Rodents Inhabiting the Southeastern Mu Us Desert May Not Have Experienced Prolonged Heat Stress in Summer 2022
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
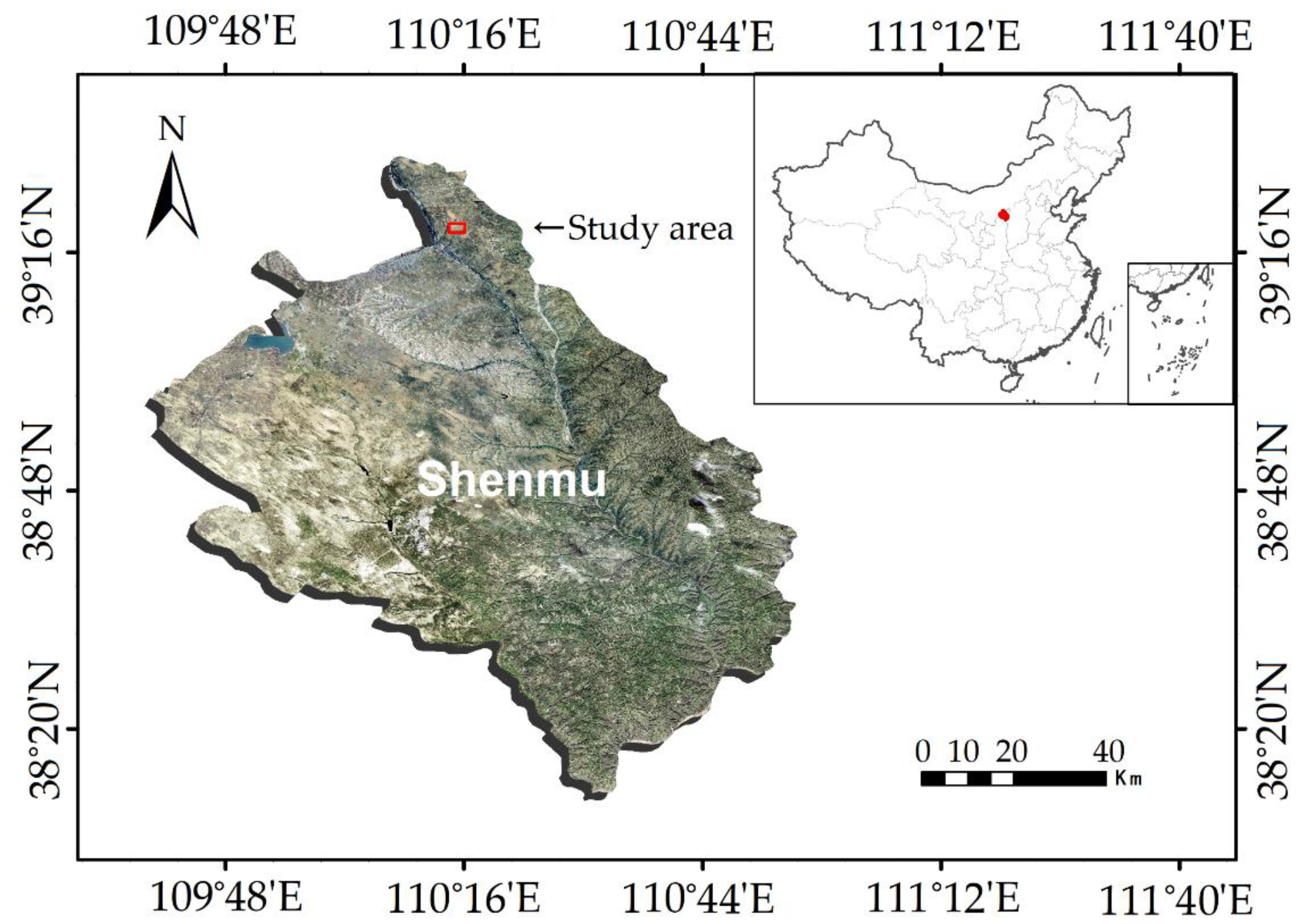
2.1. Study Area
2.2. Vegetation Analysis
2.3. Rodent Surveys
2.4. Determination and Analysis of Ambient Temperature
2.5. Determination of the Extreme Heat
2.6. Statistical Analyses
3. Results
3.1. Plant Species, Height, and Coverage
3.2. Small Mammals Living in the Study Area
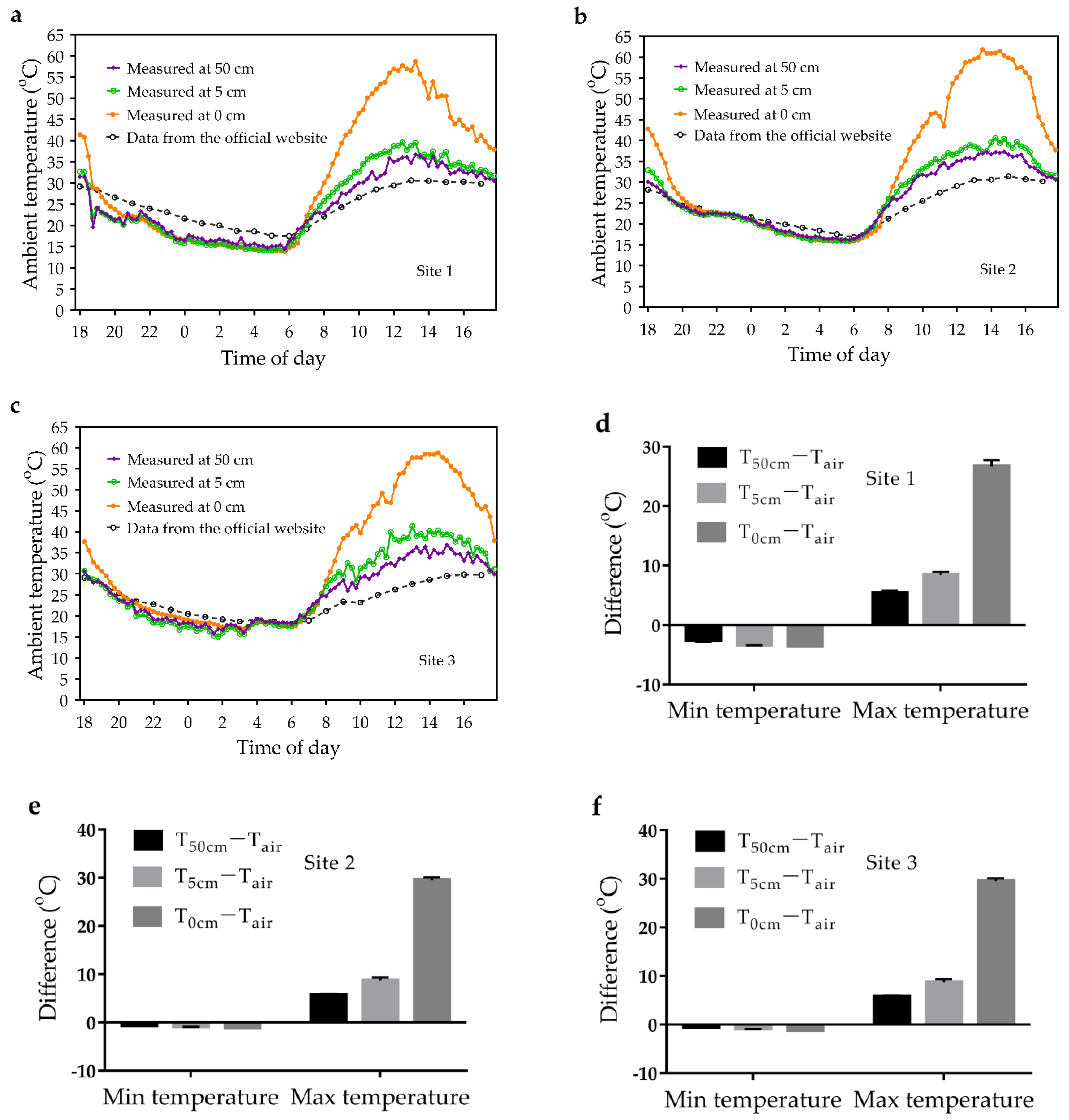
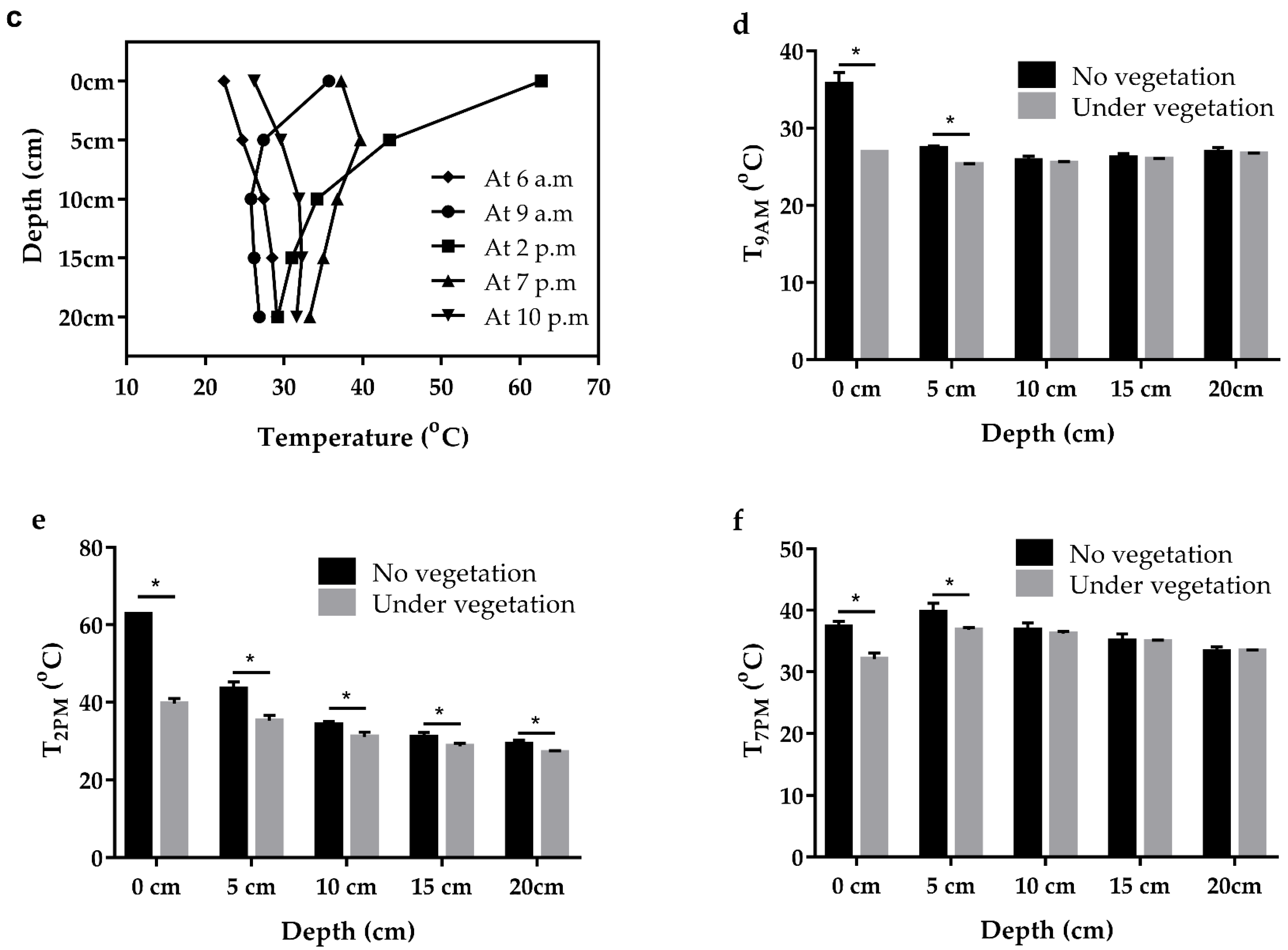
3.3. Ambient Temperature Variation in Near-Surface Space
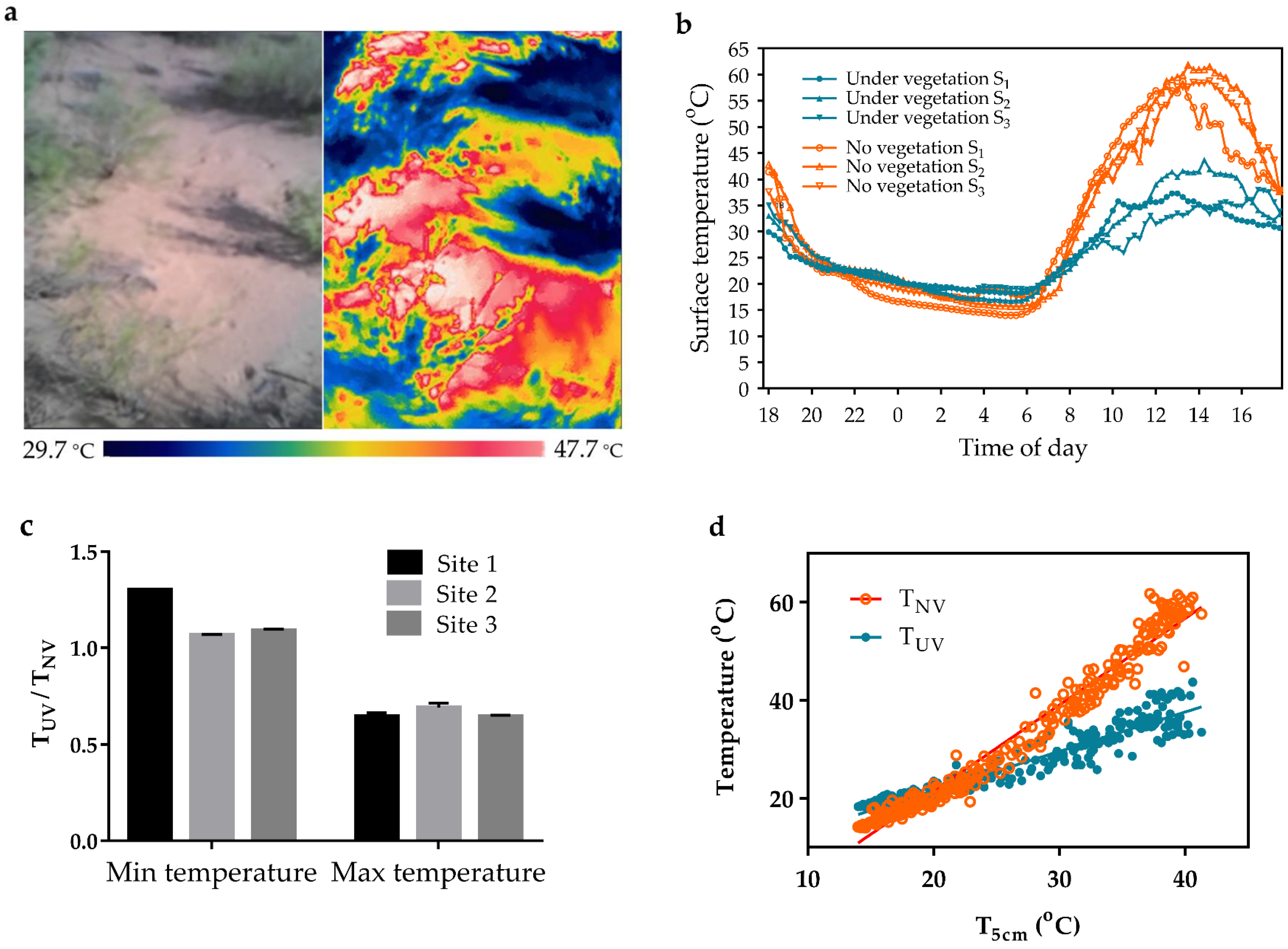
3.4. Effect of Vegetation on Desert Surface Temperature
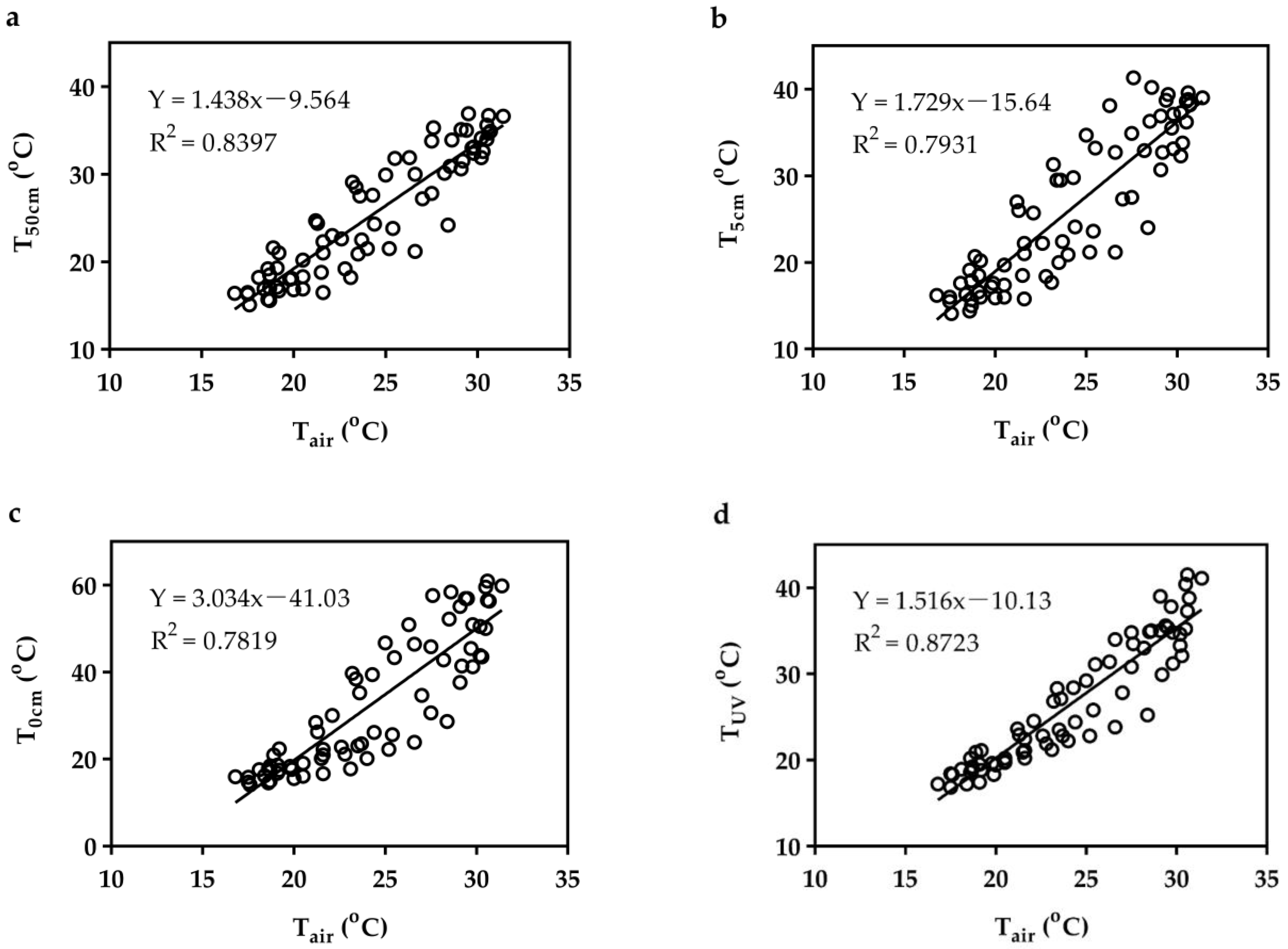
3.5. Linear Relationship between Tair and Near-Surface Ambient Temperature
3.6. Ambient Temperature in Rodent Burrow
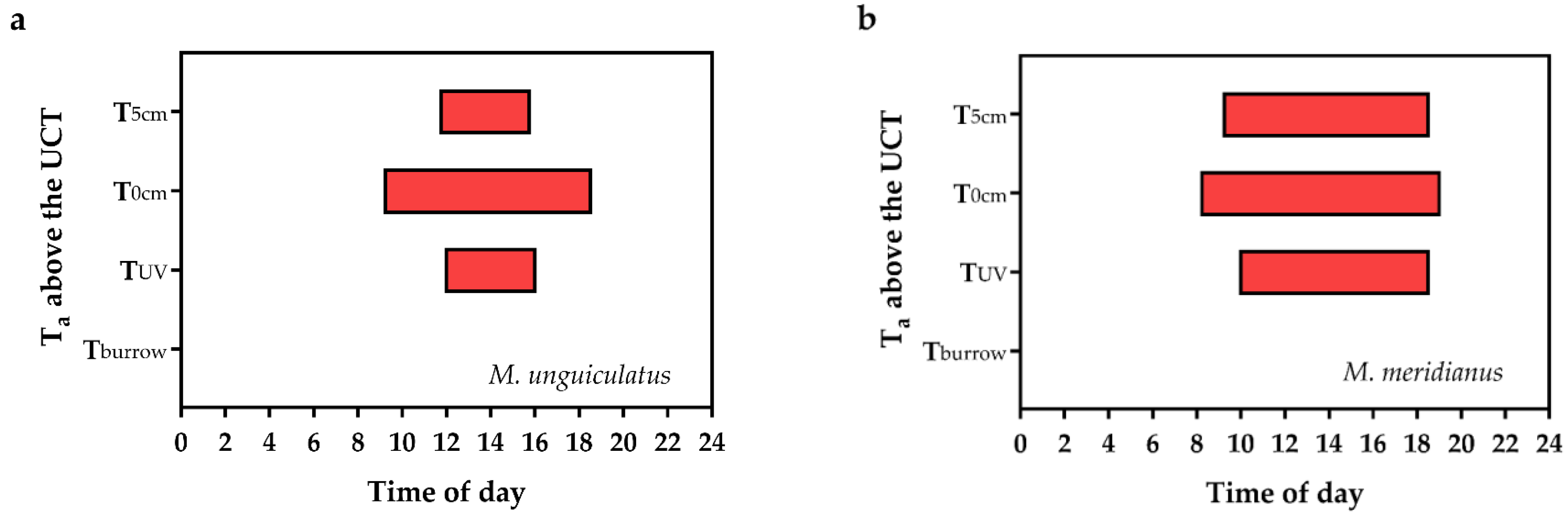
3.7. Distribution of Extreme Heat
4. Discussion
4.1. Vegetation Coverage and Rodent Species in the Mu Us Desert
4.2. Temperature Variation of Near-Surface Space in Summer
4.3. Strategies for Rodent Response to Summer Heat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stillman, J.H. Heat waves, the new normal: Summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 2019, 34, 86–100. [Google Scholar] [CrossRef]
- Khaliq, I.; Hof, C.; Prinzinger, R.; Böhning-Gaese, K.; Pfenninger, M. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141097. [Google Scholar] [CrossRef]
- Lu, R.; Xu, K.; Chen, R.; Chen, W.; Li, F.; Lv, C. Heat waves in summer 2022 and increasing concern regarding heat waves in general. Atmos. Ocean. Sci. Lett. 2023, 16, 100290. [Google Scholar] [CrossRef]
- Morit, A. Extreme heatwaves: Surprising lessons from the record warmth. Nature 2022, 608, 464–465. [Google Scholar]
- Rousi, E.; Kornhuber, K.; Beobide-Arsuaga, G.; Luo, F.; Coumou, D. Accelerated western European heatwave trends linked to more-persistent double jets over Eurasia. Nat. Commun. 2022, 13, 3851. [Google Scholar] [CrossRef]
- Fischer, E.M.; Sippel, S.; Knutti, R. Increasing probability of record-shattering climate extremes. Nat. Clim. Chang. 2021, 11, 689–695. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci.-Landmark 2011, 16, 1428–1444. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Fuente, V.C.; Wang, D. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Sareh, H.; Tulapurkar, M.E.; Shah, N.G.; Singh, I.S.; Hasday, J.D. Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress Chaperones 2011, 16, 297–307. [Google Scholar] [CrossRef]
- Sawka, M.N.; Wenger, C.B.; Pandolf, K.B. Thermoregulatory responses to acute exercise-heat stress and heat acclimation. Compr. Physiol. 2010, 157–185. [Google Scholar] [CrossRef]
- Horowitz, M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 475–483. [Google Scholar] [CrossRef]
- Sun, Z.; Mao, Z.; Yang, L.; Liu, Z.; Han, J.; Wanag, H.; He, W. Impacts of climate change and afforestation on vegetation dynamic in the Mu Us Desert, China. Ecol. Indic. 2021, 129, 108020. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.H.; Dong, Z.; Xia, D. Evolution of the southern Mu Us Desert in North China over the past 50 years: An analysis using proxies of human activity and climate parameters. Land Degrad. Dev. 2005, 16, 351–366. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, H. Historical human activities accelerated climate-driven desertification in China’s Mu Us Desert. Sci. Total Environ. 2020, 708, 134771. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, K.; Liu, H.; Zhang, C.; Yue, Y.; Qi, X. Effect of ecological engineering projects on ecosystem services in a karst region: A case study of northwest Guangxi, China. J. Clean. Prod. 2018, 183, 831–842. [Google Scholar] [CrossRef]
- Li, X.; Ren, G.; Wang, S.; You, Q.; Sun, Y.; Ma, Y.; Wang, D.; Zhang, W. Change in the heatwave statistical characteristics over China during the climate warming slowdown. Atmos. Res. 2021, 247, 105152. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Li, H.; Lou, J.; Hua, T.; Liu, W.; Jiao, L.; Ma, W.; Li, D.; Zhu, B. Key driving forces of desertification in the Mu Us Desert, China. Sci. Rep. 2017, 7, 3933. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Yan, C. Assessing the role of policies on land-use/cover change from 1965 to 2015 in the Mu Us Sandy Land, northern China. Sustainability 2017, 9, 1164. [Google Scholar] [CrossRef]
- Wang, T.; Wu, W.; Xue, X.; Zhang, W.M.; Han, Z.W.; Sun, Q.W. Time-space evolution of desertification land in northern China. J. Desert Res. 2003, 23, 230. [Google Scholar]
- Limin, W.; Yanlin, Z.; Lijun, Z. Component analysing for rodents of Erdos plateau sandland. Acta Sci. Nat. Univ. Neimonggol (Nat. Sci. Ed.) 1999, 30, 377–379. [Google Scholar]
- Robinson, P.F. Metabolism of the Gerbil, Meriones unguiculatus. Science 1959, 130, 502–503. [Google Scholar] [CrossRef]
- Pan, Q.; Li, M.; Shi, Y.L.; Liu, H.; Speakman, J.R.; Wang, D.H. Lipidomics reveals mitochondrial membrane remodeling associated with acute thermoregulation in a rodent with a wide thermoneutral zone. Lipids 2014, 49, 715–730. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Hao, S.; Zhang, M.; Zhang, X.; Wang, D. Aquaporins, evaporative water loss and thermoregulation in heat-acclimated Mongolian gerbils (Meriones unguiculatus). J. Therm. Biol. 2020, 91, 102641. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wang, Z. Metabolism and thermoregulation in the Mongolian gerbil Meriones unguiculatus. Acta Theriol. 2000, 45, 183–192. [Google Scholar] [CrossRef]
- Xu, M.M.; Wang, D.H. Water deprivation up-regulates urine osmolality and renal aquaporin 2 in Mongolian gerbils (Meriones unguiculatus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 194, 37–44. [Google Scholar] [CrossRef]
- Agren, G.; Zhong, W.; Zhou, Q. Activity pattern in Mongolian gerbils (Meriones unguiculatus) in summer. In Reports from the Inner Mongolia Grassland Ecosystem Research Station of Academia Sinica (1979–1988); Yu, B., Peng, K.L., Eds.; Science Press: Beijing, China, 1990; pp. 239–246. [Google Scholar]
- Ding, B.Y.; Chi, Q.S.; Liu, W.; Shi, Y.L.; Wang, D.H. Thermal biology of two sympatric gerbil species: The physiological basis of temporal partitioning. J. Therm. Biol. 2018, 74, 241–248. [Google Scholar] [CrossRef]
- Kai, S.O.N.G.; Rongtang, L.I.U. The ecology of midday gerbil (Meriones meridianus Pallas). Acta Theriol. Sin. 2011, 4, 291. [Google Scholar]
- Lebedev, V.S.; Maslova, N.S.; Lisenkova, A.A.; Bannikova, A.A.; Sheftel, B.I.; Feoktystova, N.Y.; Qu, J.; Zhu, Y.; Fang, Y.; Sun, Y.; et al. Phylogeographic pattern and Pleistocene range reconstruction in the long-tailed hamster Cricetulus longicaudatus (Rodentia, Cricetidae) support its Tibetan origin. Mammal Res. 2021, 66, 635–648. [Google Scholar] [CrossRef]
- Giraudoux, P.; Quéré, J.P.; Delattre, P.; Bao, G.; Wang, X.; Shi, D.; Vuitton, D.; Craig, P.S. Distribution of small mammals along a deforestation gradient in southern Gansu, central China. Acta Theriol. 1998, 43, 349–362. [Google Scholar] [CrossRef]
- Gordon, C.J. Thermal physiology of laboratory mice: Defining thermoneutrality. J. Therm. Biol. 2012, 37, 654–685. [Google Scholar] [CrossRef]
- Liu, W.; Wan, X.; Zhong, W. Population dynamics of the Mongolian gerbils: Seasonal patterns and interactions among density, reproduction and climate. J. Arid Environ. 2007, 68, 383–397. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Cao, J.; Meng, X.L.; Li, Y.B. Seasonal variations in metabolism and thermoregulation in the striped hamster (Cricetulus barabensis). J. Therm. Biol. 2010, 35, 52–57. [Google Scholar] [CrossRef]
- Zhan, X.M.; Wang, D.H. Energy metabolism and thermoregulation of the desert hamster (Phodopus roborovskii) in hunshandake desert of inner mongolia, China. Acta Theriol. Sin. 2004, 24, 152–159. [Google Scholar]
- Guo, Z.C.; Wang, T.; Liu, S.L.; Kang, W.P.; Chen, X.; Feng, K.; Zhang, X.; Zhi, Y. Biomass and vegetation coverage survey in the Mu Us sandy land-based on unmanned aerial vehicle RGB images. Int. J. Appl. Earth Obs. Geoinf. 2021, 94, 102239. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Huang, W. Weakened East Asian summer monsoon triggers increased precipitation in Northwest China. Sci. China Earth Sci. 2021, 64, 835–837. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Chen, Z.; Xiong, H.; Lian, L. Why does precipitation in northwest China show a significant increasing trend from 1960 to 2010? Atmos. Res. 2016, 167, 275–284. [Google Scholar] [CrossRef]
- Yang, P.; Xia, J.; Zhang, Y.; Hong, S. Temporal and spatial variations of precipitation in Northwest China during 1960–2013. Atmos. Res. 2017, 183, 283–295. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, X.; Ma, L.; Wang, H.; Huang, Q.; Leng, G.; Meng, E.; Guo, Y. Quantitative contribution of climate change and human activities to vegetation cover variations based on GA-SVM model. J. Hydrol. 2020, 584, 124687. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q.; Yan, Y.; Zhang, X.; Niu, J.; Svenning, J.C. Ecological restoration is the dominant driver of the recent reversal of desertification in the Mu Us Desert (China). J. Clean. Prod. 2020, 268, 122241. [Google Scholar] [CrossRef]
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World. In Rodentia; Johns Hopkins University Press: Baltimore, MD, USA, 2005. [Google Scholar]
- Liu, W.; Deng, K. Population Dynamics of Wild Mongolian Gerbils: Quadratic Temperature Effects on Survival and Density-Dependent Effects on Recruitment. Diversity 2022, 14, 586. [Google Scholar] [CrossRef]
- Scheibler, E.; Wollnik, F.; Brodbeck, D.; Hummel, E.; Yuan, S.; Zhang, F.S.; Zhang, X.D.; Fu, H.P.; Wu, X.D. Species composition and interspecific behavior affects activity pattern of free-living desert hamsters in the Alashan Desert. J. Mammal. 2013, 94, 448–458. [Google Scholar] [CrossRef]
- Commission, I.T. Glossary of terms for thermal physiology. Jpn. J. Physiol. 2001, 51, 245–280. [Google Scholar]
- Guo, Y.Y.; Chi, Q.S.; Zhang, X.Y.; Liu, W.; Hao, S.Y.; Wang, D.H. Brown adipose tissue plays thermoregulatory role within the thermoneutral zone in Mongolian gerbils (Meriones unguiculatus). J. Therm. Biol. 2019, 81, 137–145. [Google Scholar] [CrossRef]


| Study Site | Scientific Name | Plant Height (m) | Plant Coverage (%) |
|---|---|---|---|
| Site 1 | Salix cheilophila | 1.904 ± 0.256 | 40.44 |
| Artemisia ordosica | 0.7 | 0.38 | |
| Site 2 | Caragana intermedia | 1.625 ± 0.178 | 22.17 |
| Artemisia ordosica | 0.535 ± 0.176 | 11.36 | |
| Cynanchum thesioides | - | - | |
| Site 3 | Artemisia ordosica | 0.610 ± 0.188 | 26.40 |
| Caragana intermedia | 1.165 ± 0.191 | 7.85 | |
| Amygdalus pedunculata Pall. | 0.700 ± 0.100 | 2.42 | |
| Cynanchum thesioides | - | - | |
| Psammochloa villosa | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.-Y.; Wang, S.-S.; Wang, X.; Liu, W.; Xu, D. Rodents Inhabiting the Southeastern Mu Us Desert May Not Have Experienced Prolonged Heat Stress in Summer 2022. Animals 2023, 13, 2114. https://doi.org/10.3390/ani13132114
Guo Y-Y, Wang S-S, Wang X, Liu W, Xu D. Rodents Inhabiting the Southeastern Mu Us Desert May Not Have Experienced Prolonged Heat Stress in Summer 2022. Animals. 2023; 13(13):2114. https://doi.org/10.3390/ani13132114
Chicago/Turabian StyleGuo, Yang-Yang, Shan-Shan Wang, Xinyue Wang, Wei Liu, and Deli Xu. 2023. "Rodents Inhabiting the Southeastern Mu Us Desert May Not Have Experienced Prolonged Heat Stress in Summer 2022" Animals 13, no. 13: 2114. https://doi.org/10.3390/ani13132114
APA StyleGuo, Y.-Y., Wang, S.-S., Wang, X., Liu, W., & Xu, D. (2023). Rodents Inhabiting the Southeastern Mu Us Desert May Not Have Experienced Prolonged Heat Stress in Summer 2022. Animals, 13(13), 2114. https://doi.org/10.3390/ani13132114







