Measuring Chronic Stress in Broiler Chickens: Effects of Environmental Complexity and Stocking Density on Immunoglobulin-A Levels
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Environmental Complexity
2.3. Stocking Density
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Day 28 Plasma IgA Concentrations
3.2. Day 48 Plasma IgA Concentrations
3.3. Day 48 Fecal IgA Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stress. Available online: https://www.who.int/news-room/questions-and-answers/item/stress (accessed on 11 December 2022).
- Stress Effects on the Body. Available online: https://www.apa.org/topics/stress/body (accessed on 11 December 2022).
- Dixon, L.M.; Brocklehurst, S.; Sandilands, V.; Bateson, M.; Tolkamp, B.J.; D’Eath, R.B. Measuring Motivation for Appetitive Behaviour: Food-Restricted Broiler Breeder Chickens Cross a Water Barrier to Forage in an Area of Wood Shavings without Food. PLoS ONE 2014, 9, e102322. [Google Scholar] [CrossRef] [PubMed]
- Monckton, V.; van Staaveren, N.; Harlander-Matauschek, A. Broiler Chicks’ Motivation for Different Wood Beddings and Amounts of Soiling. Animals 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, B.B.; Boecker, I.; Kwon, I.Y.; Jeyachanthiran, L.; McBride, P.; Harlander-Matauschek, A. How Does the Presence of Excreta Affect the Behavior of Laying Hens on Scratch Pads? Poult. Sci. 2018, 97, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Shields, S.J.; Garner, J.P.; Mench, J.A. Dustbathing by Broiler Chickens: A Comparison of Preference for Four Different Substrates. Appl. Anim. Behav. Sci. 2004, 87, 69–82. [Google Scholar] [CrossRef]
- Vestergaard, K. Dust-Bathing in the Domestic Fowl—Diurnal Rhythm and Dust Deprivation. Appl. Anim. Ethol. 1982, 8, 487–495. [Google Scholar] [CrossRef]
- Newberry, R.C.; Estevez, I.; Keeling, L.J. Group Size and Perching Behaviour in Young Domestic Fowl. Appl. Anim. Behav. Sci. 2001, 73, 117–129. [Google Scholar] [CrossRef]
- Arnould, C.; Faure, J.M. Use of Pen Space and Activity of Broiler Chickens Reared at Two Different Densities. Appl. Anim. Behav. Sci. 2004, 87, 155–170. [Google Scholar] [CrossRef]
- Dozier, W.A.; Thaxton, J.P.; Branton, S.L.; Morgan, G.W.; Miles, D.M.; Roush, W.B.; Lott, B.D.; Vizzier-Thaxton, Y. Stocking Density Effects on Growth Performance and Processing Yields of Heavy Broilers. Poult. Sci. 2005, 84, 1332–1338. [Google Scholar] [CrossRef]
- Sørensen, P.; Su, G.; Kestin, S.C. Effects of Age and Stocking Density on Leg Weakness in Broiler Chickens. Poult. Sci. 2000, 79, 864–870. [Google Scholar] [CrossRef]
- Blokhuis, H.J. Rest in Poultry. Appl. Anim. Behav. Sci. 1984, 12, 289–303. [Google Scholar] [CrossRef]
- Buijs, S.; Keeling, L.; Vangestel, C.; Baert, J.; Vangeyte, J.; Tuyttens, F. Resting or Hiding? Why Broiler Chickens Stay near Walls and How Density Affects This. Appl. Anim. Behav. Sci. 2010, 124, 97–103. [Google Scholar] [CrossRef]
- Cornetto, T.; Estevez, I.; Douglass, L.W. Using Artificial Cover to Reduce Aggression and Disturbances in Domestic Fowl. Appl. Anim. Behav. Sci. 2002, 75, 325–336. [Google Scholar] [CrossRef]
- Estevez, I. Density Allowances for Broilers: Where to Set the Limits? Poult. Sci. 2007, 86, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Febrer, K.; Jones, T.A.; Donnelly, C.A.; Dawkins, M.S. Forced to Crowd or Choosing to Cluster? Spatial Distribution Indicates Social Attraction in Broiler Chickens. Anim. Behav. 2006, 72, 1291–1300. [Google Scholar] [CrossRef]
- Thomas, D.G.; Son, J.-H.; Ravindran, V.; Thomas, D.V. The Effect of Stocking Density on the Behaviour of Broiler Chickens. Korean J. Poult. Sci. 2011, 38, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Quinteiro-Filho, W.M.; Calefi, A.S.; Cruz, D.S.G.; Aloia, T.P.A.; Zager, A.; Astolfi-Ferreira, C.S.; Piantino Ferreira, J.A.; Sharif, S.; Palermo-Neto, J. Heat Stress Decreases Expression of the Cytokines, Avian β-Defensins 4 and 6 and Toll-like Receptor 2 in Broiler Chickens Infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017, 186, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bracke, M.B.M.; Hopster, H. Assessing the Importance of Natural Behavior for Animal Welfare. J. Agric. Environ. Ethics 2006, 19, 77–89. [Google Scholar] [CrossRef]
- Riber, A.B.; van de Weerd, H.A.; de Jong, I.C.; Steenfeldt, S. Review of Environmental Enrichment for Broiler Chickens. Poult. Sci. 2018, 97, 378–396. [Google Scholar] [CrossRef]
- Anderson, M.G.; Campbell, A.M.; Crump, A.; Arnott, G.; Jacobs, L. Environmental Complexity Positively Impacts Affective States of Broiler Chickens. Sci. Rep. 2021, 11, 16966. [Google Scholar] [CrossRef]
- Anderson, M.G.; Campbell, A.M.; Crump, A.; Arnott, G.; Newberry, R.C.; Jacobs, L. Effect of Environmental Complexity and Stocking Density on Fear and Anxiety in Broiler Chickens. Animals 2021, 11, 2383. [Google Scholar] [CrossRef]
- Hall, A. The Effect of Stocking Density on the Welfare and Behavior of Broiler Chickens Reared Commercially. Animal Welfare 2001, 10, 23–40. [Google Scholar] [CrossRef]
- Dozier, W.A.; Thaxton, J.P.; Purswell, J.L.; Olanrewaju, H.A.; Branton, S.L.; Roush, W. Stocking Density Effects on Male Broilers Grown to 1.8 Kilograms of Body Weight. Poult. Sci. 2006, 85, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Najafi, P.; Zulkifli, I.; Amat Jajuli, N.; Farjam, A.S.; Ramiah, S.K.; Amir, A.A.; O’Reily, E.; Eckersall, D. Environmental Temperature and Stocking Density Effects on Acute Phase Proteins, Heat Shock Protein 70, Circulating Corticosterone and Performance in Broiler Chickens. Int. J. Biometeorol. 2015, 59, 1577–1583. [Google Scholar] [CrossRef]
- Skomorucha, I.; Muchacka, R.; Sosnówka-Czajka, E.; Herbut, E. Response of Broiler Chickens from Three Genetic Groups to Different Stocking Densities. Ann. Anim. Sci. 2009, 9, 175–184. [Google Scholar]
- Moberg, G.; Mench, J. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Mendl, M.; Burman, O.H.P.; Paul, E.S. An Integrative and Functional Framework for the Study of Animal Emotion and Mood. Proc. R. Soc. B Biol. Sci. 2010, 277, 2895–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otten, W.; Puppe, B.; Kanitz, E.; Schön, P.C.; Stabenow, B. Physiological and Behavioral Effects of Different Success during Social Confrontation in Pigs with Prior Dominance Experience. Physiol. Behav. 2002, 75, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kannan, G.; Mench, J.A. Influence of Different Handling Methods and Crating Periods on Plasma Corticosterone Concentrations in Broilers. Br. Poult. Sci. 1996, 37, 21–31. [Google Scholar] [CrossRef]
- Vosmerova, P.; Chloupek, J.; Bedanova, I.; Chloupek, P.; Kruzikova, K.; Blahova, J.; Vecerek, V. Changes in Selected Biochemical Indices Related to Transport of Broilers to Slaughterhouse under Different Ambient Temperatures. Poult. Sci. 2010, 89, 2719–2725. [Google Scholar] [CrossRef]
- Romero, L.M.; Reed, J.M. Collecting Baseline Corticosterone Samples in the Field: Is under 3 Min Good Enough? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 73–79. [Google Scholar] [CrossRef]
- Lentfer, T.L.; Pendl, H.; Gebhardt-Henrich, S.G.; Fröhlich, E.K.F.; Von Borell, E. H/L Ratio as a Measurement of Stress in Laying Hens—Methodology and Reliability. Br. Poult. Sci. 2015, 56, 157–163. [Google Scholar] [CrossRef]
- Skwarska, J. Variation of Heterophil-to-Lymphocyte Ratio in the Great Tit Parus Major—A Review. Acta Ornithol. 2019, 53, 103. [Google Scholar] [CrossRef]
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011, 4, 603. [Google Scholar] [CrossRef] [Green Version]
- Staley, M.; Conners, M.G.; Hall, K.; Miller, L.J. Linking Stress and Immunity: Immunoglobulin A as a Non-Invasive Physiological Biomarker in Animal Welfare Studies. Horm. Behav. 2018, 102, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.W.; Ding, J.L. The Unexplored Roles of Human Serum IgA. DNA Cell Biol. 2014, 33, 823–829. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Saruta, J.; To, M.; Yamamoto, Y.; Kimura, K.; Tsukinoki, K. Voluntary Exercise Increases IgA Concentration and Polymeric Ig Receptor Expression in the Rat Submandibular Gland. Biosci. Biotechnol. Biochem. 2016, 80, 2490–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monk, J.E.; Belson, S.; Lee, C. Pharmacologically-Induced Stress Has Minimal Impact on Judgement and Attention Biases in Sheep. Sci. Rep. 2019, 9, 11446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, A.M.; Johnson, A.M.; Persia, M.E.; Jacobs, L. Effects of Housing System on Anxiety, Chronic Stress, Fear, and Immune Function in Bovan Brown Laying Hens. Animals 2022, 12, 1803. [Google Scholar] [CrossRef]
- Ablimit, A.; Kühnel, H.; Strasser, A.; Upur, H. Abnormal Savda Syndrome: Long-Term Consequences of Emotional and Physical Stress on Endocrine and Immune Activities in an Animal Model. Chin. J. Integr. Med. 2013, 19, 603–609. [Google Scholar] [CrossRef]
- Guhad, F.A.; Hau, J. Salivary IgA as a Marker of Social Stress in Rats. Neurosci. Lett. 1996, 216, 137–140. [Google Scholar] [CrossRef]
- Jarillo-Luna, A.; Rivera-Aguilar, V.; Garfias, H.R.; Lara-Padilla, E.; Kormanovsky, A.; Campos-Rodríguez, R. Effect of Repeated Restraint Stress on the Levels of Intestinal IgA in Mice. Psychoneuroendocrinology 2007, 32, 681–692. [Google Scholar] [CrossRef]
- Rammal, H.; Bouayed, J.; Falla, J.; Boujedaini, N.; Soulimani, R. The Impact of High Anxiety Level on Cellular and Humoral Immunity in Mice. Neuroimmunomodulation 2009, 17, 1–8. [Google Scholar] [CrossRef]
- Eriksson, E.; Royo, F.; Lyberg, K.; Carlsson, H.E.; Hau, J. Effect of Metabolic Cage Housing on Immunoglobulin A and Corticosterone Excretion in Faeces and Urine of Young Male Rats. Exp. Physiol. 2004, 89, 427–433. [Google Scholar] [CrossRef]
- Royo, F.; Lyberg, K.; Abelson, K.; Carlsson, H.E.; Hau, J. Effect of Repeated Confined Single Housing of Young Pigs on Faecal Excretion of Cortisol and IgA. Scandanavian J. Lab. Anim. Sci. 2005, 31, 33–37. [Google Scholar]
- Bundgaard, C.; Kalliokoski, O.; Abelson, K.; Hau, J. Acclimatization of Mice to Different Cage Types and Social Groupings with Respect to Fecal Secretion of IgA and Corticosterone Metabolites. In Vivo 2012, 26, 883–888. [Google Scholar] [PubMed]
- Kimura, F.; Aizawa, K.; Tanabe, K.; Shimizu, K.; Kon, M.; Lee, H.; Akimoto, T.; Akama, T.; Kono, I.; Kono, I. A Rat Model of Saliva Secretory Immunoglobulin: A Suppression Caused by Intense Exercise. Scand. J. Med. Sci. Sport. 2007, 18, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Victoria, M.; Drago-Serrano, M.E.; Reyna-Garfias, H.; Viloria, M.; Lara-Padilla, E.; Resendiz-Albor, A.A.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Campos-Rodriguez, R. Effects on Secretory IgA Levels in Small Intestine of Mice That Underwent Moderate Exercise Training Followed by a Bout of Strenuous Swimming Exercise. Brain Behav. Immun. 2012, 26, 1300–1309. [Google Scholar] [CrossRef]
- Souza, C.M.; Miotto, B.A.; Bonin, C.P.; Camargo, M.M. Lower Serum IgA Levels in Horses Kept under Intensive Sanitary Management and Physical Training. Animal 2010, 4, 2080–2083. [Google Scholar] [CrossRef]
- Li, D.; Tong, Q.; Shi, Z.; Li, H.; Wang, Y.; Li, B.; Yan, G.; Chen, H.; Zheng, W. Effects of Chronic Heat Stress and Ammonia Concentration on Blood Parameters of Laying Hens. Poult. Sci. 2020, 99, 3784–3792. [Google Scholar] [CrossRef]
- Reiter, K.; Bessei, W. Effect of Locomotor Activity on Bone Development and Leg Disorders in Broilers. Arch. Fuer Gefluegelkunde 2000, 64, 204–206. [Google Scholar]
- Bizeray, D.; Estevez, I.; Leterrier, C.; Faure, J.M. Effects of Increasing Environmental Complexity on the Physical Activity of Broiler Chickens. Appl. Anim. Behav. Sci. 2002, 79, 27–41. [Google Scholar] [CrossRef]
- Nazar, F.N.; Marin, R.H. Chronic Stress and Environmental Enrichment as Opposite Factors Affecting the Immune Response in Japanese Quail (Coturnix Coturnix Japonica). Stress 2011, 14, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Perea, A.T.; Galindo Maldonado, F.; Antonio, J.; López, Q. Effect of Environmental Enrichment on the Behavior, Production Parameters and Immune Response in Broilers. Vet. México 2002, 33, 89–100. [Google Scholar]
- Bailie, C.L.; Ijichi, C.; O’Connell, N.E. Effects of Stocking Density and String Provision on Welfare-Related Measures in Commercial Broiler Chickens in Windowed Houses. Poult. Sci. 2018, 97, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Ventura, B.A.; Siewerdt, F.; Estevez, I. Effects of Barrier Perches and Density on Broiler Leg Health, Fear, and Performance. Poult. Sci. 2010, 89, 1574–1583. [Google Scholar] [CrossRef]
- Sanotra, G.S.; Lund, J.D.; Vestergaard, K.S. Influence of Light-Dark Schedules and Stocking Density on Behaviour, Risk of Leg Problems and Occurrence of Chronic Fear in Broilers. Br. Poult. Sci. 2002, 43, 344–354. [Google Scholar] [CrossRef]
- Jones, T.A.; Donnelly, C.A.; Stamp Dawkins, M. Environmental and Management Factors Affecting the Welfare of Chickens on Commercial Farms in the United Kingdom and Denmark Stocked at Five Densities. Poult. Sci. 2005, 84, 1155–1165. [Google Scholar] [CrossRef]
- Malleau, A.E.; Duncan, I.J.H.; Widowski, T.M.; Atkinson, J.L. The Importance of Rest in Young Domestic Fowl. Appl. Anim. Behav. Sci. 2007, 106, 52–69. [Google Scholar] [CrossRef]
- USDA. Petition-NCC-Spreadsheet Showing Average Slaughter Age for Chickens. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-07/Petition-NCC-Slaughter-Age-Chickens.pdf (accessed on 1 June 2023).
- USDA. Poultry 2010: Structure of the U.S. Poultry Industry. Available online: https://www.aphis.usda.gov/animal_health/nahms/poultry/downloads/poultry10/Poultry10_dr_Structure_1.pdf (accessed on 1 June 2023).
- Peters, I.R.; Calvert, E.L.; Hall, E.J.; Day, M.J. Measurement of Immunoglobulin Concentrations in the Feces of Healthy Dogs. Clin. Vaccine Immunol. 2004, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- Ricci, F.G.; Terkelli, L.R.; Venancio, E.J.; Justino, L.; dos Santos, B.Q.; Baptista, A.A.S.; Oba, A.; de Oliveira Souza, B.D.; Bracarense, A.P.F.R.L.; Hirooka, E.Y.; et al. Tryptophan Attenuates the Effects of OTA on Intestinal Morphology and Local IgA/IgY Production in Broiler Chicks. Toxins 2020, 13, 5. [Google Scholar] [CrossRef]
- Walker, J.; Waran, N.; Phillips, C. The Effect of Conspecific Removal on the Behavior and Physiology of Pair-Housed Shelter Dogs. Appl. Anim. Behav. Sci. 2014, 158, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Kikkawa, A.; Uchida, Y.; Suwa, Y.; Taguchi, K. A Novel Method for Estimating the Adaptive Ability of Guide Dogs Using Salivary SIgA. J. Vet. Med. Sci. 2005, 67, 707–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cengiz, Ö.; Köksal, B.H.; Tatlı, O.; Sevim, Ö.; Ahsan, U.; Üner, A.G.; Ulutaş, P.A.; Beyaz, D.; Büyükyörük, S.; Yakan, A.; et al. Effect of Dietary Probiotic and High Stocking Density on the Performance, Carcass Yield, Gut Microflora, and Stress Indicators of Broilers. Poult. Sci. 2015, 94, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Gourkow, N.; Phillips, C.J.C. Effect of Cognitive Enrichment on Behavior, Mucosal Immunity and Upper Respiratory Disease of Shelter Cats Rated as Frustrated on Arrival. Prev. Vet. Med. 2016, 131, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourkow, N.; Phillips, C.J.C. Effect of Interactions with Humans on Behaviour, Mucosal Immunity and Upper Respiratory Disease of Shelter Cats Rated as Contented on Arrival. Prev. Vet. Med. 2015, 121, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Campderrich, I.; Nazar, F.N.; Wichman, A.; Marin, R.H.; Estevez, I.; Keeling, L.J. Environmental Complexity: A Buffer against Stress in the Domestic Chick. PLoS ONE 2019, 14, e0210270. [Google Scholar] [CrossRef] [Green Version]
- Jarillo-Luna, R.A.; Rivera-Aguilar, V.; Pacheco-Yépez, J.; Godínez-Victoria, M.; Oros-Pantoja, R.; Miliar-García, A.; Campos-Rodríguez, R. Nasal IgA Secretion in a Murine Model of Acute Stress. The Possible Role of Catecholamines. J. Neuroimmunol. 2015, 278, 223–231. [Google Scholar] [CrossRef]
- Reyna-Garfias, H.; Miliar, A.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Pacheco-Yepez, J.; Baeza, I.; Campos-Rodríguez, R. Repeated Restraint Stress Increases IgA Concentration in Rat Small Intestine. Brain Behav. Immun. 2010, 24, 110–118. [Google Scholar] [CrossRef]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B Cells Responses and Cytokine Production Are Regulated by Their Immune Microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Sanders, V.M. The Beta2-Adrenergic Receptor on T and B Lymphocytes: Do We Understand It Yet? Brain Behav. Immun. 2012, 26, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Lorton, D.; Bellinger, D. Molecular Mechanisms Underlying β-Adrenergic Receptor-Mediated Cross-Talk between Sympathetic Neurons and Immune Cells. Int. J. Mol. Sci. 2015, 16, 5635–5665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Shu, G.; Liu, Y.; Qin, P.; Zheng, Y.; Tian, Y.; Zhao, X.; Du, X. Farm Environmental Enrichments Improve the Welfare of Layer Chicks and Pullets: A Comprehensive Review. Animals 2022, 12, 2610. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.; Blatchford, R.A.; de Jong, I.C.; Erasmus, M.A.; Levengood, M.; Newberry, R.C.; Regmi, P.; Riber, A.B.; Weimer, S.L. Enhancing Their Quality of Life: Environmental Enrichment for Poultry. Poult. Sci. 2023, 102, 102233. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Rausch, Q.; Vandenberg, B.; Mason, G. Hens with Benefits: Can Environmental Enrichment Make Chickens More Resilient to Stress? Physiol. Behav. 2020, 226, 113077. [Google Scholar] [CrossRef] [PubMed]
- Altan, O.; Seremet, C.; Bayraktar, O. The Effects of Early Environmental Enrichment on Performance, Fear and Physiological Responses to Acute Stress of Broiler. Arch. Für Geflügelkunde 2013, 1, 23–28. [Google Scholar]
- Kestin, S.C.; Gordon, S.; Su, G.; Sørensen, P. Relationships in Broiler Chickens between Lameness, Liveweight, Growth Rate and Age. Vet. Rec. 2001, 148, 195–197. [Google Scholar] [CrossRef]
- Beloor, J.; Kang, H.K.; Kim, Y.J.; Subramani, V.K.; Jang, I.S.; Sohn, S.H.; Moon, Y.S. The Effect of Stocking Density on Stress Related Genes and Telomeric Length in Broiler Chickens. Asian-Australas. J. Anim. Sci. 2010, 23, 437–443. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Kalogeraki, E.; Goliomytis, M.; Charismiadou, M.A.; Triantaphyllopoulos, K.; Ayoutanti, A.; Niforou, K.; Hager-Theodorides, A.L.; Deligeorgis, S.G. Impact of Stocking Density on Broiler Growth Performance, Meat Characteristics, Behavioural Components and Indicators of Physiological and Oxidative Stress. Br. Poult. Sci. 2012, 53, 721–730. [Google Scholar] [CrossRef]
- Lara-Padilla, E.; Godínez-Victoria, M.; Drago-Serrano, M.E.; Reyna-Garfias, H.; Arciniega-Martínez, I.M.; Abarca-Rojano, E.; Cruz-Hernández, T.R.; Campos-Rodríguez, R. Intermittent Fasting Modulates IgA Levels in the Small Intestine under Intense Stress: A Mouse Model. J. Neuroimmunol. 2015, 285, 22–30. [Google Scholar] [CrossRef]
- Oros-Pantoja, R.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Sánchez-Torres, L.E.; Godinez-Victoria, M.; Campos-Rodríguez, R. Effects of Restraint Stress on NALT Structure and Nasal IgA Levels. Immunol. Lett. 2011, 135, 78–87. [Google Scholar] [CrossRef]
- James, S.P. Mucosal Immunity. In Encyclopedia of Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1780–1786. [Google Scholar]
- Yang, Y.; Cong, W.; Liu, J.; Zhao, M.; Xu, P.; Han, W.; Wang, D.; Zhao, R. Constant Light in Early Life Induces Fear-Related Behavior in Chickens with Suppressed Melatonin Secretion and Disrupted Hippocampal Expression of Clock- and BDNF-Associated Genes. J. Anim. Sci. Biotechnol. 2022, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Karaffová, V.; Bobíková, K.; Husáková, E.; Levkut, M.; Herich, R.; Revajová, V.; Levkutová, M.; Levkut, M. Interaction of TGF-Β4 and IL-17 with IgA Secretion in the Intestine of Chickens Fed with E. Faecium AL41 and Challenged with S. Enteritidis. Res. Vet. Sci. 2015, 100, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Robak, O.H.; Heimesaat, M.M.; Kruglov, A.A.; Prepens, S.; Ninnemann, J.; Gutbier, B.; Reppe, K.; Hochrein, H.; Suter, M.; Kirschning, C.J.; et al. Antibiotic Treatment–Induced Secondary IgA Deficiency Enhances Susceptibility to Pseudomonas Aeruginosa Pneumonia. J. Clin. Investig. 2018, 128, 3535–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, V.; Millon, L.; Faucher, J.-F.; Bard, E.; Robinet, E.; Piarroux, R.; Vuitton, D.-A.; Meillet, D. Effects of a Short-Course of Amoxicillin/Clavulanic Acid on Systemic and Mucosal Immunity in Healthy Adult Humans. Int. Immunopharmacol. 2005, 5, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Nyangahu, D.D.; Plumlee, C.R.; Brown, B.P.; Feng, C.; Havyarimana, E.; Cohen, S.B.; Urdahl, K.B.; Jaspan, H.B. Antibiotic Treatment during Gestation Enhances Susceptibility to Mycobacterium Tuberculosis in Offspring. Microbiol. Spectr. 2022, 10, e02491-22. [Google Scholar] [CrossRef]
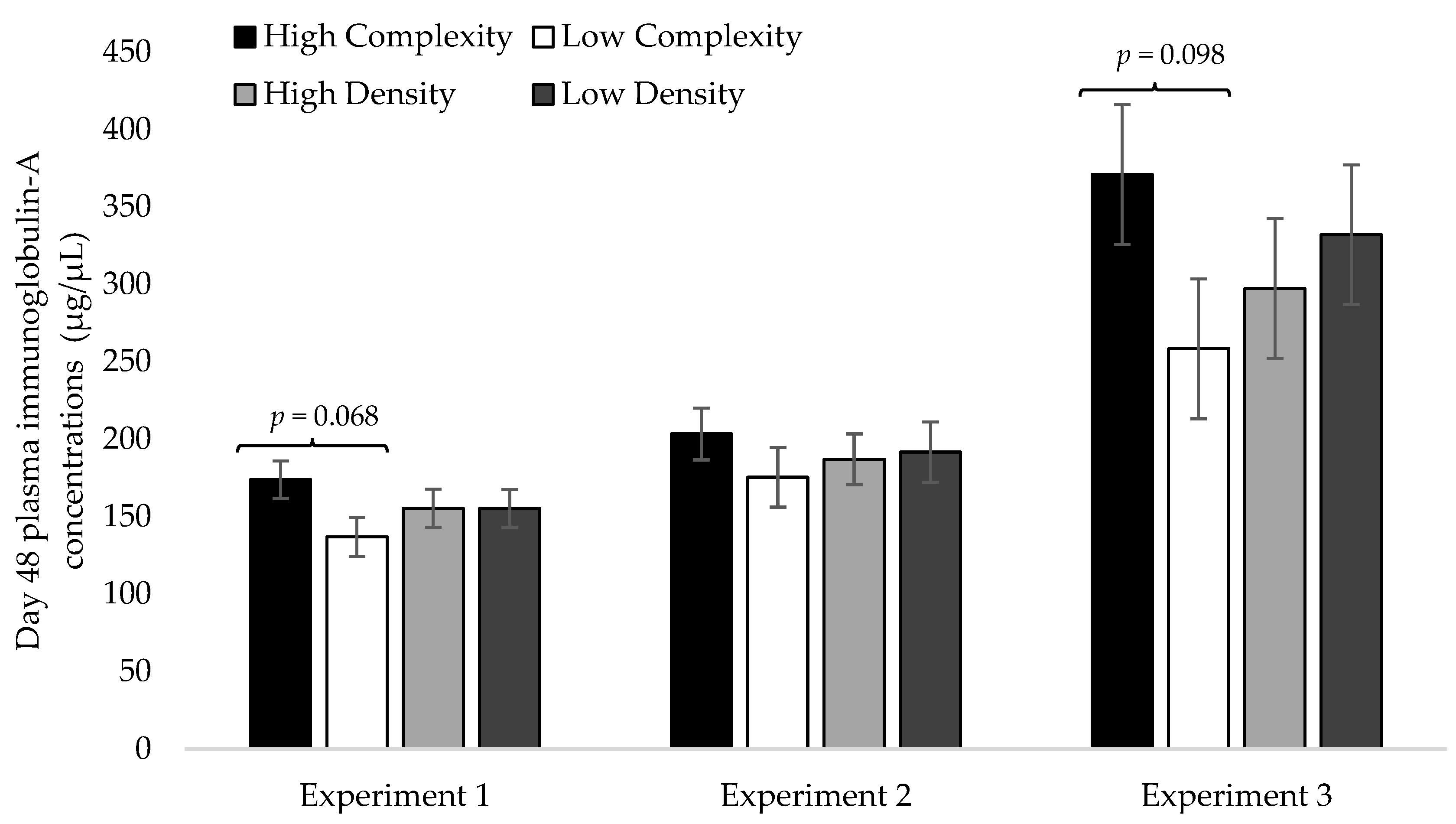
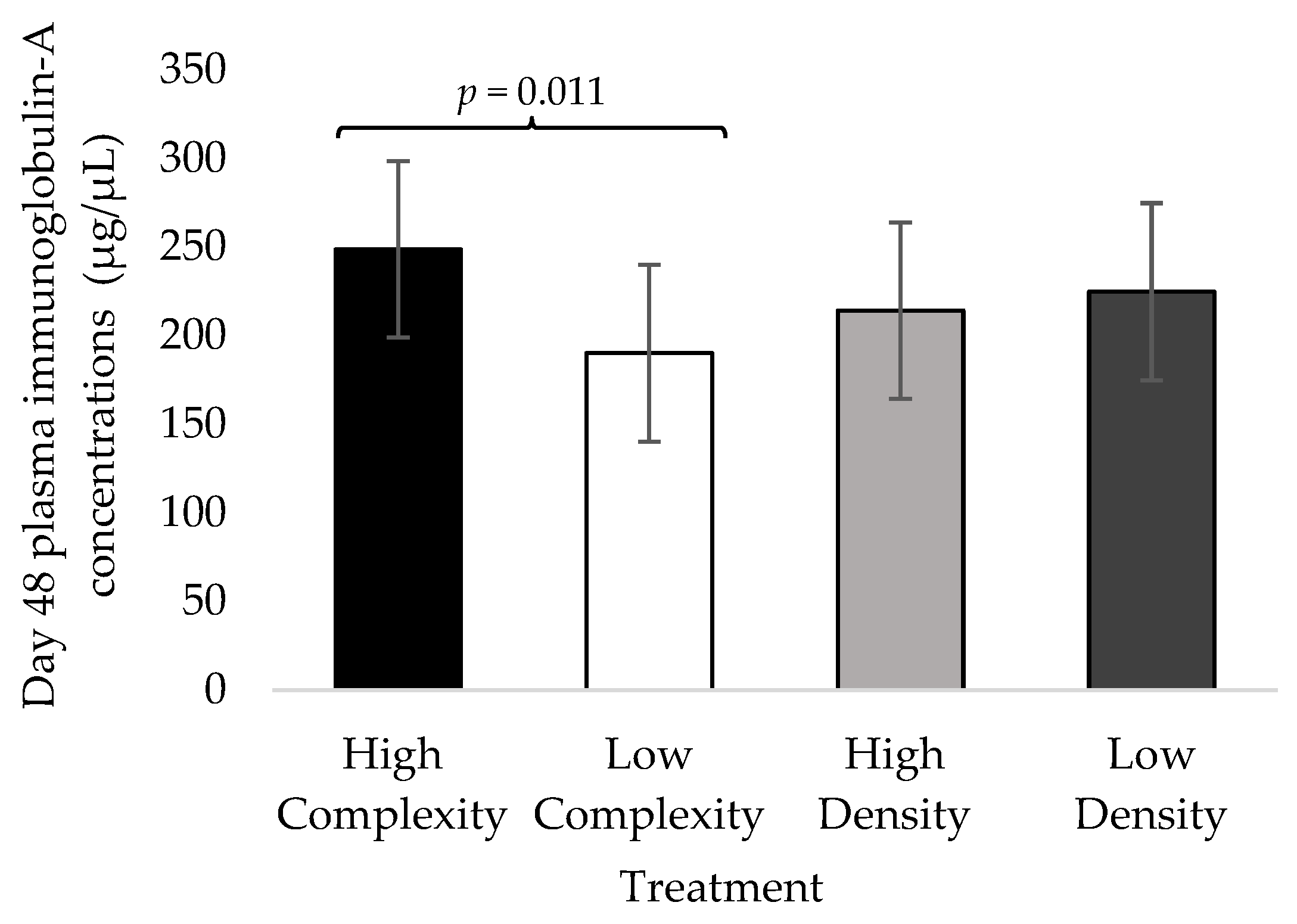

| Experiment | Day 50 Stocking Density (kg/m2) | |
|---|---|---|
| High-Density Pens | Low-Density Pens | |
| 1 | 42.1 | 23.8 |
| 2 | 42.6 | 23.3 |
| 3 | 42.1 | 22.1 |
| Plasma Immunoglobulin-A (µg/µL) on Day 28 of Age | ||||
|---|---|---|---|---|
| Treatment | Experiment 1 | Experiment 2 | Experiment 3 | Overall |
| High complexity (HC) | 139 ± 12 | 265 ± 31 | 213 ± 19 | 208 ± 29 |
| Low complexity (LC) | 156 ± 12 | 215 ± 31 | 219 ± 19 | 196 ± 29 |
| High density (HD) | 155 ± 12 | 246 ± 31 | 223 ± 19 | 207 ± 29 |
| Low density (LD) | 141 ± 12 | 234 ± 31 | 209 ± 19 | 197 ± 29 |
| HC/HD | 145 ± 18 | 327 ± 44 | 210 ± 28 | 231 ± 22 |
| HC/LD | 134 ± 18 | 204 ± 44 | 217 ± 28 | 184 ± 21 |
| LC/HD | 164 ± 18 | 165 ± 44 | 236 ± 28 | 176 ± 21 |
| LC/LD | 148 ± 18 | 264 ± 44 | 202 ± 28 | 208 ± 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, A.M.; Anderson, M.G.; Jacobs, L. Measuring Chronic Stress in Broiler Chickens: Effects of Environmental Complexity and Stocking Density on Immunoglobulin-A Levels. Animals 2023, 13, 2058. https://doi.org/10.3390/ani13132058
Campbell AM, Anderson MG, Jacobs L. Measuring Chronic Stress in Broiler Chickens: Effects of Environmental Complexity and Stocking Density on Immunoglobulin-A Levels. Animals. 2023; 13(13):2058. https://doi.org/10.3390/ani13132058
Chicago/Turabian StyleCampbell, Andrew M., Mallory G. Anderson, and Leonie Jacobs. 2023. "Measuring Chronic Stress in Broiler Chickens: Effects of Environmental Complexity and Stocking Density on Immunoglobulin-A Levels" Animals 13, no. 13: 2058. https://doi.org/10.3390/ani13132058
APA StyleCampbell, A. M., Anderson, M. G., & Jacobs, L. (2023). Measuring Chronic Stress in Broiler Chickens: Effects of Environmental Complexity and Stocking Density on Immunoglobulin-A Levels. Animals, 13(13), 2058. https://doi.org/10.3390/ani13132058





