89 | 0.94 | 0.37–2.34 |
| Days on farm (n = 82) | |||||||
| <365 days | 25 | 15 (60) | - | - | - | - | - |
| >365 days | 57 | 27 (47.4) | −0.54 | 0.524 | 0.31 | 0.58 | 0.21–1.63 |
| Number of moves (n = 83) | |||||||
| 0 | 46 | 19 (41.3) | - | - | - | - | - |
| ≥1 | 37 | 23 (62.2) | 0.92 | 0.522 | 0.08 | 2.51 | 0.90–6.98 |
| Ticks reported (n = 95) | |||||||
| Yes | 18 | 8 (44.4) | - | - | - | - | - |
| No | 77 | 37 (48.1) | 0.11 | 0.576 | 0.84 | 1.12 | 0.36–3.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McFadzean, H.; Johnson, N.; Phipps, L.P.; Swinson, V.; Boden, L.A. Surveillance and Risk Analysis for Bovine Babesiosis in England and Wales to Inform Disease Distribution. Animals 2023, 13, 2118. https://doi.org/10.3390/ani13132118
McFadzean H, Johnson N, Phipps LP, Swinson V, Boden LA. Surveillance and Risk Analysis for Bovine Babesiosis in England and Wales to Inform Disease Distribution. Animals. 2023; 13(13):2118. https://doi.org/10.3390/ani13132118
Chicago/Turabian StyleMcFadzean, Harriet, Nicholas Johnson, L. Paul Phipps, Vanessa Swinson, and Lisa A. Boden. 2023. "Surveillance and Risk Analysis for Bovine Babesiosis in England and Wales to Inform Disease Distribution" Animals 13, no. 13: 2118. https://doi.org/10.3390/ani13132118
APA StyleMcFadzean, H., Johnson, N., Phipps, L. P., Swinson, V., & Boden, L. A. (2023). Surveillance and Risk Analysis for Bovine Babesiosis in England and Wales to Inform Disease Distribution. Animals, 13(13), 2118. https://doi.org/10.3390/ani13132118






