Simple Summary
Brachycephalic dogs are affected by respiratory disorders related to abnormal anatomic conformation that can significantly affect their general health and quality of life. In this review, we address dermatological disorders in these breeds which are less recognized, but can also considerably impact welfare.
Abstract
Brachycephalic dogs are not only affected by brachycephalic obstructive airway syndrome (BOAS), but are also frequently referred to veterinary dermatologists for skin conditions, with English bulldogs and pugs particularly over-represented. Some skin diseases, such as skin fold dermatitis, are directly associated with the abnormal anatomic conformation of brachycephalic dogs, while for others, such as atopic dermatitis and viral pigmented plaques, there is an underlying genetic basis or a general predisposition. Anatomic alterations associated with brachycephaly, leading to fold formation of the skin and stenosis of the ear canal, together with primary immunodeficiencies described in some breeds, favor the development of pyoderma, Malassezia dermatitis, and otitis externa/media. In addition, the frequently neglected but often lifelong dermatological problems of brachycephalic dogs are an important consideration when discussing genetic and medical conditions affecting the welfare of those dogs. Here we review the current state of knowledge concerning dermatological problems in brachycephalic dogs and combine it with clinical experience in the management of these challenging disorders.
Keywords:
canine; BOAS; brachycephaly; congenital; skin folds; allergy; infectious diseases; immunologic disorders; otitis externa; ethical 1. Introduction
Brachycephalic dogs are very popular due to cultural and social influences, as well as their “babyface” appearance and personality traits that favor bonding and companionship with their owners [1,2]. Owners may be unaware of how seriously the welfare of these breeds can be compromised by abnormalities in anatomic conformation [3,4]. Extreme brachycephaly, i.e., foreshortening of the cranium, is associated with brachycephalic obstructive airway syndrome (BOAS), leading to stridor, stertor, dyspnoea, cyanosis, exercise intolerance, regurgitation, hyperthermia, and syncope. Non-respiratory problems, including spinal, dental, gastrointestinal, ophthalmological, dermatological, and cardiovascular disorders, as well as birthing difficulties, have also been recognized [4,5]. To mitigate these problems, several countries, including the Netherlands and Norway, have instigated legal breeding restrictions, while many professional veterinary organizations, such as the British Veterinary Association, the Australian Veterinary Association, the American Veterinary Medical Association, and the Federation of European Companion Animal Veterinary Association, have launched public education awareness initiatives and campaigns [3,4,5].
The prevalence of dermatological abnormalities in brachycephalic dogs ranges from 10% to almost 30%, depending on breed and geographic origin [6,7]. Genetic, autoimmune, and parasitic diseases, immune deficiencies, vasculitis, allergies, secondary infections, otitis externa and media, claw and anal sac diseases, skin folding, alopecia, and pruritus have all been recognized as problems in brachycephalic dog breeds [8,9,10]. Genetic aspects, skull conformation, pressure changes between the middle ear and nasopharynx, skin folding, environmental factors, and the microbiome composition may all contribute to the aetiopathogenesis of dermatological diseases in brachycephalic dogs [5,11].
Many of these skin conditions may become chronic and difficult to treat, as well as causing pain and pruritus, leading to abnormal behavior and thus negatively impacting quality of life [5,12].
In this evidence-based narrative review, we overview the dermatological diseases encountered in brachycephalic breeds of dogs, including; (i) disorders directly associated with brachycephaly that are likely to be improved if measures to prevent extreme brachycephaly are implemented, as well as (ii) disorders that are not directly linked to brachycephalic conformation. The electronic database “Web of Science” was searched using key words including “dog”, “brachycephaly/brachycephalic”, “welfare”, “genetics”, “predisposition”, “skin diseases”, “dermatologic/dermatological”, as well as individual dog breeds (e.g., pug) and individual diseases identified during the initial search (e.g., skin fold dermatitis, otitis externa, primary secretory otitis media, etc.). Selected articles were critically appraised and compared before the incorporation of relevant information in this review.
There is no definitive list of brachycephalic breeds because no uniform measure is used. Some authors use the cephalic index (CI), the ratio of the width of the skull compared with its length, while others use the craniofacial ratio or craniofacial angle [5]. In addition, the phenotypic variation within an individual breed can be very large, such that individual dogs within a “brachycephalic breed” may not be brachycephalic, while others in non-brachycephalic breeds may indeed be brachycephalic. Table 1 lists the most commonly described brachycephalic breeds of dogs [6,13], while Table 2 lists dermatological disorders reported in brachycephalic breeds.

Table 1.
The most common brachycephalic breeds in UK [6,13]. Breeds particularly associated with extreme brachycephaly are bolded.

Table 2.
Dermatological diseases of brachycephalic breeds.
2. Dermatological Diseases Directly Associated with Brachycephaly
2.1. Skin Fold Dermatitis
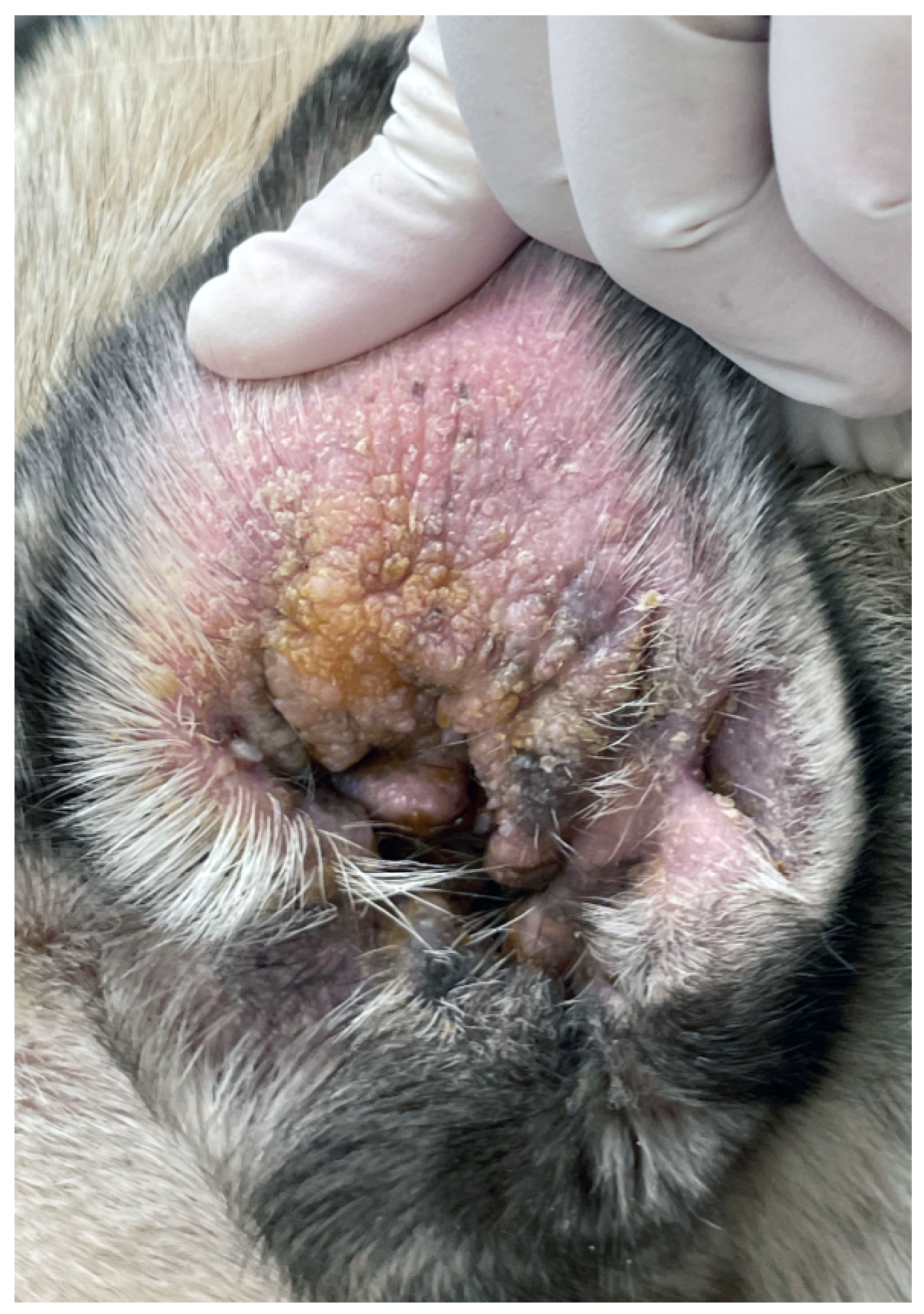
Skin fold dermatitis, or intertrigo, is a major problem in brachycephalic breeds, especially in British bulldogs, French bulldogs, Pugs, Pekingese, Boston Terriers, and Shar Peis [3,9,12,14,59,60,97,98,99]. A “big-data” study that searched the medical records of 905,553 dogs presented to veterinary clinics in the UK in 2016 for skin fold dermatitis identified 11,375 cases (1.26%). Compared to cross-breed dogs, British bulldogs (odds ratio [OR] 49.07, 95% CI [37.79–63.70]), French bulldogs (OR 25.92, 95% CI [19.62–34.26]), and Pugs (OR 16.27, 95% CI [12.20–21.69]) were predisposed [12]. Foreshortening of the skull results in the folding of excessive skin around the muzzle, eyes, and ears. The problem is exacerbated in Shar Peis by increased hyaluronic acid synthetase activity, which leads to more ground substance and mucin in the dermis and attracts water [115,116]. In addition to facial involvement, skin folds can occur in other locations, such as at the tail base in dogs with “corkscrew”, such as pugs and bulldogs [12,115]. This not only leads to secondary infections but also spine instability, nerve compression, and neurological deficiencies such as pain, ataxia, and incontinence [117].
Reduced air circulation and increased temperature, humidity, and debris within skin folds, together with intermittent friction and trauma, lead to commensal overgrowth and toxin production, followed by inflammation, maceration, and infection [14,115]. Affected areas exhibit erythema, hypotrichosis, alopecia, erosion/ulceration, crusting, lichenification, pigmentary changes, accumulation of keratosebaceous debris, and malodour (Figure 1).
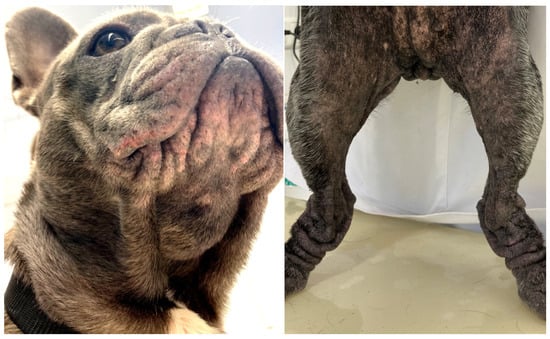
Figure 1.
French bulldog with severe skin fold dermatitis secondary to excessive skin folds on the face/muzzle that are a direct consequence of extreme brachycephalic conformation. In addition, this dog has chronic skin fold dermatitis associated with excessive folding on the distal limbs.
Involved areas can be pruritic and painful. Since the changes take place between skin folds, disease may not be noticed by owners [12,14,115]. If corrective surgery is not an option, lifelong treatment may be required with various topical preparations (e.g., antiseptics, glucocorticoids, antimicrobials, medical honey, or silver sulfadiazine). In severe cases where there is deep pyoderma, systemic antimicrobial therapy may be indicated and used according to culture and susceptibility test results [12,14,115].
2.2. Otitis Externa
Otitis externa (OE), inflammation of the ear canal and often the outer ear, is more prevalent in brachycephalic dogs, especially British bulldogs, Pugs, and Boxers, than in non-brachycephalic dogs [8,59,60,69]. Otitis externa is associated with predisposing factors (e.g., anatomic conformation, swimming), primary factors (direct induction of inflammation, e.g., parasites, food allergy, atopy, foreign body, growths, hormonal), secondary factors (e.g., secondary infection by commensals), and perpetuating factors (chronic changes of the ear canal, ear drum, or middle ear). Recently, it was shown that two brachycephalic breeds of dogs, French bulldogs and Pugs, have significantly narrower external ear canals than non-brachycephalic dogs of similar size [118]. The diameter of the horizonal ear canal was measured between its cranial and caudal bony walls on computer tomographic (CT) scan images and had a median value of 2.5 mm, 2.6 mm, and 5.0 mm in French bulldogs, Pugs and non-brachycephalic control dogs, respectively. In addition, on otoscopic examination, the tympanic membrane could only be visualized in 3.3% of brachycephalic dogs due to ear canal stenosis. Among the brachycephalic dogs examined in the study, no significant association was made between the presence of OE and ear canal diameter. However, their striking differences in ear canal diameter compared to non-brachycephalic dogs suggest that OE is likely a direct consequence of brachycephalic conformation, at least in some cases. Other predisposing factors to OE, including allergic skin diseases, are discussed in Section 3.4.2.
Clinical signs of OE include abnormal scratching of the pinnae, excoriations, head shaking, otic discharge, malodour, swelling, pain, formation of “hot-spots” (moist dermatitis), and othematoma (Figure 2) [14,119]. If left untreated, OE may further progress to involve the middle ear (otitis media), the internal ear (otitis interna), and extend into the central nervous system (CNS). Diagnosis is usually made by otoscopy and cytology, but advanced investigations (video-otoscopy, CT/MRI) may also be required [119,120].
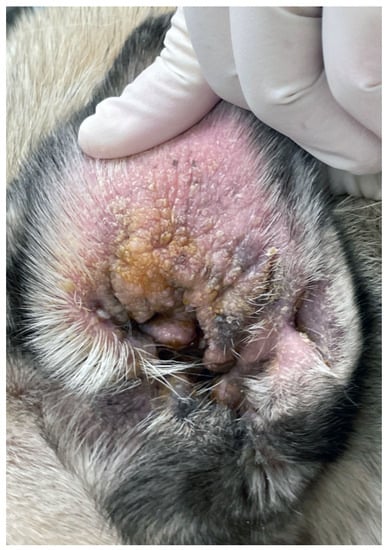
Figure 2.
Chronic otitis externa in a Pug showing erythema, lichenification, crusting and accumulation of keratosebaceous debris.
Treatment of OE typically includes a combination of topical ear drops, an ear cleaner, and, if not contra-indicated, an anti-inflammatory dose of oral glucocorticoids to reduce the stenosis, pruritus, and pain [14,121]. It is widely recognized by veterinary dermatologists that OE is painful, and consideration should be given to dispensing analgesics in all cases. Evidence-based recommendations are lacking and highlight the need for studies to evaluate and manage pain in dogs with OE. Flushing of the ear canal under anaesthesia can help to remove debris, toxins, biofilm, and exudates, but it also increases the efficacy of topical medications [14,119,120]. Biofilm can also be disrupted by topical silver nanoparticles, Tris-EDTA, and oral n-acetylcysteine or bromhexine [121,122,123]. Topical ear preparations may be ototoxic (e.g., macrolide, polypeptide, and aminoglycoside antibiotics, propylene glycol, ceruminolytics, and antiseptics) and must be used cautiously. Ototoxicity can lead to hearing loss, imbalance, or nausea by direct effect on the hair cells, stria vascularis, or cochlear nerve of the internal ear or via the formation of reactive oxygen species [124,125].
2.3. Caudal Occipital Malformation Syndrome/Chiari-like Malformation
This congenital and multifactorial inherited abnormality was first recognized and reported in Cavalier King Charles Spaniels (CKCS), with up to 95% of individuals being affected [36,37,38,39]. It is also recognized in other brachycephalic small-breed dogs [40,41,42,43]. The caudal occiput is too small-relative to the cerebellum, which may prolapse through the foramen magnum, leading to an abnormal flow of cerebrospinal fluid and the formation of a fluid-filled cyst (syrinx) within the spinal cord (syringomyelia). In chronic cases, spinal cord degeneration, including ventral horn cell or white matter damage, may complicate the situation [126,127]. Neuropathic pain results in “air-guitar” scratching, “pseudo-fly catching”, spontaneous vocalization and hopping, repeated body shaking, and severe rubbing of the face on the floor [127,128]. In more severely affected dogs, other signs may be present, including ataxia, head tilt, head tremor, facial nerve deficits, nystagmus, seizures, and scoliosis [43,127].
Magnetic resonance imaging (MRI) is the modality of choice to diagnose Chiari-like malformations, but computed tomography (CT) may also be used [129].
Medical treatment with non-steroidal inflammatory inhibitors, glucocorticoids, opioids, and anticonvulsants (gabapentin and pregabalin) helps to relieve pain, while omeprazole, acetazolamide, and methazolamide may be prescribed to reduce the formation of cerebrospinal fluid [130,131]. Alternative pain management options such as acupuncture and laser therapy are becoming more popular and may help as well, but progressive disease is common and surgical intervention may be required in severe cases [128,132]. For severe cases or dogs not responding to medical treatment, there is up to an 80% chance of clinical improvement following foramen magnum decompression and durotomy [43,133]. Duraplasty or craniotomy/cranioplasty in combination with tissue grafting/titanium prosthesis/titanium mesh/polymethylmethacrylate plate further improves the success rate [129,134]. However, despite surgical intervention, residual scratching is often reported [129,135].
2.4. Primary Secretory Otitis Media/Otitis Media with Effusion
Primary secretory otitis media, a sterile effusion of the middle ear, is mainly described in CKCS but also in other brachycephalic breeds such as Boxers and Boston Terriers [44,45,46,136]. In CKCS, around 40% may concurrently have Chiari-like malformations [45]. Auditory tube dysfunction, associated with craniofacial abnormalities, is implicated in disease pathogenesis [137,138].
Affected dogs present with head and neck pain, spontaneous vocalization, abnormal neck carriage, fatigue, aural pruritus, neurological signs (facial nerve paralysis, nystagmus, head tilt, ataxia, seizures), or reduced hearing, but may also be asymptomatic [44,45].
Diagnostic work-up includes video otoscopy (bulging, opaque pars flaccida of the tympanic membrane; can be normal in Boston Terriers), myringotomy (thick, mucoid discharge), and MRI or CT [45,136]. For an accurate diagnosis and prognosis, advanced imaging is preferred. Other conditions, such as CLM or cholesteatomas, the latter being commonly seen in French Bulldogs and Pugs, need to be excluded [136,139]. Although most of the time sterile, the secret of the middle ear should be cultured since bacterial involvement is possible [44].
Myringotomy of the pars tensa of the tympanic membrane with subsequent flushing of the bulla, systemic antibiotics, topical ear drops, and a short course of systemic anti-inflammatory glucocorticoids are common treatment steps [44,45,46]. Additional mucolytic N-acetylcysteine may be beneficial. Relapses, one to several months after treatment, are possible [45].
3. Skin Diseases in Brachycephalic Breeds That Are Not Directly Linked with Brachycephalic Conformation
3.1. Genetic Skin Diseases
3.1.1. Ichthyosis
Ichthyosis is a rare genetic disease affecting various breeds, including CKCS and American Bulldogs [29,30,31,32,33]. In the latter, a mutation in NIPAL-4 (nipa-like domain-containing 4, ICHTHYIN) is implicated in abnormal lipid metabolism in the epidermis [30]. In a multicentric study, approximately 35% of tested dogs were heterozygote carriers, and 5.4% were clinically affected. The disease was associated with an autosomal recessive insertion mutation 5781 bp upstream of NIPAL-4 [30]. Fine scaling throughout a rough hair coat, prominent erythematous to brown scales on the axillae and abdomen, together with wrinkling of the skin, are typical features described in affected American bulldogs. Secondary Malassezia dermatitis/overgrowth, pododermatitis, and otitis externa are common sequela [30,140].
In CKCS, the condition is caused by a mutation in FAM83H (family with sequence similarity 83, member H), which is yet to be further characterized [140]. In CKCS, a roughened, scaly, and curly haircoat, together with a hyperpigmented abdomen, footpad hyperkeratosis, and nail abnormalities (nail dystrophy, onychomadesis), become apparent. Affected dogs also have keratoconjunctivitis sicca and may become blind if this goes undetected [29].
In both breeds, the first clinical signs occur directly after birth [140]. A definitive diagnosis can be obtained via histopathology or genetic blood testing in cases of ichthyosis in American bulldogs [30,140]. Since ichthyosis is a congenital disease, only symptomatic treatment, including treatment of secondary infections, regular combing, mild shampoo treatment, systemic and topical fatty acids, as well as systemic retinoids (isotretinoin, etretinate), can be employed [14,140]. Affected dogs should not be used for breeding.
3.1.2. Congenital Alopecia
Congenital alopecia is a rarely observed problem in various brachycephalic and other canine breeds, including the French bulldog, Lhasa Apso, and Chihuahua [14,15,16,17]. It typically occurs within weeks to months after birth and is associated with an x-linked, autosomal dominant, or autosomal recessive trait [14,15,24]. The disease phenotype ranges from hypotrichosis to alopecia, which may be localized or generalized [14,15]. Hair loss is typically well-demarcated, occurring on the head, ears, and ventrum [14,15]. Some residual hair, symmetrically arranged, can be observed on the dorsal head, distal limbs, tail, umbilical area, and around mucocutaneous sites [15]. In more chronic cases, scaling and hyperpigmentation may occur [14]. This needs to be differentiated from ectodermal dysplasia, where other structures such as sweat glands, sebaceous glands, respiratory glands, lacrimal glands, claws, and teeth are involved as well [141]. A definitive diagnosis of congenital alopecia is made through the collection of multiple skin biopsies from different skin sites that exhibit a complete absence or a decreased number of hair follicles [14,15]. There is no specific treatment. Prevention can be effectively achieved by avoiding the breeding of affected individuals [14].
3.1.3. Colour Dilution Alopecia (CDA)/Black Hair Follicular Dysplasia/Follicular Dysplasia (FD)
These dermatopathies are reported in both brachycephalic and non-brachycephalic dog breeds, including Chihuahuas, Yorkshire Terriers, Shih Tzus, Boxers, Boston Terriers, Cavalier King Charles Spaniels, and blue Chow Chows [14,18,19,20,21,22,23]. The disease is inherited by an autosomal-recessive trait with singular or multiple mutations within or near the melanophilin gene [142,143]. Melanin precursors with cytotoxic effects and abnormal pigment clumps in the epidermis, hair shaft, hair follicle, and hair matrix lead to bulging and fracture of the hair cuticle and therefore alopecia [144]. Progressive hypotrichosis, alopecia, and scaling develop in the affected areas. In color dilution alopecia (CDA), there is also folliculitis and furunculosis. The full extent of disease is usually recognized around 2 to 3 years of age, or earlier in the case of follicular dysplasia [14,18,144].
An increased risk for cancer development has been described for CDA [145]. Trichograms, showing numerous macromelanosomes within the hair shaft leading to irregularities and distortion, and skin biopsies with histopathology exhibiting dilated hair follicles filled with keratin, hair shafts, free melanin, and abnormal melanin clumps in the epidermis and hair follicles, are important diagnostic tools. Commercially available DNA tests targeting the Ras-related protein Rab-27 (RAB27) or malanophilin (MLPH) are now also available [14,18,142,144]. There is no specific treatment, and trauma as well as intense UV-light exposure should be avoided [14,18,144]. Oral retinoic acid may be beneficial [14].
3.1.4. Canine Flank Alopecia/Seasonal Flank Alopecia
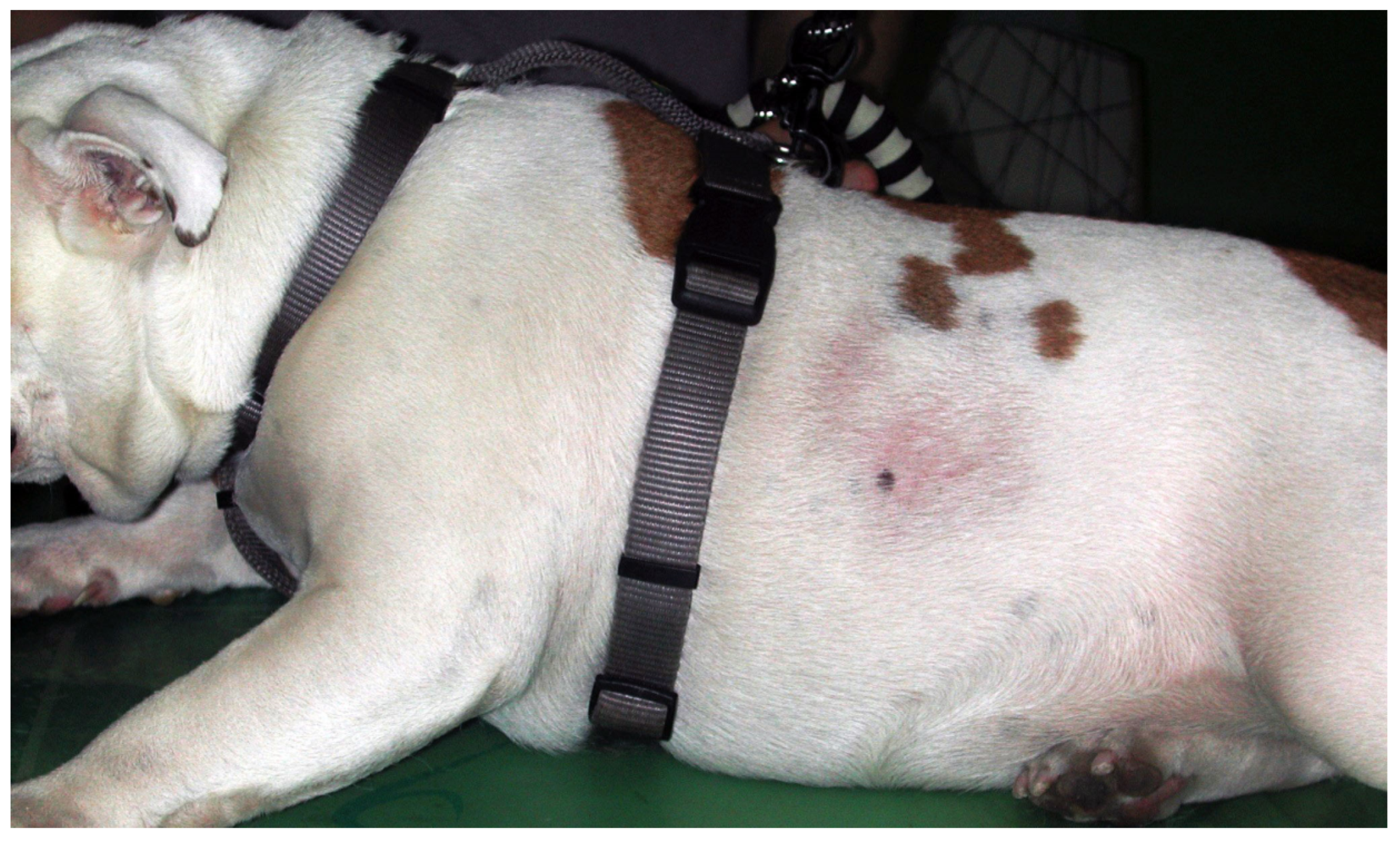
This localized, cyclic, likely polygenetic follicular dysplasia has a high prevalence in middle-aged Boxers and Affenpinschers but is also reported in other breeds, including English bulldogs, Chihuahua, and Staffordshire Bull Terrier [25,26,27,146]. The aetiology is not known, but reduced light exposure and an association with melatonin are considered likely [14,147]. Well-circumscribed, non-pruritic, hyperpigmented, mostly symmetric alopecia, forming a geographic map appearance, typically develops over the flanks during winter (Figure 3). Spontaneous hair regrowth, which may be associated with color change, occurs within 1 to 14 months. Occasionally, alopecia becomes permanent [14,146,148]. Around 20% of individuals only have one episode, whereas most dogs have recurrent alopecic episodes in the following years [146,148]. Affected individuals are otherwise healthy. The breed, history, clinical signs, and exclusion of endocrinopathies make a diagnosis very likely, but in atypical cases, histopathology may be warranted. Since this is a cosmetic problem, observation without treatment is an option, but affected individuals should not be used for breeding. Treatment success can be achieved with melatonin (oral and implanted) and increased contact with the sun/artificial light [14,146,147,148].
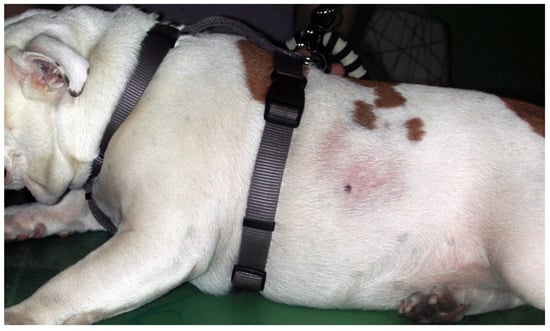
Figure 3.
English Bulldog with seasonal flank alopecia.
3.1.5. Pattern Baldness
Canine pattern baldness is an uncommon disease occurring in both brachycephalic and non-brachycephalic breeds [14,28]. Four different syndromes have been described in Dachshunds, another in American Water Spaniels, a third in Greyhounds, and a fourth syndrome in various breeds, including English bulldogs, Boston Terriers, Boxers, and Chihuahuas [14,28].
Disease often starts around 6 to 9 months of age and progresses over months or years [14,28]. The cause is not known, and an association with androgen receptor dysfunction, as has been described in humans, could not be shown [28]. The fourth syndrome is most common, especially in female dogs, and affects the periauricular skin, the ventrum, the perineal region, and the caudomedial thighs [14,28]. Affected areas do not show complete hair loss but rather miniaturized hair [14,28]. In chronic cases, hyperpigmentation and scaling may occur [14]. A trichogram can help confirm a diagnosis if the patient has normal hair in non-affected areas and miniaturized hair in affected areas. Histopathologically, hair follicles and hair shafts are smaller and thinner than normal [28]. Due to the cosmetic nature of this disease, treatment is not necessary, but oral melatonin may be beneficial [149]. If successful, improvement is typically seen within around 6 weeks [149].
3.1.6. Tyrosinase Deficiency
This genetic abnormality is rarely seen in Chow Chow puppies [14,35]. Affected dogs have a pink (instead of black) tongue, depigmentation of the buccal mucosa, and whitening of the haircoat. They are otherwise healthy [14,35]. Since tyrosinase is necessary to produce melanin, supplementation of tyrosinase to histopathologic preparations and melanin measurement after tissue staining can help with the diagnosis [14,35]. There is no specific treatment, but due to the spontaneous reappearance of melanin, improvement is seen within 2 to 4 months [14,35].
3.1.7. Cutaneous Asthenia
This rare genetic disease occurs in various canine breeds, among which boxers are more frequently affected [14,34]. Both autosomal-recessive and dominant genetic mutations are reported [14,34]. The skin is thin, hyperextensible, and can be easily torn, leaving “fish-mouth” ulcerated wounds that have minimal to no bleeding and heal quickly to leave characteristic “cigarette-paper”-like scars. Rarely, other manifestations such as widening of the bridge of the nose, inguinal and umbilical hernias, increased joint laxity, hygroma formation, and ocular changes can occur [14]. Cutaneous asthenia is associated with an increased skin fragility index, i.e., the distance between the occiput and the base of the tail divided by the length of a stretched skin fold from base to top (>14.5%) [150]. Histopathology classically shows abnormally arranged, irregular collagen fibers with atypical staining properties (Masson trichrome stain). These changes are not always visible and clear [151,152].
Since vitamin C is involved in collagen synthesis, oral supplementation may be beneficial [14]. Lifestyle and housing adjustments are needed to reduce the chance of trauma and wound formation. Such measures include soft bedding, the removal of sharp corners and rough surfaces, and reduced interactions with other animals [14]. One of the authors (SH) has successfully used special protective body suits. Affected animals should not be used for breeding [14].
3.2. Infectious Skin Diseases
3.2.1. Canine Demodicosis
Canine demodicosis is a common parasitic disease that can occur at a young age or later in life [10,153]. Adult-onset demodicosis is typically associated with an underlying disease (hormonal, neoplasia, or immunosuppression) [10,153]. Juvenile disease is the result of a mostly temporary immune alteration, leading to an overgrowth of these commensal mites [10,153,154]. Other predisposing factors include inadequate nutrition, severe stress, parturition, and post-partum oestrus and endoparasites [155,156]. Many brachycephalic breeds, including Pugs, Boxers, English bulldogs, French bulldogs, Shih Tzus, Chow Chows, Boston Terriers, Staffordshire Bull Terriers, Shar Peis, and Chihuahuas, are predisposed [10,47,48,49,50,51,52,53,54,55,56].
Various degrees of multifocal hypotrichosis, including alopecia, erythema, crusts, scales, follicular casts, papules, pustules, nodules, hyperpigmentation, lichenification, and comedones, occur on the head, trunk, limbs, and paws [10,153]. Secondary infections, especially with bacteria, are common and may lead to a mild degree of pruritus [48,153]. Ceruminous otitis externa can also be seen [157]. In severe cases, especially if immunosuppressed and left untreated, deep bacterial infections can lead to sepsis and unspecific systemic signs like fever, anorexia, lethargy, and peripheral lymphadenopathy [48].
Different stages of demodex mites (larvae, adults, and eggs) can be identified via a deep skin scrape, trichogram, or acetate tape squeezing technique [153]. Depending on the severity of the presentation, the general condition of the patient, and the form of demodicosis, active surveillance is sufficient, while medical treatment may be initiated in selected cases [48,153]. For the juvenile form, even with generalized disease, spontaneous remission is reported [153]. Additionally, since there is a genetic predisposition for juvenile-onset disease, breeding affected individuals is not recommended [153,158]. Desexing of affected intact female dogs is recommended due to flare-ups during oestrus [159]. In adult-onset disease, correction of the underlying cause is indicated [160]. Amitraz, macrocyclic lactones, and isoxazolines are efficacious, but potential adverse effects and drug legislation should be considered when selecting these drugs [47,153,160].
3.2.2. Malassezia Dermatitis
A nationwide insurance analysis in the US recognized an increased risk in brachycephalic dogs for fungal skin diseases [161]. Malassezia spp. are yeasts and are skin and mucosal commensals [162]. This fungal organism is commonly associated with dermatitis, including intertrigo, otitis externa, paronychia, and rarely keratomycosis [162]. Brachycephalic breeds predisposed to Malassezia dermatitis include the Shih Tzu, English bulldog, Boxer, Cavalier King Charles Spaniel, and Lhasa Apso [14,57,58].
Malassezia dermatitis can cause hypotrichosis, alopecia, erythema, scales, crusts, greasiness, lichenification, hyperpigmentation, and variable pruritus, especially on the concave pinnae, muzzle, ventral neck, perianal, medial thighs, axillae, inguinal, and paws [162]. Typical triggers include hypersensitivities (flea bite hypersensitivity, food allergy, atopic dermatitis), ectoparasites, superficial pyoderma, endocrinopathies, keratinization abnormalities, and autoimmune diseases [162]. Diagnosis can easily be achieved via cytological examination of affected areas, showing round to oval to peanut-shaped organisms of 3 to 8 µm [14]. Apart from addressing the underlying cause, topical treatment with chlorhexidine or azole preparations is preferred, and systemic therapy with itraconazole, terbinafine, or fluconazole should be reserved for severe, generalized cases or where topical treatment fails [162,163].
3.2.3. Viral Pigmented Plaques
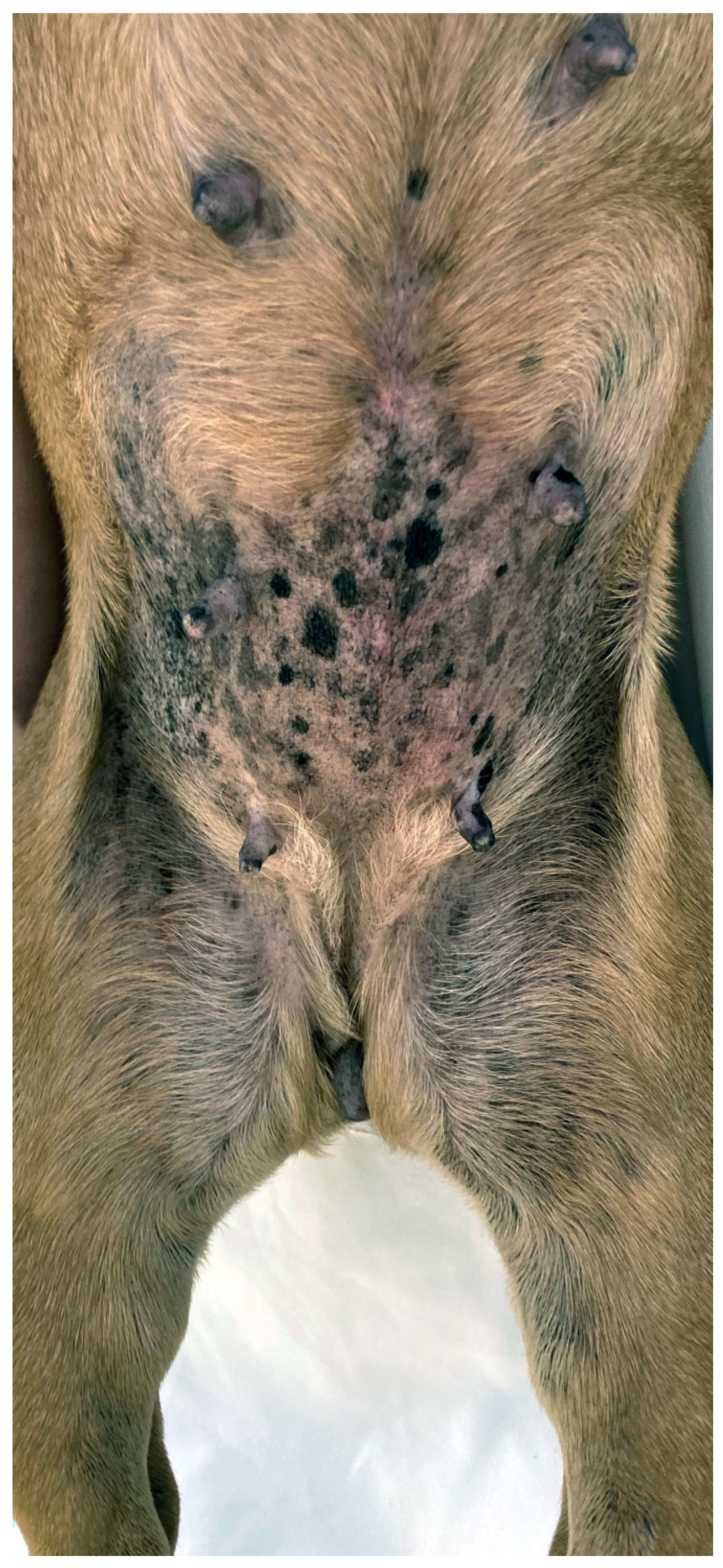
This viral skin disease associated with Chipapillomavirus is reported in many brachycephalic breeds, including the Pug, French bulldog, Chihuahua, and Boston Terrier, as well as in non-brachycephalic breeds [65,66,67,68]. The onset of the disease may be related to a genetic immunodeficiency, as reported in pugs, Vizslas, and Chihuahuas, or secondary to immunosuppression [164]. Numerous small, plaque-like, hyperpigmented lesions with an irregular and scaly surface appear on the ventral neck, thorax, abdomen, and ventro-medial, proximal limbs (Figure 4). A progression to wart-like lesions is described [164]. Depending on the location and number of lesions, discomfort and pruritus can occur [164]. Lesions further progress, especially at the beginning, and rarely transform into squamous cell carcinoma [165]. The clinical appearance together with histopathology often allow a diagnosis, but in the early stages of the disease, further workup, such as PCR, may be needed [166]. Several treatment options are described, including surgical removal, laser treatment, cryotherapy, systemic azithromycin, interferons, and retinoids, as well as topical agents such as vitamin A, imiquimod, or tigilanol tiglate gel [164,166].
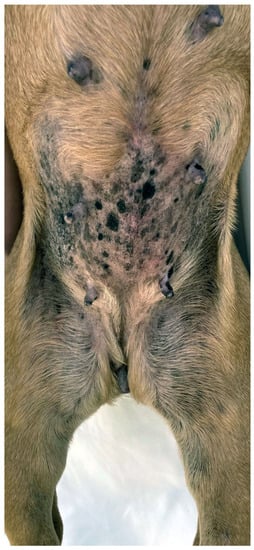
Figure 4.
Pug with multiple viral plaques caused by Chipapapillomavirus infection.
3.3. Bacterial Skin Diseases
3.3.1. Bacterial Folliculitis (Superficial Pyoderma)
Brachycephalic breeds are predisposed to bacterial skin infections, as indicated by an insurance survey in the US as well as an Australian study [3]. The British Bulldog, Pug, Boxer, Shar Pei, and Bullmastiff are predisposed to superficial bacterial folliculitis, which is usually associated with Staphylococcus pseudintermedius [9,14,59,60]. Clinical signs range from mild (loss of hair gloss, increased shedding, erect hairs, or mild scaling) to severe (alopecia, erythema, follicular papules/pustules, epidermal collarettes, and crusts). This may lead to secondary pruritus and deep pyoderma [14,167]. Common underlying triggers are allergies, trauma, ectoparasites, dermatophytes, excessive brushing, seborrhoea, and systemic diseases [14,168]. Diagnosis can be made by recognition of characteristic lesions, cytology (presence of cocci and inflammation), culture, and susceptibility testing [14,169]. Topical treatment with products containing chlorhexidine, benzoyl peroxide, or ethyl lactate is recommended. Systemic antimicrobial therapy is reserved for widespread, deep pyoderma or where topical treatment alone fails [14,168].
3.3.2. Pyotraumatic Dermatitis (Hot Spot)
This skin condition is characterized by a peracute onset of severe pruritus associated with a well-demarcated area of alopecia, erythema, swelling, papules, pustules, and crusts. British bulldogs, Pugs, and Rottweilers are predisposed [9,59,60,61,97].
3.3.3. Muzzle Folliculitis and Furunculosis
Muzzle folliculitis and furunculosis, another form of bacterial infection restricted to the skin of the muzzle, present with pruritus, alopecia, erythema, swelling, papules, pustules, erosion/ulceration, crust formation, and haemorrhagic bullae. An increased risk is recognized in the British Bulldog, Boxer, Rottweiler, and brachycephalic breeds overall [3,14,62].
3.3.4. Canine Leproid Granuloma
Boxers are predisposed to this infectious disease, suggesting a genetic predisposition [14,63,64]. Disease, caused by mycobacterial strains of the Mycobacterium simiae clade in association with trauma, previous skin lesions, and insect bites, is most prevalent in Australia, the USA, and South America (Brazil) [170]. Affected individuals show multiple, intact to ulcerated, well-demarcated nodules and plaques on the head (especially pinnae) and limbs but are otherwise healthy [170]. The diagnosis is based on clinical, cytological (acid-fast bacilli), and histopathological findings [170]. Although there is a chance for spontaneous remission within one to three months, systemic treatment with azithromycin and rifampicin with or without surgery may be needed, particularly in more severe and refractory cases [170]. Topical formulations may be supportive [171].
3.4. Immunological Skin Diseases
3.4.1. Hypersensitivities
Many brachycephalic breeds show an increased risk for different forms of allergy, including flea bite hypersensitivity (FBH; Chow Chow), food allergy (FA; Lhasa Apso, Boxer, Shar Pei), and atopic dermatitis (AD; Boxer, American bulldog, English bulldog, French bulldog, Boston Terrier, Lhasa Apso, Shih Tzu, Chow Chow, Pug, Staffordshire Bull Terrier, Shar Pei) [14,75,76,77,78,79,80,81,82,83,84]. The pathogenesis of most of these diseases is complex and still not fully understood, but likely includes a combination of genetic, skin/mucosal barrier, immunologic, and skin/mucosal microbiome abnormalities [11]. All of these conditions are characterized by variable primary pruritus associated secondary lesions and are further complicated by secondary bacterial and yeast infections (otitis externa, Malassezia dermatitis, pyoderma, pododermatitis/furunculosis).
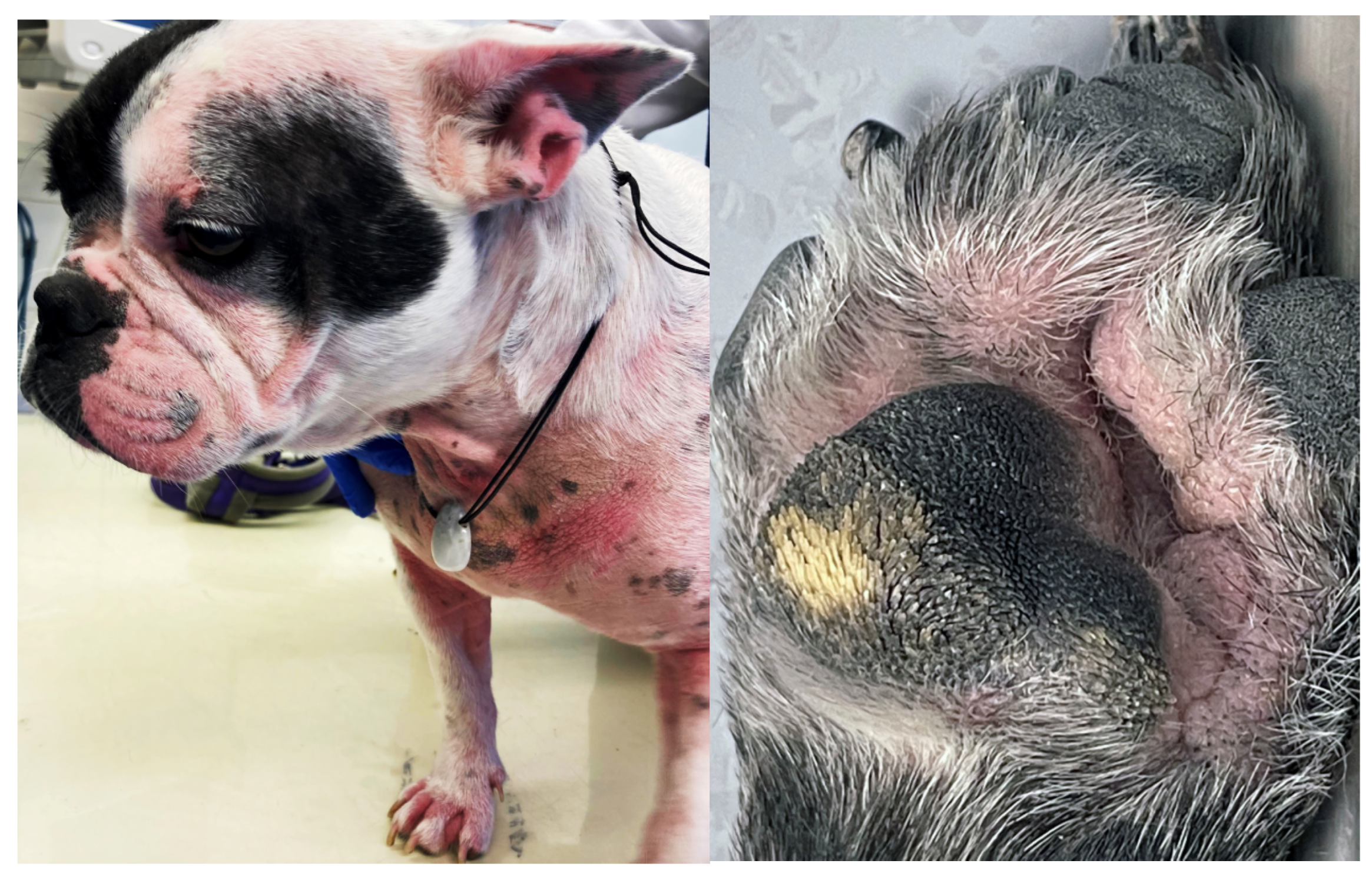
In many brachycephalic breeds, especially Pugs and French bulldogs, the nails and footpads do not wear down normally, further contributing to and worsening allergic pododermatitis [172,173]. Primary pruritus mainly affects the posterior in FBH, whereas in FA and AD the ears, face, muzzle, ventral neck, distal limbs, paws, axillae, inguinal, and perineum are commonly affected (Figure 5) [82,174,175,176,177]. Atopic dogs and dogs with FA may also present with anal sac impaction, acute moist dermatitis, acral lick dermatitis, seborrhoea, hyperhidrosis, rhinitis, reverse sneezing, gastrointestinal disturbances, and sexual cycle abnormalities [178]. Alternatively, dogs with FA may have other presentations such as erythema multiforme, cutaneous vasculitis, urticaria, anaphylaxis, seizures, and behavioral changes [14,179].
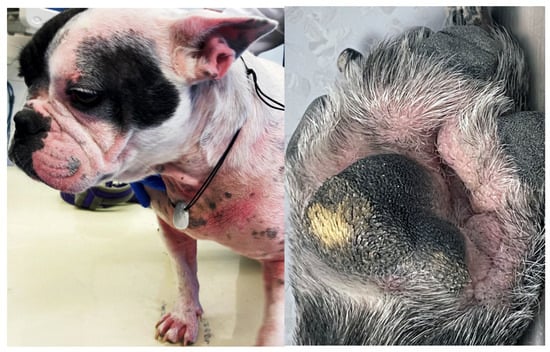
Figure 5.
Atopic French Bulldog with chronic allergic dermatitis, including mild to moderate alopecia, erythema, lichenification, and accumulation of keratosebaceous debris on the pinnae, muzzle, ventral neck, chest, dorsal elbows and paws. Fold formation as a consequence of brachycephaly as well as abnormal wear of the paw pads negatively influence allergic disease.
Diagnosis of the different forms of allergy can be achieved by a response to flea treatment, a strict elimination diet over 4 to 8 weeks with subsequent provocation, the exclusion of other causes of pruritus, and the application of specific established criteria (Favrot’s criteria) [11]. Dogs with FBH or FA can be managed with the use of appropriate flea control and/or dietary interventions [14]. A multimodal approach is often required for the treatment of AD, including addressing the pruritus, secondary infections, and skin barrier, especially if allergen-specific immunotherapy is insufficient [11,14].
3.4.2. Pemphigus Foliaceus
Pemphigus foliaceus is the most common canine autoimmune skin disease that mainly occurs in middle-aged and older animals [14,85]. Multiple breeds can be affected, but Chow Chows have an increased risk [85,86,87,88,89]. Several factors, including genetics, drugs, insects, UV-light, and chronic inflammation, may trigger an autoimmune response targeting desmocollin-1, leading to acantholysis and pustule formation [180]. The disease mainly affects the pinnae, dorsal nose, and paws but may progress to involve other sites. The distribution is often symmetrical, and affected dogs show transient papules and pustules, intense crusting, alopecia, epidermal collarettes, and fissures on the paw pads. There is variable pruritus and secondary bacterial and Malassezia infections. In severe cases, fever, lethargy, anorexia, and lymphadenopathy are also present [85,181]. Cytology of intact pustules reveals neutrophils, eosinophils, and acantholytic cells in the absence of bacteria. Since acantholytic cells can also occur with fungal (Trichophyton spp.) and bacterial infections (Staphylococcus spp.), these organisms must be excluded [14,85]. A definitive diagnosis is attained via multiple skin biopsies and histopathology [85]. Treatment typically includes topical and systemic antimicrobials as well as immunosuppressive drugs such as glucocorticoids, cyclosporine, azathioprine, chlorambucil, mycophenolate mofetil, and recently, oclacitinib [14,85,181]. Potential triggers should be eliminated. Cases with vascular involvement may show more serious clinical signs, be more challenging to treat, and take longer to achieve remission [181]. Most patients require life-long treatment, and few die or will be euthanized due to treatment failure, drug side effects, complications, and/or lack of compliance [85,181].
3.4.3. Uveodermatologic Syndrome
This rare immune-mediated disease primarily affects Akitas but also occurs in other breeds, including Chow Chows [14,90,91]. The pathogenesis is complex, including a heritable component (canine leukocyte antigen alleles) and an inflammatory response including Th17, Th1, and Th2 helper cells with the formation of associated cytokines, autoantibodies, and macrophages infiltration, targeting pigmented structures of the eyes, ears, hair, skin, and nervous system [14,182]. The disease occurs in young to middle-aged dogs, presenting as bilateral photophobia, blepharospasm, epiphora, and blindness. Skin abnormalities classically occur later on, are bilaterally symmetric, and show depigmentation, leukotrichia, leukoderma, alopecia, erythema, scaling, erosion/ulceration, crusting, hyperkeratosis, and rarely onychomadesis or swelling of the nose. The nasal planum, periocular skin, lips, oral cavity, genitals, and footpads are commonly involved [183,184]. Neurologic and auditory signs are rarely reported, might be very subtle, and are thereby underdiagnosed [183]. A rapid diagnosis is very important to avoid blindness. It includes a complete ophthalmological examination and histopathology in cases of skin involvement [183,184]. Ophthalmic glucocorticoids, together with oral immunosuppressive doses of glucocorticoids, are indicated. Initial treatment can be enhanced by the addition of systemic cyclosporine, azathioprine, or other steroid-sparing immunosuppressants in refractory cases [14,184].
3.4.4. Sterile Granuloma and Pyogranuloma Syndrome
Boxers, English bulldogs, and French Mastiffs are predisposed to this rare immune-mediated disease [14,96]. Infectious (bacteria, fungi, parasites, and protozoa) and foreign bodies must first be ruled out before inflammation can be considered sterile [185]. Usually, there are multiple lesions consisting of non-pruritic, non-painful, erythematous, haired to alopecic, often ulcerated, fistulated, and crusted, papules to nodules and plaques, especially occurring on the head and distal limbs. The lesions can spontaneously resolve but also wax and wane [185,186]. Definitive diagnosis requires bacterial and fungal culture, histopathology, including a variety of special stains, and ideally also Leishmania and mycobacterial PCR testing [185,186]. Control can be achieved by immunosuppressive drugs, including glucocorticoids, azathioprine, and cyclosporine. Oral fatty acids may have beneficial or drug-sparing effects. Tetracycline/doxycycline together with niacinamide may also be beneficial in selected cases, but as part of good antimicrobial stewardship, this approach should be avoided where possible [14,187].
3.4.5. Acute Febrile Vasculitis
This vascular disease is rare but is exclusively seen in Shar Peis [92,93,94,95]. The cause of it is not known, but vaccines, insect bites, and infectious microorganisms are discussed as potential triggers [92]. The disease occurs in young puppies, with affected individuals showing acute fever, lethargy, anorexia, lymphadenopathy, and dramatic skin changes comprising severe swelling, well-demarcated ulceration, and necrosis, as well as haemorrhagic maculae, vesicles, and bullae on the head, limbs, and trunk [92]. Diascopy is an easy, cheap, and fast test to recognize bleeding into the skin, but further workup involving comprehensive blood tests, imaging, and skin biopsies is usually warranted [92]. The described treatments include wound and pain management, immunosuppressive and antimicrobial therapy, and surgery. Potential triggers should be eliminated and avoided. The prognosis is guarded, with some affected individuals succumbing to the disease despite treatment [92,95].
3.4.6. Primary Immune Deficiencies
Very rarely, dogs are born with specific immune deficiencies, leading to recurrent infections of the skin, respiratory, urogenital, and/or gastrointestinal tract. These deficiencies include cyclic haematopoiesis (Pomeranian), T-cell dysfunction (Bull Terrier), IgA/IgG (Chow Chow, Rottweiler), and granulocyte colony stimulating factor (G-CSF) (Rottweiler) abnormalities. Affected individuals are young, and the skin might be affected by juvenile demodicosis, recurrent secondary pyoderma, and subcutaneous abscesses [14,70,71,72,73,74].
3.5. Miscellaneous Skin Diseases
3.5.1. Anal Sac Disease
Anal sac disease is common in dogs overall but is especially common in brachycephalic dogs (up to 2.62 times the odds of dolichocephalics), particularly Pugs (up to 2.23 times the odds of non-Pugs) [3,6,97,100,188]. Obesity, soft stools, intestinal disorders, changes in muscle tone, and relatively small anal sac ducts are contributing factors to disease [178]. Recurrent anal sac disease is often associated with AD or FA [14,178]. Anal sac impaction may progress to sacculitis and abscess formation. Perianal pruritus, tail chasing, scooting, tenesmus, and abscess formation are common reasons for presentation. Clinical signs, digital palpation, and perianal evaluation help with diagnosing these problems [14,178]. Anal sacs can be expressed, lavaged, topical antimicrobials instilled, or, in more severe cases, systemic antibiotics, wound treatment, and surgical excision considered. In cases of sacculitis, cannulation and flushing of the anal sac with normal saline, 0.025% chlorhexidine, or 0.4% povidone-iodine solution can be done via 22 to 24 G catheter. Often, a commercially available steroid/antifungal/antibiotic solution/ointment is instilled thereafter [189]. Since topical treatment is often effective, systemic antibiotics should only be used in refractory or severe cases [190]. In addition, underlying problems should be identified and corrected [14,178].
3.5.2. Pododermatitis and Furunculosis
This is a common and often frustrating disease complex affecting the ventral, dorsal-interdigital, pad, and/or ungual parts of the paw [14,191,192]. A predisposition to this disease is described in brachycephalic breeds, particularly English bulldogs, Staffordshire Bull Terriers, and Boxers [6,9,14,60,191,193]. Disease pathogenesis is associated with abrasive short hair coats and abnormal footpad wear, resulting in external trauma to the hair follicles of the palmar and plantar webs, leading to cyst formation, cyst rupture, foreign body reaction, fistulation, and secondary bacterial infection [192,194].
In the majority of cases, more than one foot is affected [192]. Atopy and food hypersensitivity are the most common underlying causes, followed by podo-demodicosis. Other causes include infections (bacterial, fungal, viral), immune-mediated diseases (pemphigus foliaceus, systemic lupus erythematosus, lupoid onychodystrophy, adverse cutaneous drug reaction, vasculitis, lymphocytic plasmacytic pododermatitis), endocrinopathies (hypothyroidism, hyperadrenocorticism, diabetes mellitus), metabolic diseases (superficial necrolytic dermatitis), contact reactions, obesity, foreign bodies, and neoplasms (squamous cell carcinoma, cutaneous lymphoma) [191,192]. Dermatological signs can include variable pruritus, pain, discomfort, erythema, swelling, hypotrichosis to alopecia, erosions/ulcerations, crusts, lichenification, hyperpigmentation, interdigital papule to nodule formation, interdigital fistulation, serosanguineous to purulent exudation, paw pad hyperkeratosis, fissures and ulcerations, paronychia, and nail alterations. Secondary infections with bacteria and/or Malassezia are common, complicating not only the disease but also the management [14,191,192].
Due to the multiplicity of causes, a detailed history, general and dermatological examination, and basic tests such as skin scrapes, trichogram, and cytology are warranted. Bacterial culture and sensitivity testing, fungal culture, haematology and biochemistry, specific tests for infectious organisms (leishmania, distemper, and others), and histopathology may be added, depending on the suspected underlying cause [14,191,192].
The treatment is largely influenced by the underlying disease and should be addressed accordingly, but antiseptic shampoos, mousses, sprays, or foot soaks, as well as topical and systemic anti-inflammatory and anti-pruritic products, are often used unless contra-indicated. In cases of deep bacterial or fungal infections, additional oral antimicrobials over 8 to 12 weeks may be necessary. Severe cases with chronic, fibrotic, and hyperplastic changes may need surgical interventions or CO2 laser treatment. Magnesium sulphate baths (Epsom salts) can also be beneficial. Non-medical measurements such as weight reduction, soft bedding, trauma reduction, and the temporary wearing of special, waterproof dog boots are also favorable [14,173,191,195].
The prognosis is good to guarded, depending on how easily the underlying cause can be corrected and how chronic the changes of the paws at the timepoint of the diagnosis are [191].
3.5.3. Calcinosis Circumscripta
In young dog breeds, including the Rottweiler, Boston Terrier, Boxer, and Shih Tzu, repeated trauma may cause a localized calcification of the skin called calcinosis circumscripta. In these cases, the underlying tissue as well as the calcium/phosphor homeostasis appear normal. In brachycephalic breeds, small to large, white to purple, firm, dome-shaped, sometimes ulcerated papules, nodules, or plaques filled with a chalky material often occur at the cheek and base of the ear. Cytology and histopathology are diagnostic options, and treatment is usually done by surgical excision [14,101,102,103].
3.5.4. Dermoid Sinus/Cyst
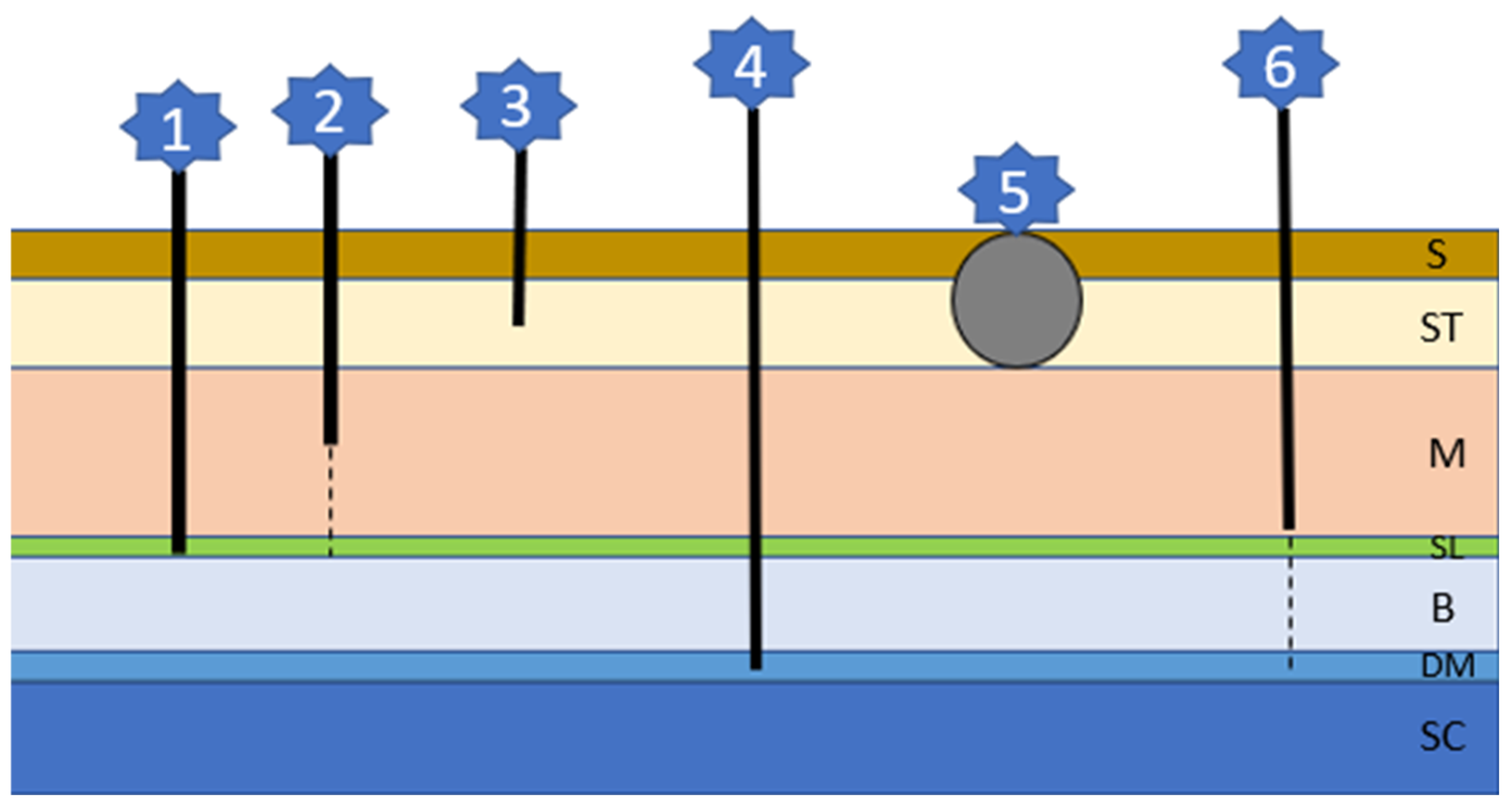
This inherited problem is associated with an abnormal separation of the skin and the neural tube, leading to cyst or tube formation of different depths and lengths (Figure 6) [14,104]. Each type of cyst/tube represents involuted skin with surrounding hair follicles and glands and a lumen filled with keratin, sebum, debris, and hairs [14]. There is an association between an autosomal-dominant mutation involving fibroblast growth factors (FGF) 3, 4, and 19 being responsible for the ridge formation and oral cancer overexpressed 1 factor (ORAOV1) [196]. Although Rhodesian Ridgebacks are most commonly affected, brachycephalic breeds include Boxers, Victorian bulldogs, English Bull Terriers, French bulldogs, Shih Tzus, and Chow Chows [104,105,106,107,108,109,110,111,112,113,114]. There can be singular or multiple sinuses, mostly occurring in the cervical or thoracic region, although head involvement is described in Rottweilers [108]. Lesions are often not recognized by the owner since they occur very concealed as tufts of hair or very small openings. When secondarily infected, a fistulous wound may develop. Neurological signs occur if the defect includes the dura mater and the spinal cord and are associated with a more guarded prognosis [104,106]. A diagnosis can be made via history, clinical signs, palpation, fistulogram, myelogram, CT, or MRI. Depending on the type of sinus and possible complications, considerations between observation and conservative treatment or surgical interventions need to be made [104,106].
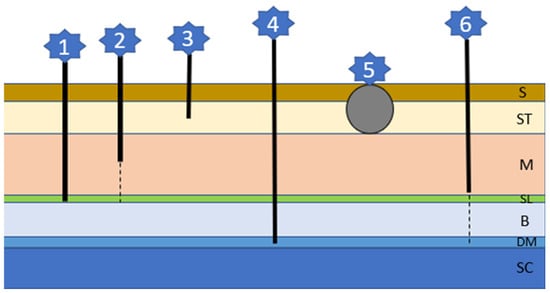
Figure 6.
The six different sinus types of dermoid cysts (refer to text for details); S: skin; ST: Subcutaneous tissue; M: muscle; SL: supraspinous ligament; B: bone; DM: dura mater; SC: spinal cord.
3.6. Other Skin Diseases
Brachycephalic breeds are predisposed to skin cyst formation and nail overgrowth, the latter especially in British bulldogs and Pugs [6,59,60]. Boxers are predisposed to gingival hyperplasia, solar dermatitis, and sternal callus [14], English bulldogs to idiopathic nasodigital hyperkeratosis [197], Boston Terriers to localized parakeratotic hyperkeratosis [198], French Mastiffs to footpad hyperkeratosis [199], and Chow Chows to post-clipping alopecia [200,201].
4. General Discussion and Ethical Considerations
Dermatological disorders are common among brachycephalic breeds. While some are a direct consequence of the anatomic abnormalities that have been selected for over generations of breeding, others are not linked to brachycephaly but highlight the consequences of small gene pool diversity within dog breeds. As breeding programs are modified to select for less extreme brachycephalic confirmation, the prevalence and expression of unrelated genetic disorders need to be carefully monitored to prevent their unwitting selection.
5. Conclusions
Brachycephalic dogs are not only adversely affected by their airway problems, chronic hypoxia, hypertension, sleep disorders, ophthalmologic, dental, and gastrointestinal problems, but also by lifelong dermatological dilemmas, which are often challenging to treat and negatively affect their quality of life. These are enough arguments to suggest that revised breed standards for those dogs are desperately needed.
Author Contributions
Conceptualization, S.H. and P.M.B.; methodology, S.H. and P.M.B.; investigation, S.H.; writing-original draft preparation, S.H.; writing-review and editing, S.H., V.R.B., and P.M.B.; supervision, V.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the City University of Hong Kong, UGC Grant 9610519.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Tamara Weitzer, Alexandra Dehesa, Nellie Choi, and Ramon Almela for the image contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Packer, R.; Murphy, D.; Farnworth, M. Purchasing popular purebreds: Investigating the influence of breed-type on the pre-purchase motivations and behaviour of dog owners. Anim. Wefare 2017, 26, 191–201. [Google Scholar] [CrossRef]
- Kenny, D.D.; Freemantle, R.; Jeffery, A.; Tivers, M.S. Impact of an educational intervention on public perception of brachycephalic obstructive airway syndrome in brachycephalic dogs. Vet. Rec. 2022, 190, e1430. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, A.; Barrs, V.; Awad, M.; Child, G.; Brunel, L.; Mooney, E.; Martinez-Taboada, F.; McDonald, B.; McGreevy, P. Consequences and Management of Canine Brachycephaly in Veterinary Practice: Perspectives from Australian Veterinarians and Veterinary Specialists. Animals 2018, 9, 3. [Google Scholar] [CrossRef]
- Mitze, S.; Barrs, V.R.; Beatty, J.A.; Hobi, S.; Bęczkowski, P.M. Brachycephalic Obstructive Airway Syndrome: Much more than a surgical problem. Vet. Q. 2022, 42, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ekenstedt, K.; Crosse, K.; Risselada, M. Canine brachycephaly: Anatomy, pathology, genetics and welfare. J. Comp. Pathol. 2020, 176, 109–115. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Pegram, C.; Crocker, P.; Brodbelt, D.C.; Church, D.B.; Packer, R.M.A. Unravelling the health status of brachycephalic dogs in the UK using multivariable analysis. Sci. Rep. 2020, 10, 17251. [Google Scholar] [CrossRef]
- Schroers, M.; Meyer-Lindenberg, A. Assessment of clinical signs of brachycephalic obstructive airway syndrome and other breed-specific diseases in pug dogs—An online survey. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2022, 50, 261–268. [Google Scholar]
- O’Neill, D.G.; Volk, A.V.; Soares, T.; Church, D.B.; Brodbelt, D.C.; Pegram, C. Frequency and predisposing factors for canine otitis externa in the UK—A primary veterinary care epidemiological view. Canine Med. Genet. 2021, 8, 7. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Skipper, A.; Packer, R.M.A.; Lacey, C.; Brodbelt, D.C.; Church, D.B.; Pegram, C. English Bulldogs in the UK: A VetCompass study of their disorder predispositions and protections. Canine Med. Genet. 2022, 9, 5. [Google Scholar] [CrossRef]
- O'Neill, D.G.; Turgoose, E.; Church, D.B.; Brodbelt, D.C.; Hendricks, A. Juvenile-onset and adult-onset demodicosis in dogs in the UK: Prevalence and breed associations. J. Small Anim. Pract. 2020, 61, 32–41. [Google Scholar] [CrossRef]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzales, A.J.; Fadok, V.A. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J. Am. Vet. Med. Assoc. 2019, 254, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, I.D.; Rowe, D.; Brodbelt, D.C.; Pegram, C.; Hendricks, A. Ironing out the wrinkles and folds in the epidemiology of skin fold dermatitis in dog breeds in the UK. Sci. Rep. 2022, 12, 10553. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.D.; Georgevsky, D.; Carrasco, J.; Valenzuela, M.; Duffy, D.L.; Serpell, J.A. Dog behavior co-varies with height, bodyweight and skull shape. PLoS ONE 2013, 8, e80529. [Google Scholar] [CrossRef]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L. Muller and Kirk's Small Animal Dermatology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ihrke, P.J.; Mueller, R.S.; Stannard, A.A. Generalized congenital hypotrichosis in a female Rottweiler. Vet. Dermatol. 1993, 4, 65–69. [Google Scholar] [CrossRef]
- Marks, A.; van den Broek, A.; Else, R. Congenital hypotrichosis in a French bulldog. J. Small Anim. Pract. 1992, 33, 450–452. [Google Scholar] [CrossRef]
- O'Neill, C. Hereditary skin disease in the dog and the cat. Compend. Contin. Educ. Pract. Vet. 1981, 3, 791–801. [Google Scholar]
- Perego, R.; Proverbio, D.; Roccabianca, P.; Spada, E. Color dilution alopecia in a blue Doberman pinscher crossbreed. Can. Vet. J. 2009, 50, 511–514. [Google Scholar]
- Kim, S.R.; Kim, Y.I.; Seo, J.A.; Park, J.W.; Jeong, A.Y.; Lee, K.W.; Oh, T.H. Black Hair Follicular Dysplasia in a Shih Tzu. J. Vet. Clin. 2005, 22, 157–159. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, K.I.; Sohn, H.J.; Woo, G.H.; Jean, Y.H.; Hwang, E.K. Color-dilution alopecia in dogs. J. Vet. Sci. 2005, 6, 259–261. [Google Scholar] [CrossRef]
- Rachid, M.A.; Demaula, C.D.; Scott, D.W.; Miller, W.H.; Senter, D.A.; Myers, S. Concurrent follicular dysplasia and interface dermatitis in Boxer dogs. Vet. Dermatol. 2003, 14, 159–166. [Google Scholar] [CrossRef]
- Beco, L.; Fontaine, J.; Gross, T.L.; Charlier, G. Colour dilution alopecia in seven Dachshunds. A clinical study and the hereditary, microscopical and ultrastructural aspect of the disease. Vet. Dermatol. 1996, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Roperto, F.; Cerundolo, R.; Restucci, B.; Vincensi, M.R.; Caprariis, D.D.; Vico, G.D.; Maiolino, P. Colour dilution alopecia (CDA) in ten Yorkshire Terriers. Vet. Dermatol. 1995, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, L. An overview on congenital alopecia in domestic animals. Vet. Dermatol. 2006, 17, 393–410. [Google Scholar] [CrossRef]
- Vandenabeele, S.; Declercq, J.; De Cock, H.; Daminet, S. Canine recurrent flank alopecia: A synthesis of theory and practice. Vlaams Diergeneeskd. Tijdschr. 2014, 83, 275–283. [Google Scholar] [CrossRef]
- Fontaine, J.; Beco, L.; Paradis, M. Alopécie récidivante des flancs: Étude de douze cas chez le griffon Korthals. Point Vét. 1998, 29, 445–449. [Google Scholar]
- Miller, M.; Dunstan, R. Seasonal flank alopecia in boxers and Airedale terriers: 24 cases (1985–1992). J. Am. Vet. Med. Assoc. 1993, 203, 1567–1572. [Google Scholar]
- Paradis, M. 3.3. 8 Canine pattern alopecia. In Hair Loss Disorders in Domestic Animals; Wiley: Hoboken, NJ, USA, 2009; p. 164. [Google Scholar]
- Hartley, C.; Donaldson, D.; Smith, K.C.; Henley, W.; Lewis, T.W.; Blott, S.; Mellersh, C.; Barnett, K.C. Congenital keratoconjunctivitis sicca and ichthyosiform dermatosis in 25 Cavalier King Charles spaniel dogs–part I: Clinical signs, histopathology, and inheritance. Vet. Ophthalmol. 2012, 15, 315–326. [Google Scholar] [CrossRef]
- Mauldin, E.; Wang, P.; Evans, E.; Cantner, C.; Ferracone, J.; Credille, K.; Casal, M. Autosomal recessive congenital ichthyosis in American Bulldogs is associated with NIPAL4 (ICHTHYIN) deficiency. Vet. Pathol. 2015, 52, 654–662. [Google Scholar] [CrossRef]
- Mauldin, E.A. Canine ichthyosis and related disorders of cornification. Vet. Clin. Small Anim. Pract. 2013, 43, 89–97. [Google Scholar] [CrossRef]
- Barnett, K. Congenital keratoconjunctivitis sicca and ichthyosiform dermatosis in the cavalier King Charles spaniel. J. Small Anim. Pract. 2006, 47, 524–528. [Google Scholar] [CrossRef]
- Alhaidari, Z.; Ortonne, J.P.; Pisani, A. Congenital ichthyosis in two cavalier King Charles spaniel littermates. Vet. Dermatol. 1994, 5, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Caldini, E.; Scapinelli, M.; Simões, M.; Machado, D.; Nürmberg, R. Increased elastic microfibrils and thickening of fibroblastic nuclear lamina in canine cutaneous asthenia. Vet. Dermatol. 2009, 20, 139–143. [Google Scholar] [CrossRef]
- Engstrom, D.; Kirk, R. Tyrosinase deficiency in the chow chow. In Current Veterinary Therapy Small Animal Practice; WB Saunders, Philadelphia: Philadelphia, PA, USA, 1966; p. 350. [Google Scholar]
- Rusbridge, C.; Knowler, P.; Rouleau, G.A.; Minassian, B.A.; Rothuizen, J. Inherited occipital hypoplasia/syringomyelia in the cavalier King Charles spaniel: Experiences in setting up a worldwide DNA collection. J. Hered. 2005, 96, 745–749. [Google Scholar] [CrossRef]
- Rusbridge, C.; Knowler, S.P. Inheritance of occipital bone hypoplasia (Chiari type I malformation) in Cavalier King Charles Spaniels. J. Vet. Intern. Med. 2004, 18, 673–678. [Google Scholar] [CrossRef]
- Lewis, T.; Rusbridge, C.; Knowler, P.; Blott, S.; Woolliams, J.A. Heritability of syringomyelia in Cavalier King Charles spaniels. Vet. J. 2010, 183, 345–347. [Google Scholar] [CrossRef]
- Rusbridge, C.; Knowler, S. Hereditary aspects of occipital bone hypoplasia and syringomyelia (Chiari type I malformation) in cavalier King Charles spaniels. Vet. Rec. 2003, 153, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Cagle, L. Concurrent occipital hypoplasia, occipital dysplasia, syringohydromyelia, and hydrocephalus in a Yorkshire terrier. Can. Vet. J. 2010, 51, 904. [Google Scholar] [PubMed]
- Sanchis-Mora, S.; Pelligand, L.; Thomas, C.; Volk, H.; Abeyesinghe, S.; Brodbelt, D.; Church, D.; Thomson, P.; McGreevy, P.; O'Neill, D. Dogs attending primary-care practice in England with clinical signs suggestive of Chiari-like malformation/syringomyelia. Vet. Rec. 2016, 179, 436. [Google Scholar] [CrossRef]
- Rusbridge, C.; Knowler, S.; Pieterse, L.; McFadyen, A. Chiari-like malformation in the Griffon Bruxellois. J. Small Anim. Pract. 2009, 50, 386–393. [Google Scholar] [CrossRef]
- Dewey, C.W.; Berg, J.M.; Barone, G.; Marino, D.J.; Stefanacci, J.D. Foramen magnum decompression for treatment of caudal occipital malformation syndrome in dogs. J. Am. Vet. Med. Assoc. 2005, 227, 1270–1275. [Google Scholar] [CrossRef]
- Souza, C.P.; Foss, K.D.; Mascarenhas, M.B.; Clegg, J.L. Otitis media with effusion in two Boston terrier dogs. Vet. Med. Sci. 2023, 9, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.K. Primary secretory otitis media in Cavalier King Charles spaniels. Vet. Clin. Small Anim. Pract. 2012, 42, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S. Otitis media with effusion in the boxer: A report of seven cases. J. Small Anim. Pract. 2018, 59, 646–650. [Google Scholar] [CrossRef]
- Mueller, R.S.; Meyer, D.; Bensignor, E.; Sauter-Louis, C. Treatment of canine generalized demodicosis with a ‘spot-on’formulation containing 10% moxidectin and 2.5% imidacloprid (Advocate®, Bayer Healthcare). Vet. Dermatol. 2009, 20, 441–446. [Google Scholar] [CrossRef]
- Kuznetsova, E.; Bettenay, S.; Nikolaeva, L.; Majzoub, M.; Mueller, R. Influence of systemic antibiotics on the treatment of dogs with generalized demodicosis. Vet. Parasitol. 2012, 188, 148–155. [Google Scholar] [CrossRef]
- Wright, I. Case study: Generalised demodicosis in a Chihuahua. Companion Anim. 2014, 19, 342–344. [Google Scholar] [CrossRef]
- Barrientos, L.S.; Crespi, J.A.; Peral Garcia, P.; Castellano, M.C.; Giovambattista, G. Prevalence of canine juvenile generalized demodicosis in the Buenos Aires region, Argentina. Jpn. J. Vet. Dermatol. 2013, 19, 57–61. [Google Scholar] [CrossRef][Green Version]
- It, V.; Barrientos, L.; López Gappa, J.; Posik, D.; Díaz, S.; Golijow, C.; Giovambattista, G. Association of canine juvenile generalized demodicosis with the dog leukocyte antigen system. Tissue Antigens 2010, 76, 67–70. [Google Scholar] [CrossRef]
- Holm, B.R. Efficacy of milbemycin oxime in the treatment of canine generalized demodicosis: A retrospective study of 99 dogs (1995–2000). Vet. Dermatol. 2003, 14, 189–195. [Google Scholar] [CrossRef]
- Plant, J.D.; Lund, E.M.; Yang, M. A case–control study of the risk factors for canine juvenile-onset generalized demodicosis in the USA. Vet. Dermatol. 2011, 22, 95–99. [Google Scholar] [CrossRef]
- Lemarie, S.; Hosgood, G.; Foil, C. A retrospective study of juvenile-and adult-onset generalized demodicosis in dogs (1986–91). Vet. Dermatol. 1996, 7, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Day, M. An immunohistochemical study of the lesions of demodicosis in the dog. J. Comp. Pathol. 1997, 116, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. A Short-tailed Demodectic Mite and Demodex canis Infestation in a Chihuahua Dog. Vet. Dermatol. 1995, 6, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, J. Canine Malassezia dermatitis. Can. Vet. J. 2017, 58, 1119–1121. [Google Scholar]
- Mauldin, E.A.; Scott, D.W.; Miller, W.H.; Smith, C.A. Malassezia dermatitis in the dog: A retrospective histopathological and immunopathological study of 86 cases (1990–95). Vet. Dermatol. 1997, 8, 191–202. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Darwent, E.C.; Church, D.B.; Brodbelt, D.C. Demography and health of Pugs under primary veterinary care in England. Canine Genet. Epidemiol. 2016, 3, 5. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Skipper, A.M.; Kadhim, J.; Church, D.B.; Brodbelt, D.C.; Packer, R.M. Disorders of Bulldogs under primary veterinary care in the UK in 2013. PLoS ONE 2019, 14, e0217928. [Google Scholar] [CrossRef]
- Holm, B.R.; Rest, J.R.; Seewald, W. A prospective study of the clinical findings, treatment and histopathology of 44 cases of pyotraumatic dermatitis. Vet. Dermatol. 2004, 15, 369–376. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Pooch, A.S.; Liu, H. A genetic assessment of the English bulldog. Canine Genet. Epidemiol. 2016, 3, 6. [Google Scholar] [CrossRef]
- Conceição, L.G.; Acha, L.M.R.; Borges, A.S.; Assis, F.G.; Loures, F.H.; e Silva, F.F. Epidemiology, clinical signs, histopathology and molecular characterization of canine leproid granuloma: A retrospective study of cases from Brazil. Vet. Dermatol. 2011, 22, 249–256. [Google Scholar] [CrossRef]
- Malik, R.; Love, D.; Wigney, D.; Martin, P. Mycobacterial nodular granulomas affecting the subcutis and skin of dogs (canine leproid granuloma syndrome). Aust. Vet. J. 1998, 76, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Rosenkrantz, W. Cutaneous viral dermatoses in dogs and cats. Compendium 2013, 35, E1. [Google Scholar] [PubMed]
- Luff, J.A.; Affolter, V.K.; Yeargan, B.; Moore, P.F. Detection of six novel papillomavirus sequences within canine pigmented plaques. J. Vet. Diagn. Investig. 2012, 24, 576–580. [Google Scholar] [CrossRef]
- Nagata, M.; Nanko, H.; Moriyama, A.; Washizu, T.; Ishida, T. Pigmented plaques associated with papillomavirus infection in dogs: Is this epidermodysplasia verruciformis? Vet. Dermatol. 1995, 6, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Narama, I.; Kobayashi, Y.; Yamagami, T.; Ozaki, K.; Ueda, Y. Pigmented cutaneous papillomatosis (pigmented epidermal nevus) in three pug dogs; histopathology, electron microscopy and analysis of viral DNA by the polymerase chain reaction. J. Comp. Pathol. 2005, 132, 132–138. [Google Scholar] [CrossRef]
- Sapierzyński, R. Otitis externa in dogs. Med. Weter. 2009, 65, 552–556. [Google Scholar]
- Ellis, J.A. Canine IgA and IgA deficiency: Implications for immunization against respiratory pathogens. Can. Vet. J. 2019, 60, 1305. [Google Scholar]
- Olsson, M.; Tengvall, K.; Frankowiack, M.; Kierczak, M.; Bergvall, K.; Axelsson, E.; Tintle, L.; Marti, E.; Roosje, P.; Leeb, T. Genome-wide analyses suggest mechanisms involving early B-cell development in canine IgA deficiency. PLoS ONE 2015, 10, e0133844. [Google Scholar]
- Day, M. Possible immunodeficiency in related rottweiler dogs. J. Small Anim. Pract. 1999, 40, 561–568. [Google Scholar] [CrossRef]
- Lanevschi, A.; Daminet, S.; Niemeyer, G.P.; Lothrop, C.D., Jr. Granulocyte Colony-Stimulating Factor Deficiency in a Rottweiler with Chronic Idiopathic Neutropenia. J. Vet. Intern. Med. 1999, 13, 72–75. [Google Scholar] [CrossRef]
- Rivas, A.L.; Tintle, L.; Argentieri, D.; Kimball, E.S.; Goodman, M.G.; Anderson, D.W.; Capetola, R.J.; Quimby, F.W. A primary immunodeficiency syndrome in Shar-Pei dogs. Clin. Immunol. Immunopathol. 1995, 74, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis: Pathogenesis and Treatment. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mazrier, H.; Vogelnest, L.J.; Thomson, P.C.; Taylor, R.M.; Williamson, P. Canine atopic dermatitis: Breed risk in Australia and evidence for a susceptible clade. Vet. Dermatol. 2016, 27, 167-e42. [Google Scholar] [CrossRef] [PubMed]
- Theerawatanasirikul, S.; Sailasuta, A.; Thanawongnuwech, R.; Suriyaphol, G. Alterations of keratins, involucrin and filaggrin gene expression in canine atopic dermatitis. Res. Vet. Sci. 2012, 93, 1287–1292. [Google Scholar] [CrossRef]
- Jaeger, K.; Linek, M.; Power, H.; Bettenay, S.; Zabel, S.; Rosychuk, R.; Mueller, R.S. Breed and site predispositions of dogs with atopic dermatitis: A comparison of five locations in three continents. Vet. Dermatol. 2010, 21, 119–123. [Google Scholar] [CrossRef]
- Picco, F.; Zini, E.; Nett, C.; Naegeli, C.; Bigler, B.; Rüfenacht, S.; Roosje, P.; Gutzwiller, M.E.; Wilhelm, S.; Pfister, J.; et al. A prospective study on canine atopic dermatitis and food-induced allergic dermatitis in Switzerland. Vet. Dermatol. 2008, 19, 150–155. [Google Scholar] [CrossRef]
- Počta, S.; Svoboda, M. Approach to the diagnostics of atopic dermatitis in dogs in conditions of clinical practice. Acta Vet. Brno 2007, 76, 461–468. [Google Scholar] [CrossRef][Green Version]
- Nødtvedt, A.; Egenvall, A.; Bergval, K.; Hedhammar, Å. Incidence of and risk factors for atopic dermatitis in a Swedish population of insured dogs. Vet. Rec. 2006, 159, 241–246. [Google Scholar] [CrossRef]
- Verlinden, A.; Hesta, M.; Millet, S.; Janssens, G. Food allergy in dogs and cats: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 259–273. [Google Scholar] [CrossRef]
- Prélaud, P.; Guaguere, E.; Alhaidari, Z.; Faivre, N.; Heripret, D.; Gayerie, A. Reevaluation of diagnostic criteria of canine atopic dermatitis. Rev. Med. Vet. 1998, 149, 1057–1064. [Google Scholar]
- Harvey, R. Food allergy and dietary intolerance in dogs: A report of 25 cases. J. Small Anim. Pract. 1993, 34, 175–179. [Google Scholar] [CrossRef]
- Goodale, E. Pemphigus foliaceous. Can. Vet. J. 2019, 60, 311–313. [Google Scholar] [PubMed]
- Bizikova, P.; Dean, G.A.; Hashimoto, T.; Olivry, T. Cloning and establishment of canine desmocollin-1 as a major autoantigen in canine pemphigus foliaceus. Vet. Immunol. Immunopathol. 2012, 149, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T. A review of autoimmune skin diseases in domestic animals: I–superficial pemphigus. Vet. Dermatol. 2006, 17, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves-Hubers, T. Pemphigus erythematosus in a chow chow. Can. Vet. J. 2005, 46, 925. [Google Scholar] [PubMed]
- Kuhl, K.; Shofer, F.; Goldschmidt, M. Comparative histopathology of pemphigus foliaceus and superficial folliculitis in the dog. Vet. Pathol. 1994, 31, 19–27. [Google Scholar] [CrossRef]
- Zarfoss, M.K.; Tusler, C.A.; Kass, P.H.; Montgomery, K.; Lim, C.C.; Mowat, F.; Thomasy, S.M. Clinical findings and outcomes for dogs with uveodermatologic syndrome. J. Am. Vet. Med. Assoc. 2018, 252, 1263–1271. [Google Scholar] [CrossRef]
- Blackwood, S.E.; Barrie, K.P.; Plummer, C.E.; Taylor, D.; Nunnery, C.M.; Seltzer, J.D.; Ben-Shlomo, G.; Brooks, D.E. Uveodermatologic syndrome in a rat terrier. J. Am. Anim. Hosp. Assoc. 2011, 47, e56–e63. [Google Scholar] [CrossRef]
- Weingart, C.; Kershaw, O.; Kohn, B.; Rohwedder, T. Life-threatening acute neutrophilic vasculitis in a Shar-Pei puppy. Tierarztl. Praxis. Ausg. K Kleintiere Heimtiere 2022, 50, 57–63. [Google Scholar]
- Malik, R.; Foster, S.; Martin, P.; Canfield, P.; Mason, K.; Bosward, K.; Gough, A.; Rippon, G. Acute febrile neutrophilic vasculitis of the skin of young Shar-Pei dogs. Aust. Vet. J. 2002, 80, 200–206. [Google Scholar] [CrossRef]
- Tellier, L.A. Immune-mediated vasculitis in a shar-pei with swollen hock syndrome. Can. Vet. J. 2001, 42, 137–139. [Google Scholar] [PubMed]
- Innerå, M. Cutaneous vasculitis in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 113–134. [Google Scholar] [CrossRef]
- Panich, R.; Scott, D.; Miller, W., Jr. Canine cutaneous sterile pyogranuloma/granuloma syndrome: A retrospective analysis of 29 cases (1976 to 1988). J. Am. Anim. Hosp. Assoc. 1991, 27, 519–528. [Google Scholar]
- O’Neill, D.G.; Sahota, J.; Brodbelt, D.C.; Church, D.B.; Packer, R.M.A.; Pegram, C. Health of Pug dogs in the UK: Disorder predispositions and protections. Canine Med. Genet. 2022, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.; O’Neill, D. Health and Welfare of Brachycephalic (Flat-Faced) Companion Animals: A Complete Guide for Veterinary and Animal Professionals; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Beco, L.; Guaguère, E.; Lorente Méndez, C.; Noli, C.S.; Nuttall, T.; Vroom, M. Suggested guidelines for using systemic antimicrobials in bacterial skin infections (1): Diagnosis based on clinical presentation, cytology and culture. Vet. Rec. 2013, 172, 72–78. [Google Scholar] [CrossRef]
- Feng, T.; McConnell, C.; O’Hara, K.; Chai, J.; Spadafori, G. Nationwide’s Brachycephalic Breed Disease Prevalence Study; Nationwide: Columbus, OH, USA, 2017. [Google Scholar]
- Doerr, K.A.; Outerbridge, C.A.; White, S.D.; Kass, P.H.; Shiraki, R.; Lam, A.T.; Affolter, V.K. Calcinosis cutis in dogs: Histopathological and clinical analysis of 46 cases. Vet. Dermatol. 2013, 24, 355-e79. [Google Scholar] [CrossRef]
- Tafti, A.; Hanna, P.; Bourque, A.C. Calcinosis circumscripta in the dog: A retrospective pathological study. J. Vet. Med. Ser. A 2005, 52, 13–17. [Google Scholar] [CrossRef]
- Scott, D.; Buerger, R. Idiopathic calcinosis circumscripta in the dog: A retrospective analysis of 130 cases. J. Am. Anim. Hosp. Assoc. 1988, 24, 651–658. [Google Scholar]
- Barrios, N.; Gómez, M.; Mieres, M.; Vera, F.; Alvial, G. Spinal dermoid sinus in a Dachshund with vertebral and thoracic limb malformations. BMC Vet. Res. 2014, 10, 54. [Google Scholar] [CrossRef]
- Motta, L.; Skerritt, G.; Denk, D.; Leeming, G.; Saulnier, F. Dermoid sinus type IV associated with spina bifida in a young Victorian bulldog. Vet. Rec. -Engl. Ed. 2012, 170, 127. [Google Scholar] [CrossRef]
- Ployart, S.; Doran, I.; Bomassi, E.; Bille, C.; Libermann, S. Myelomeningocoele and a dermoid sinus-like lesion in a French bulldog. Can. Vet. J. 2013, 54, 1133–1136. [Google Scholar] [PubMed]
- Sturgeon, C. Nasal dermoid sinus cyst in a shih tzu. Vet. Rec. 2008, 163, 219. [Google Scholar] [CrossRef] [PubMed]
- Bornard, N.; Pin, D.; Carozzo, C. Bilateral parieto-occipital dermoid sinuses in a Rottweiler. J. Small Anim. Pract. 2007, 48, 107–110. [Google Scholar] [CrossRef]
- Colón, J.A.; Maritato, K.C.; Mauterer, J.V. Dermoid sinus and bone defects of the fifth thoracic vertebrae in a shih-tzu. J. Small Anim. Pract. 2007, 48, 180. [Google Scholar] [CrossRef]
- Bowens, A.L.; Ducoté, J.M.; Early, P.J. What is your neurologic diagnosis? J. Am. Vet. Med. Assoc. 2005, 227, 713–715. [Google Scholar] [CrossRef]
- Burrow, R. A nasal dermoid sinus in an English bull terrier. J. Small Anim. Pract. 2004, 45, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Fatone, G.; Brunetti, A.; Lamagna, F.; Potena, A. Dermoid sinus and spinal malformations in a Yorkshire terrier: Diagnosis and follow-up. J. Small Anim. Pract. 1995, 36, 178–180. [Google Scholar] [CrossRef]
- Booth, M. Atypical dermoid sinus in a chow chow dog: Case report. J. S. Afr. Vet. Assoc. 1998, 69, 102–104. [Google Scholar] [CrossRef]
- Selcer, E. Dermoid sinus in a shih tzu and a boxer. J. Am. An. Hosp. Assoc. 1984, 20, 634–636. [Google Scholar]
- Banovic, F.; Strzok, E. Skin Fold Dermatitis (Intertrigo) in Dogs. Todays Vet. Pract. 2019, 9, 63–67. [Google Scholar]
- Zanna, G.; Docampo, M.J.; Fondevila, D.; Bardagí, M.; Bassols, A.; Ferrer, L. Hereditary cutaneous mucinosis in shar pei dogs is associated with increased hyaluronan synthase-2 mRNA transcription by cultured dermal fibroblasts. Vet. Dermatol. 2009, 20, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.A.; Saiyad, S.; Rao, N. Common health issues related to brachycephalic dogs. Pharma Innov. 2022, 11, 786–796. [Google Scholar]
- Töpfer, T.; Köhler, C.; Rösch, S.; Oechtering, G. Brachycephaly in French bulldogs and pugs is associated with narrow ear canals. Vet. Dermatol. 2022, 33, 214-e260. [Google Scholar] [CrossRef] [PubMed]
- Pye, C. Pseudomonas otitis externa in dogs. Can. Vet. J. 2018, 59, 1231–1234. [Google Scholar] [PubMed]
- Gotthelf, L.N. Diagnosis and treatment of otitis media in dogs and cats. Vet. Clin. Small Anim. Pract. 2004, 34, 469–487. [Google Scholar] [CrossRef]
- Nuttall, T. Successful management of otitis externa. Practice 2016, 38, 17–21. [Google Scholar] [CrossRef]
- Chan, W.Y.; Hickey, E.E.; Page, S.W.; Trott, D.J.; Hill, P.B. Biofilm production by pathogens associated with canine otitis externa, and the antibiofilm activity of ionophores and antimicrobial adjuvants. J. Vet. Pharmacol. Ther. 2019, 42, 682–692. [Google Scholar] [CrossRef]
- Seo, M.; Oh, T.; Bae, S. Antibiofilm activity of silver nanoparticles against biofilm forming Staphylococcus pseudintermedius isolated from dogs with otitis externa. Vet. Med. Sci. 2021, 7, 1551–1557. [Google Scholar] [CrossRef]
- Pickrell, J.; Oehme, F.; Cash, W. Ototoxicity in dogs and cats. Semin. Vet. Med. Surg. Small Anim. 1993, 8, 42–49. [Google Scholar]
- Oishi, N.; Talaska, A.E.; Schacht, J. Ototoxicity in dogs and cats. Vet. Clin. Small Anim. Pract. 2012, 42, 1259–1271. [Google Scholar] [CrossRef]
- Cerda-Gonzalez, S.; Olby, N.; Pease, T.; McCullough, S.; Massoud, N.; Broadstone, R. Morphology of the caudal fossa in Cavalier King Charles Spaniels. J. Vet. Intern. Med. 2006, 20, 736. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Lamb, C.; Pfeiffer, D.; Targett, M. Neurological signs and results of magnetic resonance imaging in 40 cavalier King Charles spaniels with Chiari type 1-like malformations. Vet. Rec. 2003, 153, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Plessas, I.; Rusbridge, C.; Driver, C.; Chandler, K.; Craig, A.; McGonnell, I.; Brodbelt, D.; Volk, H. Long-term outcome of Cavalier King Charles spaniel dogs with clinical signs associated with Chiari-like malformation and syringomyelia. Vet. Rec. 2012, 171, 501. [Google Scholar] [CrossRef] [PubMed]
- Loughin, C.A. Chiari-like Malformation. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 231–242. [Google Scholar] [CrossRef]
- Salazar, V.; Dewey, C.W.; Schwark, W.; Badgley, B.L.; Gleed, R.D.; Horne, W.; Ludders, J.W. Pharmacokinetics of single-dose oral pregabalin administration in normal dogs. Vet. Anaesth. Analg. 2009, 36, 574–580. [Google Scholar] [CrossRef]
- Grubb, T. Chronic neuropathic pain in veterinary patients. Top. Companion Anim. Med. 2010, 25, 45–52. [Google Scholar] [CrossRef]
- Silva, N.; Luna, S.P.L.; Joaquim, J.G.F.; Coutinho, H.D.; Possebon, F.S. Effect of acupuncture on pain and quality of life in canine neurological and musculoskeletal diseases. Can. Vet. J. 2017, 58, 941–951. [Google Scholar]
- Rusbridge, C. Chiari-like malformation with syringomyelia in the Cavalier King Charles spaniel: Long-term outcome after surgical management. Vet. Surg. 2007, 36, 396–405. [Google Scholar] [CrossRef]
- Colverde, A.S.; Nicetto, T.; Falzone, C. Occipital cranioplasty using customized titanium prosthesis yields successful outcome in association with foramen magnum decompression in dogs suffering by Chiari-like malformation. Am. J. Vet. Res. 2021, 83, 275–282. [Google Scholar] [CrossRef]
- Ortinau, N.; Vitale, S.; Akin, E.Y.; Beasley, M.; Shores, A. Foramen magnum decompression surgery in 23 Chiari-like malformation patients 2007–2010: Outcomes and owner survey results. Can. Vet. J. 2015, 56, 288–291. [Google Scholar]
- Stern-Sertholtz, W.; Sjöström, L.; Hårkanson, N.W. Primary secretory otitis media in the Cavalier King Charles spaniel: A review of 61 cases. J. Small Anim. Pract. 2003, 44, 253–256. [Google Scholar] [CrossRef]
- Kubba, H.; Pearson, J.; Birchall, J. The aetiology of otitis media with effusion: A review. Clin. Otolaryngol. Allied Sci. 2000, 25, 181–194. [Google Scholar] [CrossRef]
- Hayes, G.; Friend, E.; Jeffery, N. Relationship between pharyngeal conformation and otitis media with effusion in Cavalier King Charles spaniels. Vet. Rec. 2010, 167, 55–58. [Google Scholar] [CrossRef]
- Imai, A.; Kondo, H.; Suganuma, T.; Nagata, M. Clinical analysis and nonsurgical management of 11 dogs with aural cholesteatoma. Vet. Dermatol. 2019, 30, 42-e12. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, E.A.; Elias, P.M. Ichthyosis and hereditary cornification disorders in dogs. Vet. Dermatol. 2021, 32, 567-e154. [Google Scholar] [CrossRef] [PubMed]
- Moura, E.; Daltro, S.; Sás, D.; Engracia Filho, J.; Farias, M.; Pimpão, C. Genetic analysis of a possible case of canine X-linked ectodermal dysplasia. J. Small Anim. Pract. 2021, 62, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Caramalac, S.M.; Caramalac, S.M.; Babo-Terra, V.J.; Ramos, C.A.; Palumbo, M.I. PCR-RFLP molecular confirmation of color dilution alopecia in dogs in Brazil. J. Vet. Diagn. Investig. 2021, 33, 984–986. [Google Scholar] [CrossRef]
- Welle, M.; Philipp, U.; Rüfenacht, S.; Roosje, P.; Scharfenstein, M.; Schütz, E.; Brenig, B.; Linek, M.; Mecklenburg, L.; Grest, P. MLPH genotype—melanin phenotype correlation in dilute dogs. J. Hered. 2009, 100, S75–S79. [Google Scholar] [CrossRef]
- Von Bomhard, W.; Mauldin, E.A.; Schmutz, S.M.; Leeb, T.; Casal, M.L. Black hair follicular dysplasia in Large Münsterländer dogs: Clinical, histological and ultrastructural features. Vet. Dermatol. 2006, 17, 182–188. [Google Scholar] [CrossRef][Green Version]
- Antunes, M.I.P.P.; Fabris, V.E.; Machado, L.H.d.A. Carcinoma de células escamosas em um cão com alopecia por diluição de cor. Vet. Zootec. 2012, 19, 507–512. [Google Scholar]
- Mecklenburg, L.; Linek, M.; Tobin, D.J. Hair Loss Disorders in Domestic Animals; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Verschuuren, M.; Schlotter, Y.M.; van Geijlswijk, I.M.; van der Lugt, J.J.; Gehring, R. The efficacy of subcutaneous slow-release melatonin implants in the prevention of canine flank alopecia recurrence is uncertain: A double-blind, randomized, placebo-controlled study. Vet. Dermatol. 2022, 33, 553–558. [Google Scholar] [CrossRef]
- Paradis, M. An approach to symmetrical alopecia in the dog. In BSAVA Manual of Canine and Feline Dermatology; BSAVA Library: Gloucester, UK, 2012; pp. 91–102. [Google Scholar]
- Paradis, M. Melatonin in the Treatment of Canine Pattern Baldness; Butterworth-Heinemann Ltd.: Oxford, UK, 1998. [Google Scholar]
- Freeman, L.; Hegreberg, G.; Robinette, J. Ehlers-Danlos syndrome in dogs and cats. Semin. Vet. Med. Surg. Small Anim. 1987, 2, 221–227. [Google Scholar]
- Patterson, D.; Minor, R. Hereditary fragility and hyperextensibility of the skin of cats. A defect in collagen fibrillogenesis. Lab. Investig. J. Tech. Methods Pathol. 1977, 37, 170–179. [Google Scholar]
- Fernandez, C.J.; Scott, D.W.; Erb, H.N.; Minor, R.R. Staining abnormalities of dermal collagen in cats with cutaneous asthenia or acquired skin fragility as demonstrated with Masson's trichrome stain. Vet. Dermatol. 1998, 9, 49–54. [Google Scholar] [CrossRef]
- Mueller, R.S.; Rosenkrantz, W.; Bensignor, E.; Karaś-Tęcza, J.; Paterson, T.; Shipstone, M.A. Diagnosis and treatment of demodicosis in dogs and cats: Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 4-e2. [Google Scholar] [CrossRef]
- Ferrer, L.; Ravera, I.; Silbermayr, K. Immunology and pathogenesis of canine demodicosis. Vet. Dermatol. 2014, 25, 427-e465. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Bostami, M.B.; Datta, A.; Sabuj, A.A.M.; Rana, E.A.; Mannan, A.; Hossain, M.M.A.; Chowdhury, M.Y.E. Estimation of the prevalence and determination of risk factors associated with demodicosis in dogs. J. Adv. Vet. Anim. Res. 2021, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Taylan-Ozkan, A.; Mumcuoglu, K.Y. Immune mechanisms in human and canine demodicosis: A review. Parasite Immunol. 2019, 41, e12673. [Google Scholar] [CrossRef] [PubMed]
- Saridomichelakis, M.N.; Farmaki, R.; Leontides, L.S.; Koutinas, A.F. Aetiology of canine otitis externa: A retrospective study of 100 cases. Vet. Dermatol. 2007, 18, 341–347. [Google Scholar] [CrossRef]
- Bowden, D.G.; Outerbridge, C.A.; Kissel, M.B.; Baron, J.N.; White, S.D. Canine demodicosis: A retrospective study of a veterinary hospital population in California, USA (2000–2016). Vet. Dermatol. 2018, 29, 19-e10. [Google Scholar] [CrossRef]
- Mueller, R.; Hastie, K.; Bettenay, S. Daily oral ivermectin for treatment of generalised demodicosis in 23 dogs. Aust. Vet. Pract. 1999, 29, 132. [Google Scholar]
- Duangkaew, L.; Larsuprom, L.; Anukkul, P.; Lekcharoensuk, C.; Chen, C. A field trial in Thailand of the efficacy of oral fluralaner for the treatment of dogs with generalized demodicosis. Vet. Dermatol. 2018, 29, 208-e74. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; McConnell, C.; O’hara, K.; Chai, J.; Spadafori, G. Brachycephalic Breed Disease Prevalence Study. Can. Vet. J. 2017, 58, 777–780. [Google Scholar]
- Hobi, S.; Cafarchia, C.; Romano, V.; Barrs, V.R. Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals. J. Fungi 2022, 8, 708. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Morris, D.O.; Guillot, J.; Bensignor, E.J.; Robson, D.; Mason, K.V.; Kano, R.; Hill, P.B. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 28–74. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Lam, A.T.; Sakai, M. Extensive progressive pigmented viral plaques in a Chihuahua dog. Vet. Dermatol. 2022, 33, 252–254. [Google Scholar] [CrossRef]
- Luff, J.; Rowland, P.; Mader, M.; Orr, C.; Yuan, H. Two canine papillomaviruses associated with metastatic squamous cell carcinoma in two related Basenji dogs. Vet. Pathol. 2016, 53, 1160–1163. [Google Scholar] [CrossRef]
- Hansen, N.; Nicholas, N.; Pack, G.; Mackie, J.T.; Shipstone, M.; Munday, J.S.; Reddell, P.; Orbell, G.; Malik, R. Progressive cutaneous viral pigmented plaques in three Hungarian Vizslas and the response of lesions to topical tigilanol tiglate gel. Vet. Med. Sci. 2018, 4, 53–62. [Google Scholar] [CrossRef]
- Banovic, F.; Linder, K.; Olivry, T. Clinical, microscopic and microbial characterization of exfoliative superficial pyoderma-associated epidermal collarettes in dogs. Vet. Dermatol. 2017, 28, 107-e23. [Google Scholar] [CrossRef]
- Bajwa, J. Canine superficial pyoderma and therapeutic considerations. Can. Vet. J. 2016, 57, 204. [Google Scholar]
- Hillier, A.; Lloyd, D.H.; Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet. Dermatol. 2014, 25, 163-e43. [Google Scholar] [CrossRef] [PubMed]
- Biezus, G.; de Cristo, T.G.; Ikuta, C.Y.; Carniel, F.; Volpato, J.; de Souza Teixeira, M.B.; Neto, J.S.F.; Casagrande, R.A. Canine leproid granuloma (CLG) caused by mycobacterial species closely related to members of Mycobacterium simiae complex in a dog in Brazil. Top. Companion Anim. Med. 2022, 50, 100672. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Martin, P.; Wigney, D.; Swan, D.; Sattler, P.; Cibilic, D.; Allen, J.; Mitchell, D.; Chen, S.; Hughes, M. Treatment of canine leproid granuloma syndrome: Preliminary findings in seven dogs. Aust. Vet. J. 2001, 79, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Jeandel, A.; Garosi, L. Gait abnormalities in brachycephalic breeds: Should we be more concerned? Vet. Rec. 2018, 182, 164. [Google Scholar] [CrossRef]
- Nuttall, T. Chronic pododermatitis and interdigital furunculosis in dogs. Companion Anim. 2019, 24, 194–200. [Google Scholar] [CrossRef]
- Laffort-Dassot, C. Flea allergy in dogs: Clinical signs and diagnosis. Eur. J. Companion Anim. Pract. 2009, 19, 242–248. [Google Scholar]
- Zur, G.; Ihrke, P.J.; White, S.D.; Kass, P.H. Canine atopic dermatitis: A retrospective study of 266 cases examined at the University of California, Davis, 1992–1998. Part I. Clinical features and allergy testing results. Vet. Dermatol. 2002, 13, 89–102. [Google Scholar] [CrossRef]
- Griffin, C.; DeBoer, D. The ACVD task force on canine atopic dermatitis (XIV): Clinical manifestations of canine atopic dermatitis. Vet. Immunol. Immunopathol. 2001, 81, 255–269. [Google Scholar] [CrossRef]
- Favrot, C. Clinical signs and diagnosis of canine atopic dermatitis. In Proceedings of the 3. Congresso Latinoamericano de Dermatologia Veterinaria, Buenos Aires, Argentina, 26–27 November 2015. [Google Scholar]
- Corbee, R.J.; Woldring, H.H.; van den Eijnde, L.M.; Wouters, E.G.H. A Cross-Sectional Study on Canine and Feline Anal Sac Disease. Animals 2022, 12, 95. [Google Scholar] [CrossRef]
- Mueller, R.S.; Olivry, T. Critically appraised topic on adverse food reactions of companion animals (6): Prevalence of noncutaneous manifestations of adverse food reactions in dogs and cats. BMC Vet. Res. 2018, 14, 341. [Google Scholar] [CrossRef]
- White, A.; Hicks, K.; Bizikova, P.; Bailey, J.; Linder, K. Probable drug-triggered pemphigus foliaceus in a dog following administration of afoxolaner (NexGard). Vet. Rec. Case Rep. 2019, 7, e000735. [Google Scholar] [CrossRef]
- Zhou, Z.; Corner, S.; Petersen, A.; Rosser, E.; Noland, E.L. Clinical presentation, treatment and outcome in dogs with pemphigus foliaceus with and without vasculopathic lesions: An evaluation of 41 cases. Vet. Dermatol. 2021, 32, 503-e139. [Google Scholar] [CrossRef]
- Egbeto, I.A.; Garelli, C.J.; Piedra-Mora, C.; Wong, N.B.; David, C.N.; Robinson, N.A.; Richmond, J.M. Case Series: Gene Expression Analysis in Canine Vogt-Koyanagi-Harada/Uveodermatologic Syndrome and Vitiligo Reveals Conserved Immunopathogenesis Pathways Between Dog and Human Autoimmune Pigmentary Disorders. Front. Immunol. 2020, 11, 590558. [Google Scholar] [CrossRef] [PubMed]
- Tham, H.L.; Linder, K.E.; Olivry, T. Autoimmune diseases affecting skin melanocytes in dogs, cats and horses: Vitiligo and the uveodermatological syndrome: A comprehensive review. BMC Vet. Res. 2019, 15, 251. [Google Scholar] [CrossRef]
- Oliveira, A.T.C.; de Oliveira, A.R.F.; Santiago, I.L.T.; de Lima, Y.B.S.; Ferreira, T.C. Clinical, diagnostic and therapeutic approach of uveodermatologic syndrome in dogs: A review. Rev. Bras. Hig. Sanid. Anim. 2020, 14, 248–261. [Google Scholar] [CrossRef]
- Santoro, D.; Prisco, M.; Ciaramella, P. Cutaneous sterile granulomas/pyogranulomas, leishmaniasis and mycobacterial infections. J. Small Anim. Pract. 2008, 49, 552–561. [Google Scholar] [CrossRef]
- Diaz, S. Canine Sterile Papular and Nodular Skin Diseases. Clin. Small Anim. Intern. Med. 2020, 30, 1441–1448. [Google Scholar]
- Moosavian, H.; Mashayekhi-Goyonlo, V.; Rajayee Mousavi, S.A. Long-term successful management of an idiopathic interstitial pyogranulomatous/granulomatous dermatitis and folliculitis by omega 3 fatty acid in a dog. Comp. Clin. Pathol. 2021, 30, 335–339. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Hendricks, A.; Phillips, J.A.; Brodbelt, D.C.; Church, D.B.; Loeffler, A. Non-neoplastic anal sac disorders in UK dogs: Epidemiology and management aspects of a research-neglected syndrome. Vet. Rec. 2021, 189, e203. [Google Scholar] [CrossRef]
- Rutherford, L.; Lee, K. Anal sac disease in dogs. Practice 2015, 37, 435–444. [Google Scholar] [CrossRef]
- Lundberg, A.; Koch, S.N.; Torres, S.M.F. Local treatment for canine anal sacculitis: A retrospective study of 33 dogs. Vet. Dermatol. 2022, 33, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, J. Canine pododermatitis. Can. Vet. J. 2016, 57, 991–993. [Google Scholar] [PubMed]
- Bouza-Rapti, P.; Kaltsogianni, F.; Koutinas, A.; Farmaki, R. Canine pododermatitis: A retrospective study of 300 cases. J. Hell. Vet. Med. Soc. 2023, 74, 5355–5362. [Google Scholar]
- Breathnach, R.M.; Fanning, S.; Mulcahy, G.; Bassett, H.F.; Jones, B.R. Canine pododermatitis and idiopathic disease. Vet. J. 2008, 176, 146–157. [Google Scholar] [CrossRef]
- Duclos, D.D.; Hargis, A.M.; Hanley, P.W. Pathogenesis of canine interdigital palmar and plantar comedones and follicular cysts, and their response to laser surgery. Vet. Dermatol. 2008, 19, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Perego, R.; Proverbio, D.; Zuccaro, A.; Spada, E. Low-level laser therapy: Case-control study in dogs with sterile pyogranulomatous pododermatitis. Vet. World 2016, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Salmon Hillbertz, N.H.; Isaksson, M.; Karlsson, E.K.; Hellmén, E.; Pielberg, G.R.; Savolainen, P.; Wade, C.M.; Von Euler, H.; Gustafson, U.; Hedhammar, Å. Duplication of FGF3, FGF4, FGF19 and ORAOV1 causes hair ridge and predisposition to dermoid sinus in Ridgeback dogs. Nat. Genet. 2007, 39, 1318–1320. [Google Scholar] [CrossRef]
- Scott, D.; Miller Jr, W.H. Idiopathic nasodigital hyperkeratosis in dogs: A retrospective analysis of 35 cases (1988–1998). Jpn. J. Vet. Dermatol. 2012, 18, 169–170. [Google Scholar] [CrossRef][Green Version]
- Lee, F.F.; Bradley, C.W.; Cain, C.L.; White, S.D.; Outerbridge, C.A.; Murphy, L.A.; Mauldin, E.A. Localized parakeratotic hyperkeratosis in sixteen Boston terrier dogs. Vet. Dermatol. 2016, 27, 384-e96. [Google Scholar] [CrossRef]
- Lachaume, P.; Hitte, C.; Jouquand, S.; Priat, C.; Galibert, F. Identification and analysis of the dog keratin 9 (KRT9) gene. Anim. Genet. 1998, 29, 173–177. [Google Scholar] [CrossRef]
- Scott, D.W.; Miller, W.H. Retrospective record review of canine postclipping hair follicle arrest. Vet. Dermatol. 2012, 23, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.C.; Schick, R.O.; Savage, M.Y.; Fadok, V.A. A small scale study to evaluate the efficacy of microneedling in the presence or absence of platelet-rich plasma in the treatment of post-clipping alopecia in dogs. Vet. Dermatol. 2020, 31, 214-e45. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).