Disentangling Exploitative and Interference Competition on Forest Dwelling Salamanders
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Framework
2.2. Study Species
2.3. Study Site
2.4. Salamanders’ Sampling
2.5. Environmental Covariates
2.6. Data Analysis
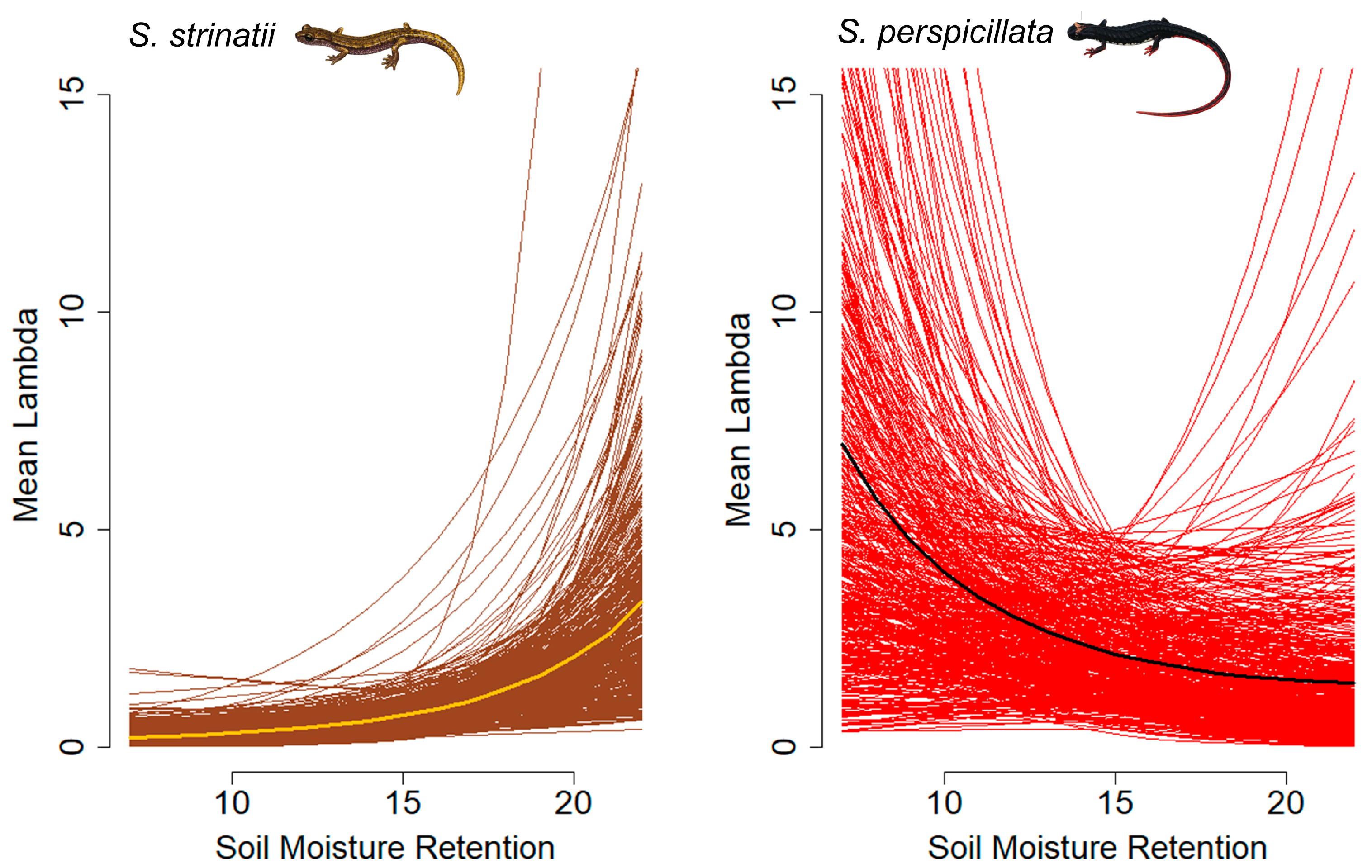
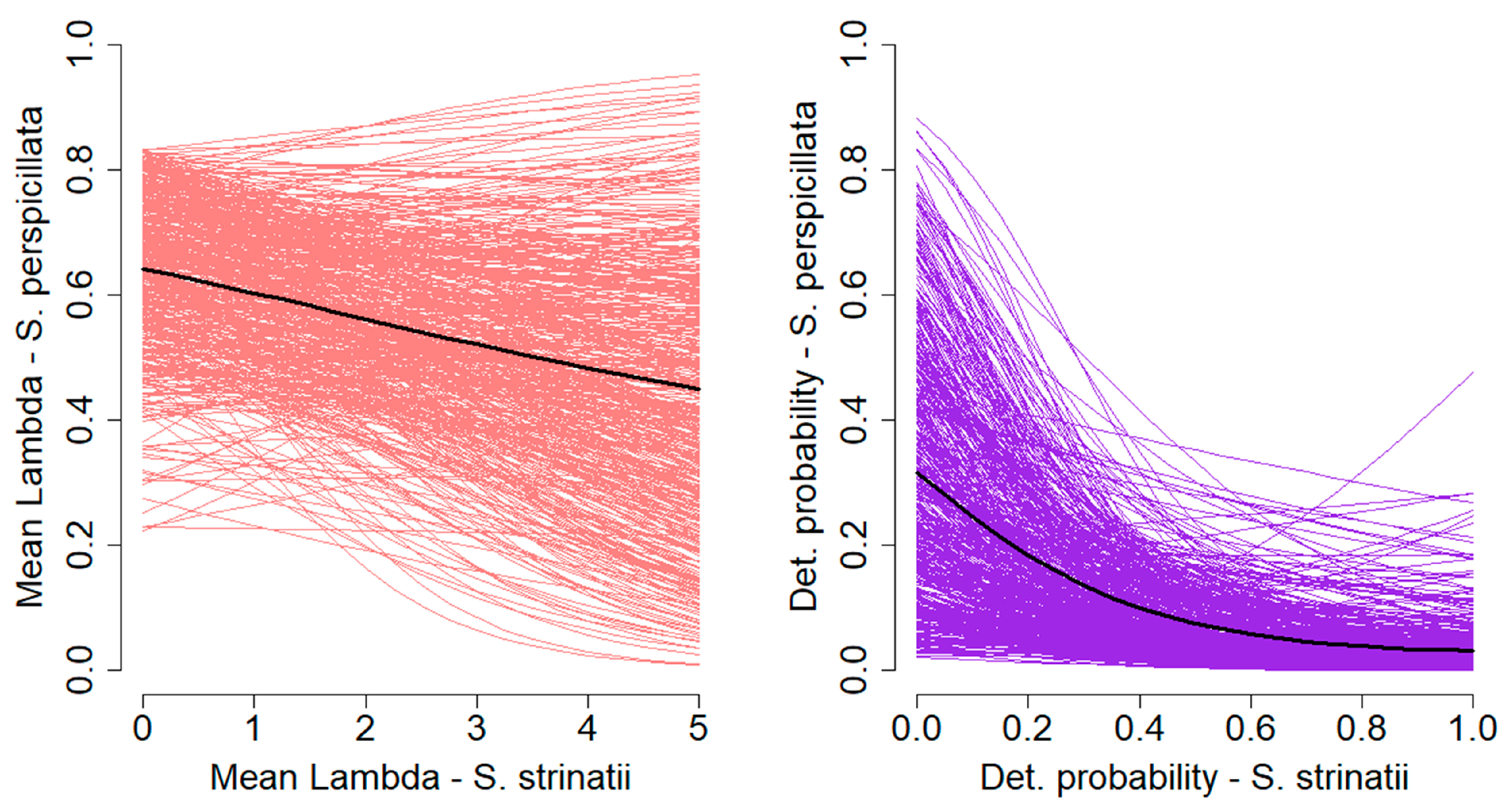
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fretwell, S.D.; Lucas, H.L. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 1970, 19, 16–36. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Connell, J.H. On the prevalence and relative importance of interspecific competition: Evidence from field experiments. Am. Nat. 1983, 122, 661–696. [Google Scholar] [CrossRef]
- Houston, A.I.; McNamara, J.M. The ideal free distribution when competitive abilities differ: An approach based on statistical mechanics. Anim. Behav. 1988, 36, 166–174. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance, 5th ed.; Benjamin Cummings: San Francisco, CA, USA, 2001. [Google Scholar]
- Périquet, S.; Fritz, H.; Revilla, E. The Lion King and the Hyaena Queen: Large carnivore interactions and coexistence. Biol. Rev. 2015, 90, 1197–1214. [Google Scholar] [CrossRef]
- Grether, G.F.; Okamoto, K.W. Eco-evolutionary dynamics of interference competition. Ecol. Lett. 2022, 25, 2167–2176. [Google Scholar] [CrossRef]
- Grether, G.F.; Peiman, K.S.; Tobias, A.; Robinson, B.W. Causes and consequences of behavioral interference between species. Trends Ecol. Evol. 2017, 32, 760–772. [Google Scholar] [CrossRef]
- Smallegange, I.M.; Van Der Meer, J.; Kurvers, R.H. Disentangling interference competition from exploitative competition in a crab–bivalve system using a novel experimental approach. Oikos 2006, 113, 157–167. [Google Scholar] [CrossRef]
- Lang, B.; Rall, B.C.; Brose, U. Warming effects on consumption and intraspecific interference competition depend on predator metabolism. J. Anim. Ecol. 2012, 81, 516–523. [Google Scholar] [CrossRef]
- Daly, M. The cost of mating. Am. Nat. 1978, 112, 771–774. [Google Scholar] [CrossRef]
- Parker, G.A. Sperm competition and the evolution of animal mating strategies. In Sperm Competition and the Evolution of Animal Mating Systems; Elsevier Academic Press: Cambdridge, MA, USA, 1984; pp. 1–60. [Google Scholar]
- Schoener, T.W. Resource Partitioning in Ecological Communities: Research on how similar species divide resources helps reveal the natural regulation of species diversity. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Gerhardt, H.C.; Huber, F. Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions; University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Case, T.J.; Gilpin, M.E. Interference competition and niche theory. Proc. Natl. Acad. Sci. USA 1974, 71, 3073–3077. [Google Scholar] [CrossRef]
- Carothers, J.H.; Jaksić, F.M. Time as a niche difference: The role of interference competition. Oikos 1984, 42, 403–406. [Google Scholar] [CrossRef]
- Hardin, G. The Competitive Exclusion Principle: An idea that took a century to be born has implications in ecology, economics, and genetics. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef]
- Adler, F.R.; Mosquera, J. Is space necessary? Interference competition and limits to biodiversity. Ecology 2000, 81, 3226–3232. [Google Scholar] [CrossRef]
- Amarasekare, P. Interference competition and species coexistence. Proc. R. Soc. Lond. B 2002, 269, 2541–2550. [Google Scholar] [CrossRef]
- Petren, K.; Case, T.J. An experimental demonstration of exploitation competition in an ongoing invasion. Ecology 1996, 77, 118–132. [Google Scholar] [CrossRef]
- Schoener, T.W. Competition and the Niche. In Biology of the Reptilia. Ecology; Gans, A.C., Tinkle, D.W., Eds.; Academic Press: New York, NY, USA, 1977; Volume 7, pp. 35–136. [Google Scholar]
- Schoener, T.W. Field experiments on interspecific competition. Am. Nat. 1983, 122, 240–285. [Google Scholar] [CrossRef]
- Albrecht, M.; Gotelli, N.J. Spatial and temporal niche partitioning in grassland ants. Oecologia 2001, 126, 134–141. [Google Scholar] [CrossRef]
- Andrewartha, H.G.; Birch, L.C. The Distribution and Abundance of Animals; University of Chicago Press: Chicago, IL, USA, 1954. [Google Scholar]
- Sinclair, A.R.E. Population regulation in animals. In Ecological Concept; Cherret, J.M., Ed.; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Valeix, M.; Chamaillé-Jammes, S.; Fritz, H. Interference competition and temporal niche shifts: Elephants and herbivore communities at waterholes. Oecologia 2007, 153, 739–748. [Google Scholar] [CrossRef]
- Olea, P.P.; Iglesias, N.; Mateo-Tomás, P. Temporal resource partitioning mediates vertebrate coexistence at carcasses: The role of competitive and facilitative interactions. Basic Appl. Ecol. 2022, 60, 63–75. [Google Scholar] [CrossRef]
- Salvidio, S.; Romano, A.; Oneto, F.; Ottonello, D.; Michelon, R. Different season, different strategies: Feeding ecology of two syntopic forest-dwelling salamanders. Acta Oecol. 2012, 43, 42–50. [Google Scholar]
- Costa, A.; Salvidio, S.; Posillico, M.; Matteucci, G.; De Cinti, B.; Romano, A. Generalisation within specialization: Inter-individual diet variation in the only specialized salamander in the world. Sci. Rep. 2015, 5, 13260. [Google Scholar] [CrossRef]
- Royle, J.A. N-mixture models for estimating Population size from spatially replicated counts. Biometrics 2004, 60, 108–115. [Google Scholar] [CrossRef]
- Kéry, M.; Royle, J.A. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS: Volume 2: Dynamic and Advanced Models; Academic Press: London, UK, 2020. [Google Scholar]
- Waddle, J.; Dorazio, R.M.; Walls, S.; Rice, K.; Beauchamp, J.; Schuman, M. A new parameterization for estimating co-occurrence of interacting species. Ecol. Appl. 2010, 20, 1467–1475. [Google Scholar] [CrossRef]
- Clare, J.D.; Linden, D.W.; Anderson, E.M.; MacFarland, D.M. Do the antipredator strategies of shared prey mediate intraguild predation and mesopredator suppression? Ecol. Evol. 2016, 6, 3884–3897. [Google Scholar] [CrossRef]
- Roth, T.; Allan, E.; Pearman, P.B.; Amrhein, V. Functional ecology and imperfect detection of species. Methods Ecol. Evol. 2018, 9, 917–928. [Google Scholar] [CrossRef]
- Brodie, J.F.; Helmy, O.E.; Mohd-Azlan, J.; Granados, A.; Bernard, H.; Giordano, A.J.; Zipkin, E. Models for assessing local-scale co-abundance of animal species while accounting for differential detectability and varied responses to the environment. Biotropica 2018, 50, 5–15. [Google Scholar] [CrossRef]
- Rosa, G.; Salvidio, S.; Costa, A. European plethodontid salamanders on the forest floor: Testing for age-class segregation and habitat selection. J. Herpetol. 2022, 56, 27–33. [Google Scholar] [CrossRef]
- Romano, A.; Rosa, G.; Salvidio, S.; Novaga, R.; Costa, A. How landscape and biotic interactions shape a Mediterranean reptile community. Landsc. Ecol. 2022, 37, 2915–2927. [Google Scholar] [CrossRef]
- Angelini, C.; Vanni, S.; Vignoli, L. Salamandrina terdigitata (Bonnaterre, 1789) Salamandrina perspicillata (Savi, 1821). In Fauna d’Italia. 42. Amphibia; Lanza, B., Ed.; Edizioni Calderini: Bologna, Italy, 2007. [Google Scholar]
- Lanza, B. Speleomantes strinatii (Aellen, 1958). In Fauna d’Italia. 42. Amphibia; Lanza, B., Andreone, F., Bologna, M., Corti, C., Razzetti, E., Eds.; Edizioni Calderini: Bologna, Italy, 2007. [Google Scholar]
- Costa, A.; Crovetto, F.; Salvidio, S. European plethodontid salamanders on the forest floor: Local abundance is related to fine-scale environmental factors. Herpetol. Conserv. Biol. 2016, 11, 344–349. [Google Scholar]
- Blondel, J.; Aronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Romano, A.; Costa, A.; Basile, M.; Raimondi, R.; Posillico, M.; Scinti Roger, D.; De Cinti, B. Conservation of salamanders in managed forests: Methods and costs of monitoring abundance and habitat selection. For. Ecol. Manag. 2017, 400, 12–18. [Google Scholar] [CrossRef]
- Guisan, A.; Weiss, S.B.; Weiss, A.D. GLM versus CCA spatial modelling of plant species distribution. Plant Ecol. 1999, 143, 107–122. [Google Scholar] [CrossRef]
- Conrad, O.; Bechtel, B.; Bock, M.; Dietrich, H.; Fischer, E.; Gerlitz, L.; Böhner, J. System for automated geoscientific analyses (SAGA) v. 2.1.4. Geosci. Model Dev. 2015, 8, 1991–2007. [Google Scholar] [CrossRef]
- Amir, Z.; Sovie, A.; Luskin, M.S. Inferring predator–prey interactions from camera traps: A Bayesian co-abundance modeling approach. Ecol. Evol. 2022, 12, e9627. [Google Scholar] [CrossRef]
- Mac Nally, R. Multiple regression and inference in ecology and conservation biology: Further comments on identifying important predictor variables. Biodiv. Conserv. 2002, 11, 1397–1401. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; García Marquéz, R. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Kéry, M.; Schaub, M. Bayesian Population Analysis Using WinBUGS: A Hierarchical Perspective; Academic Press: London, UK, 2011. [Google Scholar]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
- Duarte, A.; Adams, M.J.; Peterson, J.T. Fitting N-mixture models to count data with unmodeled heterogeneity: Bias, diagnostics, and alternative approaches. Ecol. Model. 2018, 374, 51–59. [Google Scholar] [CrossRef]
- Knape, J.; Arlt, D.; Barraquand, F.; Berg, Å.; Chevalier, M.; Pärt, T.; Żmihorski, M. Sensitivity of binomial N-mixture models to overdispersion: The importance of assessing model fit. Met. Ecol. Evol. 2018, 9, 2102–2114. [Google Scholar] [CrossRef]
- Costa, A.; Salvidio, S.; Penner, J.; Basile, M. Time-for-space substitution in N-mixture models for estimating population trends: A simulation-based evaluation. Sci. Rep. 2021, 11, 4581. [Google Scholar] [CrossRef]
- Plummer, M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vienna, Austria, 20–22 March 2003; Volume 124, pp. 1–10. [Google Scholar]
- Kellner, K. jagsUI: A Wrapper around Rjags to Streamline JAGSanalyses. R Package Version 1.5.1. Available online: https://github.com/kenkellner/jagsUI (accessed on 1 March 2023).
- Rosa, G.; Bosio, M.; Salvidio, S.; Costa, A. Foraging success is differently affected by local climate in two syntopic forest-dwelling salamanders. Ethol. Ecol. Evol. 2022, 1–10. [Google Scholar] [CrossRef]
- Costa, A.; Rosa, G.; Salvidio, S. Size-Mediated Trophic Interactions in two Syntopic Forest Salamanders. Animals 2023, 13, 1281. [Google Scholar] [CrossRef]
- Jaeger, R.G.; Gollmann, B.; Anthony, C.D.; Gabor, C.R.; Kohn, N.R. Behavioral Ecology of the Eastern Red-Backed Salamander: 50 Years of Research; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Alatalo, R.V.; Gustafsson, L.; Linden, M.; Lundberg, A. Interspecific competition and niche shifts in tits and the goldcrest: An experiment. J. Anim. Ecol. 1985, 54, 977–984. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dayan, T. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 153–181. [Google Scholar] [CrossRef]
- Jaeger, R.G.; Kalvarsky, D.; Shimizu, N. Territorial behaviour of the red-backed salamander: Expulsion of intruders. Anim. Behav. 1983, 31, 490–496. [Google Scholar] [CrossRef]
- Jaeger, R.G.; Wicknick, J.A.; Griffis, M.R.; Anthony, C.D. Socioecology of a terrestrial salamander: Juveniles enter adult territories during stressful foraging periods. Ecology 1995, 76, 533–543. [Google Scholar] [CrossRef]
- Hairston, N.G.; Smith, F.E.; Slobodkin, L.B. Community structure, population control, and competition. Am. Nat. 1995, 146, 796–824. [Google Scholar] [CrossRef]
- Petranka, J.W.; Smith, C.K. Interspecific competition between two terrestrial salamanders: Effects on individual growth and survivorship. Oecologia 1987, 73, 507–511. [Google Scholar]
- Lunghi, E.; Corti, C.; Biaggini, M.; Zhao, Y.; Cianferroni, F. The trophic niche of two sympatric species of salamanders (Plethodontidae and Salamandridae) from Italy. Animals 2022, 12, 2221. [Google Scholar] [CrossRef] [PubMed]

| Speleomantes strinatii | Salamandrina perspicillata | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Mean | 90% CRI | pd | Parameter | Mean | 90% CRI | pd |
| Detection | Detection | ||||||
| Mean p | 0.43 | 0.21–0.66 | - | Mean p | 0.32 | 0.08–0.62 | – |
| DAY | 0.04 | −3.9–−4.3 | 50.7 | DAY | −0.43 | −4.85–3.61 | 57.0 |
| TEMP | 1.32 | −1.7–4.4 | 76.6 | TEMP | −2.59 | −5.88–0.97 | 89.7 |
| RAIN | 1.33 | −1.56–4.32 | 77.0 | RAIN | −1.81 | −5.2–1.37 | 82.0 |
| Abundance | Abundance | ||||||
| Mean λ | 0.70 | 0.25–1.15 | - | Mean λ | 2.10 | 0.48–4.02 | - |
| MOIST | 0.88 | 0.25–1.57 | 98.7 | MOIST | −0.48 | −1.30–0.29 | 85.0 |
| INSOL | −0.76 | −1.31–−0.20 | 99.2 | INSOL | −1.41 | −2.34–−0.37 | 99.5 |
| TPI | −1.11 | −0.48–0.24 | 70.0 | TPI | −0.33 | −0.99–0.36 | 79.6 |
| Interactions | |||||||
| ε | −3.76 | −7.66–−0.09 | 94.8 | ||||
| γ | −0.18 | −0.56–0.20 | 79.9 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, G.; Salvidio, S.; Costa, A. Disentangling Exploitative and Interference Competition on Forest Dwelling Salamanders. Animals 2023, 13, 2003. https://doi.org/10.3390/ani13122003
Rosa G, Salvidio S, Costa A. Disentangling Exploitative and Interference Competition on Forest Dwelling Salamanders. Animals. 2023; 13(12):2003. https://doi.org/10.3390/ani13122003
Chicago/Turabian StyleRosa, Giacomo, Sebastiano Salvidio, and Andrea Costa. 2023. "Disentangling Exploitative and Interference Competition on Forest Dwelling Salamanders" Animals 13, no. 12: 2003. https://doi.org/10.3390/ani13122003
APA StyleRosa, G., Salvidio, S., & Costa, A. (2023). Disentangling Exploitative and Interference Competition on Forest Dwelling Salamanders. Animals, 13(12), 2003. https://doi.org/10.3390/ani13122003








