Evaluation of the Genetic Diversity, Population Structure and Selection Signatures of Three Native Chinese Pig Populations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Sequencing
2.2. Quality Control and Genotyping
2.3. Genetic Diversity and Linkage Disequilibrium
2.4. Genetic Differentiation Analysis
2.5. Population Genetic Structure
2.6. Detection of the Selection Signatures
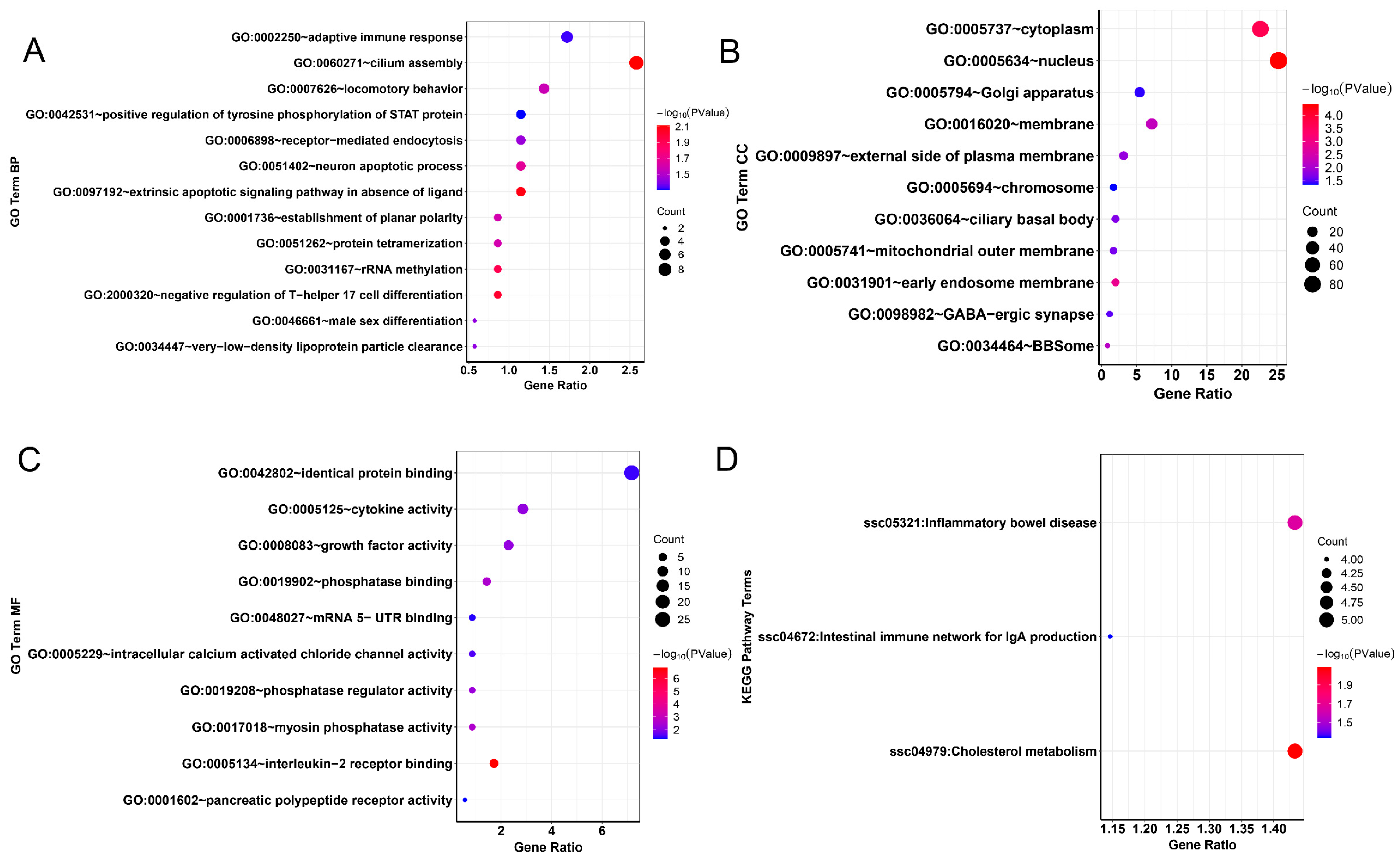
2.7. Functional Enrichment Analysis of Candidate Genes
3. Results
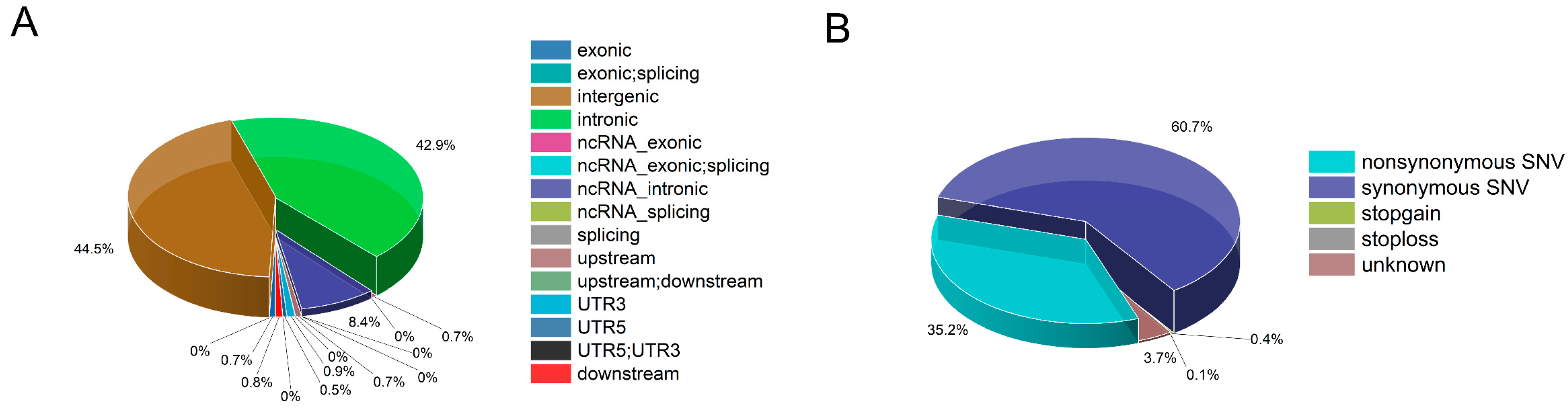
3.1. Single Nucleotide Polymorphism Discovery
3.2. Analysis of Genetic Diversity and Linkage Disequilibrium
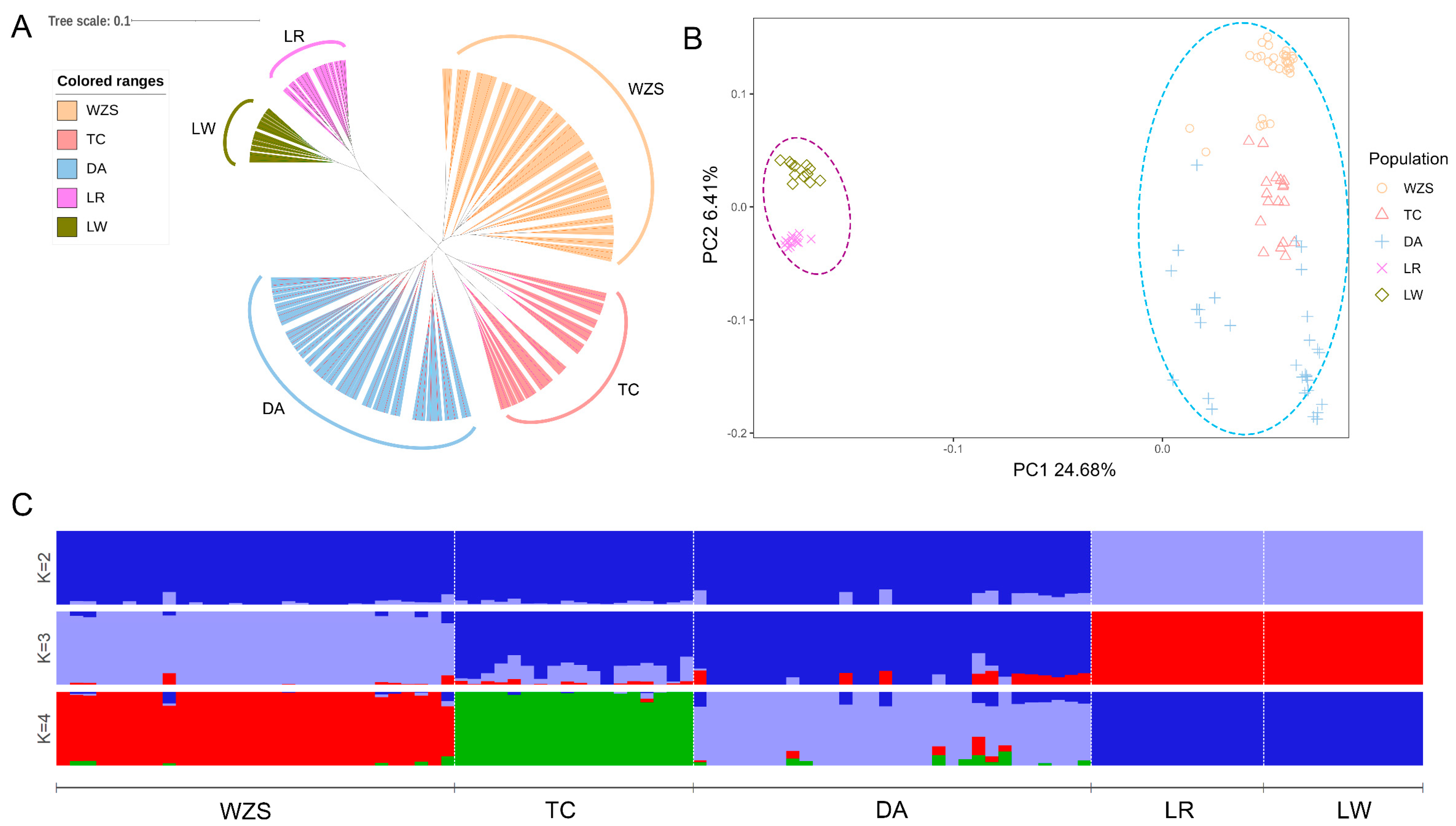
3.3. Analysis of Genetic Structure
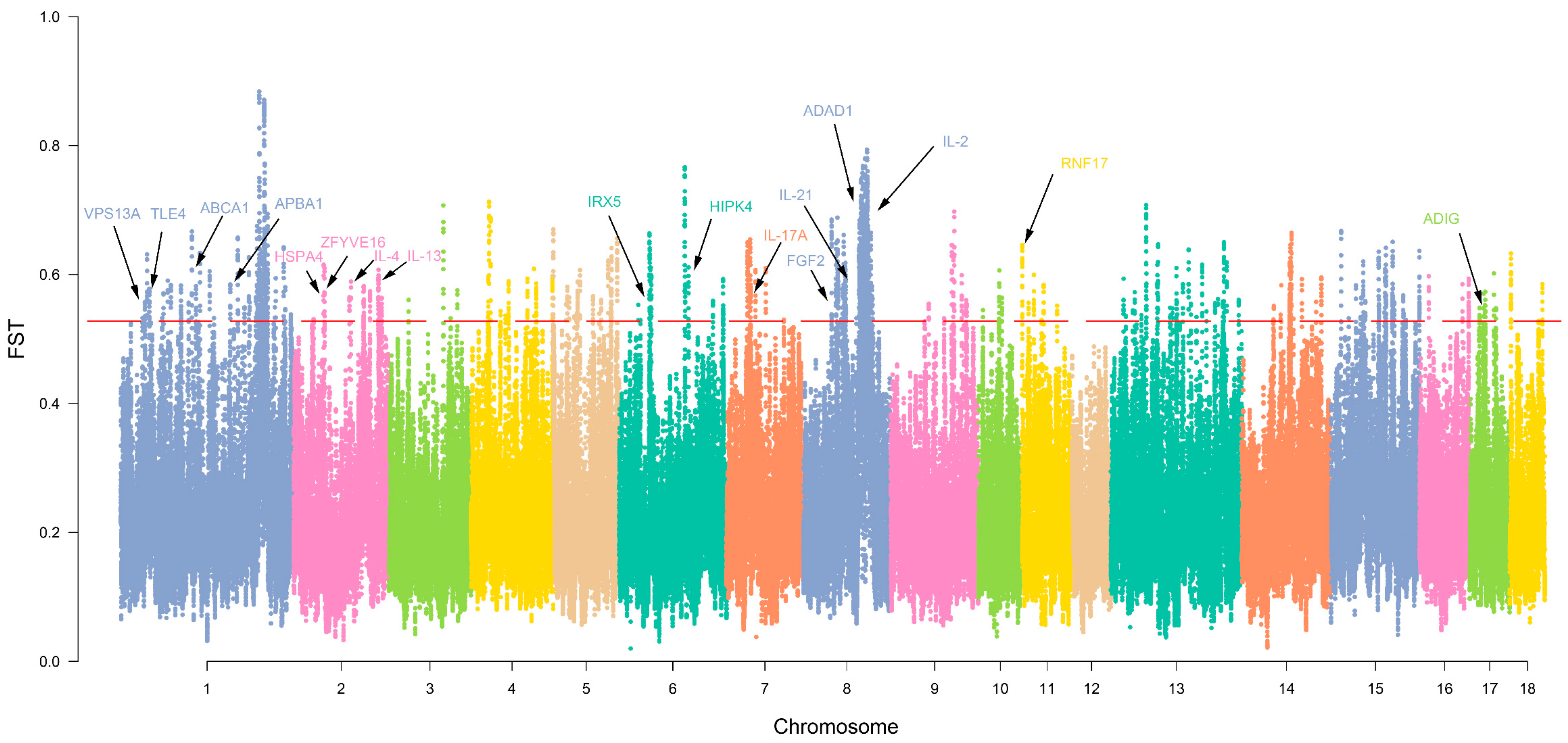
3.4. Selection Signature and Functional Clustering of Variant Genes
4. Discussion
4.1. Genetic Diversity and Linkage Disequilibrium Analysis of Chinese Indigenous Pigs and Commercial Pigs
4.2. Population Structure of Indigenous Pigs and Commercial Pigs
4.3. Gene Annotation and Functional Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.L.; Chai, Y.; Zhang, W.C.; Cheng, Y.W.; Zhang, Z.X.; An, Q.; Chen, S.; Man, C.; Du, L.; Zhang, W.G.; et al. Whole-Genome Sequencing Reveals the Genomic Characteristics and Selection Signatures of Hainan Black Goat. Genes 2022, 13, 1539. [Google Scholar] [CrossRef]
- China National Commission of Animal Genetic Resources. Animal Genetic Resources in China Pigs; China Agriculture Press: Beijing, China, 2011. [Google Scholar]
- Fang, X.; Mou, Y.; Huang, Z.; Li, Y.; Han, L.; Zhang, Y.; Feng, Y.; Chen, Y.; Jiang, X.; Zhao, W.; et al. The sequence and analysis of a Chinese pig genome. Gigascience 2012, 1, 16. [Google Scholar] [CrossRef]
- Li, C.Q.; Liu, G.P.; Tong, K.; Wang, Y.; Li, T.; Tan, X.; Yang, J.; Yang, X.L.; Guo, L.W.; Zeng, J.G. Pathogenic ecological characteristics of PCV2 in large-scale pig farms in China affected by African swine fever in the surroundings from 2018 to 2021. Front. Microbiol. 2023, 13, 1013617. [Google Scholar] [CrossRef]
- Qiao, R.; Li, X.; Han, X.; Wang, K.; Lv, G.; Ren, G. Population structure and genetic diversity of four Henan pig populations. Anim. Genet. 2019, 50, 262–265. [Google Scholar] [CrossRef]
- Alhusain, L.; Hafez, A.M. Nonparametric approaches for population structure analysis. Hum. Genom. 2018, 12, 25. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.J.; Jiang, Y.; Zhou, M.; Liu, L.Q.; Su, S.G.; Xu, C.L.; Li, X.T.; Wang, C.L. Genetic architecture and selection of Anhui autochthonous pig population revealed by whole genome resequencing. Front. Genet. 2022, 13, 1022261. [Google Scholar] [CrossRef]
- Qiao, R.M.; Zhang, M.H.; Zhang, B.; Li, X.J.; Han, X.L.; Wang, K.J.; Li, X.L.; Yang, F.; Hu, P.Y. Population genetic structure analysis and identification of backfat thickness loci of Chinese synthetic Yunan pigs. Front. Genet. 2022, 13, 1039838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, M.; Zhou, M.; Wang, Y.L.; Wu, X.D.; Zhang, X.D.; Ding, Y.Y.; Zhao, G.Y.; Yin, Z.J.; Wang, C.L. Identification of Signatures of Selection by Whole-Genome Resequencing of a Chinese Native Pig. Front. Genet. 2020, 11, 566255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.T.; Ye, X.W.; Zhang, Z.; Zhao, Q.B.; Xiang, Y.; Xu, N.Y.; Wang, Q.S.; Pan, Y.C.; Guo, X.L.; Wang, Z. Genetic diversity and selection signatures of four indigenous pig breeds from eastern China. Anim. Genet. 2022, 53, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, P.; Zheng, X.; Zhou, L.; Wang, C.; Liu, J.F. Genome-Wide Detection of Selection Signatures in Duroc Revealed Candidate Genes Relating to Growth and Meat Quality. G3 Genes Genomes Genet. 2020, 10, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Huang, M.; Tang, J.; Yang, L.; Yu, Z.; Li, D.; Li, G.; Jiang, Y.; Sun, Y.; et al. Whole-genome SNP markers reveal conservation status, signatures of selection, and introgression in Chinese Laiwu pigs. Evol. Appl. 2021, 14, 383–398. [Google Scholar] [CrossRef]
- Schiavo, G.; Bertolini, F.; Galimberti, G.; Bovo, S.; Dall’Olio, S.; Nanni Costa, L.; Gallo, M.; Fontanesi, L. A machine learning approach for the identification of population-informative markers from high-throughput genotyping data: Application to several pig breeds. Animal 2020, 14, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, A.M.; Cardoso, D.F.; Carvalheiro, R.; Junior, G.A.F.; de Albuquerque, L.G.; de Oliveira, H.N. Signatures of selection in Nelore cattle revealed by whole-genome sequencing data. Genomics 2022, 114, 110304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Chen, Y.S.; Shi, C.M.; Huang, Z.B.; Zhang, Y.; Li, S.K.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2017, 7, gix120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010, 38, e164. [Google Scholar] [CrossRef]
- Yuan, J.; Zhou, X.; Xu, G.Q.; Xu, S.P.; Liu, B. Genetic diversity and population structure of Tongcheng pigs in China using whole-genome SNP chip. Front. Genet. 2022, 13, 910521. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, S.S.; Xu, J.Y.; He, W.M.; Yang, T.L. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, K.E.; Weir, B.S. Genetics in geographically structured populations: Defining, estimating and interpreting F-ST. Nat. Rev. Genet. 2009, 10, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Hu, Z.L.; Park, C.A.; Reecy, J.M. Bringing the Animal QTLdb and CorrDB into the future: Meeting new challenges and providing updated services. Nucleic Acids Res. 2022, 50, D956–D961. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.Y.; Zhang, Y.; Tang, Z.L.; Li, K.; Liu, B. Genome-wide analysis reveals artificial selection on coat colour and reproductive traits in Chinese domestic pigs. Mol. Ecol. Resour. 2015, 15, 414–424. [Google Scholar] [CrossRef]
- Ai, H.S.; Huang, L.S.; Ren, J. Genetic Diversity, Linkage Disequilibrium and Selection Signatures in Chinese and Western Pigs Revealed by Genome-Wide SNP Markers. PLoS ONE 2013, 8, e56001. [Google Scholar] [CrossRef]
- Chen, J.; Peng, J.; Xiao, Q.; Pan, Y.; Zhang, X.; Lo, L.J.; Xu, N. The genetic diversity and population structures of indigenous pig breeds in Zhejiang Province revealed by GGRS sequencing. Anim. Genet. 2018, 49, 36–42. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Huang, M.; Tang, J.; Fan, Y.; Li, Y.; Li, X.; Ji, H.; Ren, J.; Ding, N. Genetic diversity, population structure and phylogenetic relationships of three indigenous pig breeds from Jiangxi Province, China, in a worldwide panel of pigs. Anim. Genet. 2018, 49, 275–283. [Google Scholar] [CrossRef]
- Diao, S.Q.; Huang, S.W.; Xu, Z.T.; Ye, S.P.; Yuan, X.L.; Chen, Z.M.; Zhang, H.; Zhang, Z.; Li, J.Q. Genetic Diversity of Indigenous Pigs from South China Area Revealed by SNP Array. Animals 2019, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Li, P.H.; Zhou, W.D.; Ma, X.; Wang, X.P.; Xu, Y.; Jiang, N.J.; Zhao, M.R.; Zhou, T.W.; Yin, Y.Z.; et al. Genome Data Uncover Conservation Status, Historical Relatedness and Candidate Genes Under Selection in Chinese Indigenous Pigs in the Taihu Lake Region. Front. Genet. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef]
- Ren, H.M.; Lukacher, A.E.; Rahman, Z.S.M.; Olsen, N.J. New developments implicating IL-21 in autoimmune disease. J. Autoimmun. 2021, 122, 102689. [Google Scholar] [CrossRef] [PubMed]
- McGinley, A.M.; Sutton, C.E.; Edwards, S.C.; Leane, C.M.; DeCourcey, J.; Teijeiro, A.; Hamilton, J.A.; Boon, L.; Djouder, N.; Mills, K.H.G. Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1 beta-Producing Myeloid Cells that Promote Pathogenic T Cells. Immunity 2020, 52, 342–356. [Google Scholar] [CrossRef]
- Zhao, X.M.; Li, D.H.; Qiu, Q.S.; Jiao, B.; Zhang, R.H.; Liu, P.; Ren, R.B. Zfyve16 regulates the proliferation of B-lymphoid cells. Front. Med. 2018, 12, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Marioni, R.E.; Liewald, D.C.; Hill, W.D.; Hagenaars, S.P.; Harris, S.E.; Ritchie, S.J.; Luciano, M.; Fawns-Ritchie, C.; Lyall, D.; et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112151). Mol. Psychiatry 2016, 21, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.; Loffredo, C.A.; Simhadri, J.; Nunlee-Bland, G.; Korba, B.; Johnson, J.; Cotin, S.; Moses, G.; Quartey, R.; Howell, C.D.; et al. Insights on the pathogenesis of type 2 diabetes as revealed by signature genomic classifiers in an African American population in the Washington, DC area. Diabetes/Metab. Res. 2023, 39, e3589. [Google Scholar] [CrossRef]
- Pan, J.; Goodheart, M.; Chuma, S.; Nakatsuji, N.; Page, D.C.; Wang, P.J. RNF17, a component of the mammalian germ cell nuage, is essential for spermiogenesis. Development 2005, 132, 4029–4039. [Google Scholar] [CrossRef]
- Du, C.; Davis, J.S.; Chen, C.; Li, Z.; Cao, Y.; Sun, H.; Shao, B.S.; Lin, Y.X.; Wang, Y.S.; Yang, L.G.; et al. FGF2/FGFR signaling promotes cumulus-oocyte complex maturation in vitro. Reproduction 2021, 161, 205–214. [Google Scholar] [CrossRef]
- Snyder, E.; Chukrallah, L.; Seltzer, K.; Goodwin, L.; Braun, R.E. ADAD1 and ADAD2, testis-specific adenosine deaminase domain-containing proteins, are required for male fertility. Sci. Rep. 2020, 10, 11536. [Google Scholar] [CrossRef]
- Crapster, J.A.; Rack, P.G.; Hellmann, Z.J.; Le, A.D.; Adams, C.M.; Leib, R.D.; Elias, J.E.; Perrino, J.; Behr, B.; Li, Y.F.; et al. HIPK4 is essential for murine spermiogenesis. eLife 2020, 9, e50209. [Google Scholar] [CrossRef]
- Qiu, F.F.; Xie, L.; Ma, J.E.; Luo, W.; Zhang, L.; Chao, Z.; Chen, S.H.; Nie, Q.H.; Lin, Z.M.; Zhang, X.Q. Lower Expression of SLC27A1 Enhances Intramuscular Fat Deposition in Chicken via Down-Regulated Fatty Acid Oxidation Mediated by CPT1A. Front. Physiol. 2017, 8, 449. [Google Scholar] [CrossRef]
- Alvarez-Guaita, A.; Patel, S.; Lim, K.; Haider, A.; Dong, L.; Conway, O.J.; Ma, M.K.L.; Chiarugi, D.; Saudek, V.; O’Rahilly, S.; et al. Phenotypic characterization of Adig null mice suggests roles for adipogenin in the regulation of fat mass accrual and leptin secretion. Cell Rep. 2021, 34, 108810. [Google Scholar] [CrossRef]
- Zhang, F.W.; Hanif, Q.; Luo, X.Y.; Jin, X.D.; Zhang, J.C.; He, Z.X.; Lei, C.Z.; Liu, J.Y.; Huang, B.Z.; Qu, K.X. Muscle transcriptome analysis reveal candidate genes and pathways related to fat and lipid metabolism in Yunling cattle. Anim. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Agarwal, M.; Bharadwaj, A.; Mathew, S.J. TLE4 regulates muscle stem cell quiescence and skeletal muscle differentiation. J. Cell Sci. 2022, 135, jcs256008. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.L.; Huang, L.Y.; Tian, T.; Wu, H.L.; Yao, H.T.; Marmo, T.; Song, F.F.; Huang, C. IRX5 promotes adipogenesis of hMSCs by repressing glycolysis. Cell Death Discov. 2022, 8, 204. [Google Scholar] [CrossRef]
- Asadollahi, H.; Vaez Torshizi, R.; Ehsani, A.; Masoudi, A.A. An association of CEP78, MEF2C, VPS13A and ARRDC3 genes with survivability to heat stress in an F2 chicken population. J. Anim. Breed Genet. 2022, 139, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.M.; Schmid, E.; Munzer, P.; Hermann, A.; Eyrich, A.K.; Russo, A.; Walker, B.; Gu, S.; vom Hagen, J.M.; Faggio, C.; et al. Chorein sensitivity of cytoskeletal organization and degranulation of platelets. FASEB J. 2013, 27, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Sakurai, T.; Kashida, H.; Mine, H.; Hagiwara, S.; Matsui, S.; Yoshida, K.; Nishida, N.; Watanabe, T.; Itoh, K.; et al. Involvement of Heat Shock Protein A4/Apg-2 in Refractory Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Lv, Y.J.; Zhang, M.; Yue, Z.H.; Tang, S.; Islam, A.; Rehana, B.; Bao, E.D.; Hartung, J. Hsp110 expression changes in rat primary myocardial cells exposed to heat stress in vitro. Genet. Mol. Res. 2012, 11, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
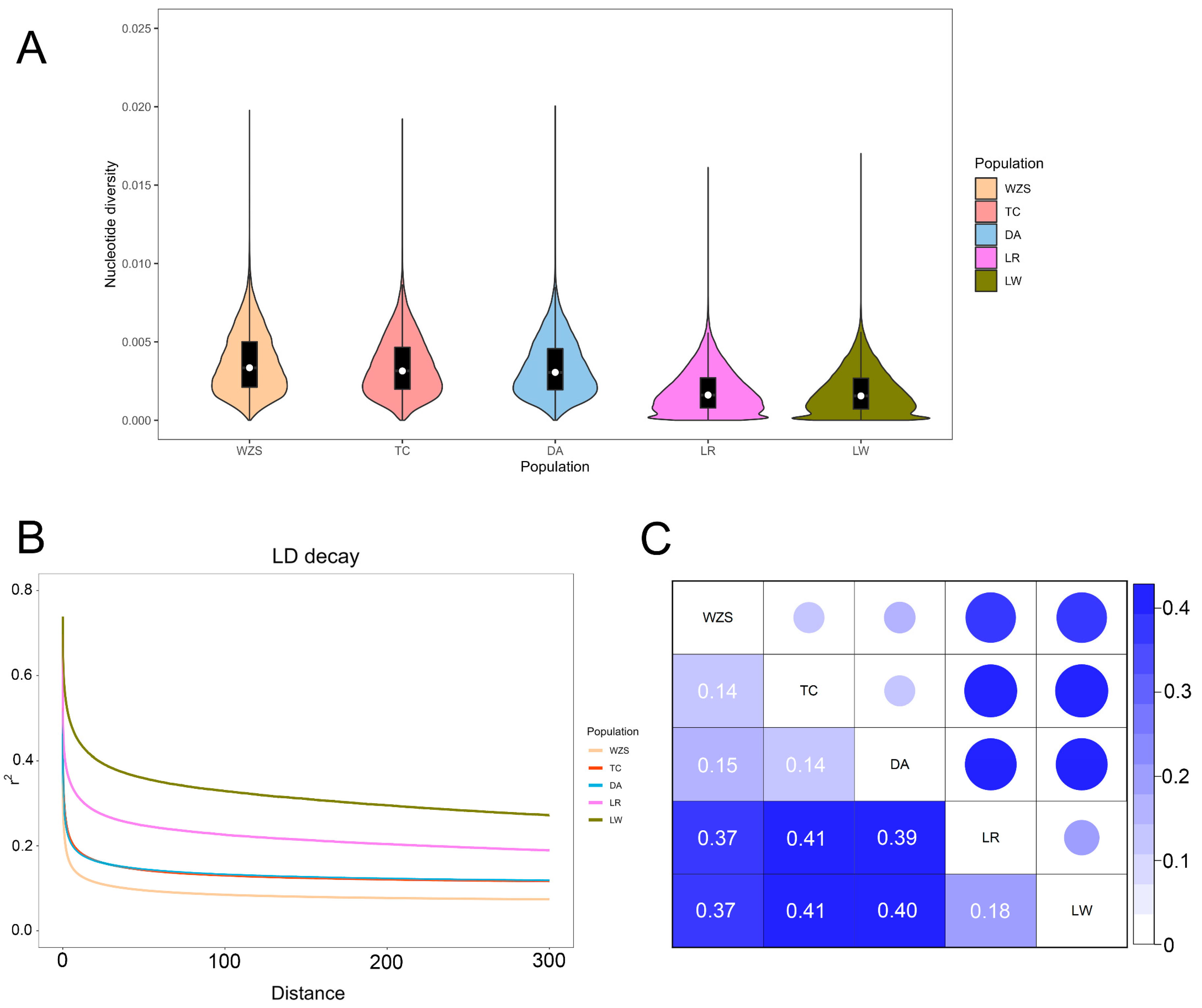
| Population | Abbreviation | Size | MAF | HO | HE | Nucleotide Diversity (pi) |
|---|---|---|---|---|---|---|
| Wuzhishan | WZS | 30 | 0.22 ± 0.13 | 0.30 ± 0.15 | 0.31 ± 0.14 | 0.0037 |
| Tunchang | TC | 18 | 0.23 ± 0.13 | 0.33 ± 0.18 | 0.32 ± 0.14 | 0.0034 |
| Dingan | DA | 30 | 0.23 ± 0.14 | 0.34 ± 0.17 | 0.32 ± 0.14 | 0.0034 |
| Landrace | LR | 13 | 0.24 ± 0.13 | 0.29 ± 0.15 | 0.34 ± 0.12 | 0.0019 |
| Large White | LW | 12 | 0.26 ± 0.13 | 0.39 ± 0.18 | 0.35 ± 0.12 | 0.0018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Z.; Wang, Z.; Xie, X.; Tian, S.; Wang, F.; Wang, Q.; Ni, S.; Pan, Y.; Xiao, Q. Evaluation of the Genetic Diversity, Population Structure and Selection Signatures of Three Native Chinese Pig Populations. Animals 2023, 13, 2010. https://doi.org/10.3390/ani13122010
Zhong Z, Wang Z, Xie X, Tian S, Wang F, Wang Q, Ni S, Pan Y, Xiao Q. Evaluation of the Genetic Diversity, Population Structure and Selection Signatures of Three Native Chinese Pig Populations. Animals. 2023; 13(12):2010. https://doi.org/10.3390/ani13122010
Chicago/Turabian StyleZhong, Ziqi, Ziyi Wang, Xinfeng Xie, Shuaishuai Tian, Feifan Wang, Qishan Wang, Shiheng Ni, Yuchun Pan, and Qian Xiao. 2023. "Evaluation of the Genetic Diversity, Population Structure and Selection Signatures of Three Native Chinese Pig Populations" Animals 13, no. 12: 2010. https://doi.org/10.3390/ani13122010
APA StyleZhong, Z., Wang, Z., Xie, X., Tian, S., Wang, F., Wang, Q., Ni, S., Pan, Y., & Xiao, Q. (2023). Evaluation of the Genetic Diversity, Population Structure and Selection Signatures of Three Native Chinese Pig Populations. Animals, 13(12), 2010. https://doi.org/10.3390/ani13122010





