Simple Summary
The price of feed ingredients has been drastically increasing over the last decade, and thus the methods for improving nutrient digestibility and utilization with various feed ingredients have been widely studied in the field of the swine industry. One of the most efficacious approaches to enhancing nutrient digestibility and utilization is the manipulation of feed particle size. Nonetheless, limited research has been conducted on the impact of varying particle sizes of feeds with high fiber content. Dietary fiber is composed of several components, including β-glucan, arabinoxylan, and cellulose, which exhibit distinct physiological effects on the small and large intestines. The compositions and proportions of these components can influence and modulate nutrient digestibility in the small and large intestines of pigs. The objective of the present investigation was to examine the potential for enhanced nutrient and fiber digestibility in growing pigs through the reduction of particle size in barley and wheat diets. Similar ileal and total nutrient digestibility were observed in pigs fed diets containing fine and coarse wheat; however, pigs consuming coarse barley exhibited reduced nutrient digestibility compared to the other diets. Consequently, the present study shows that nutrient digestibility was more influenced by reduced particle size in barley than wheat, most likely because of the rigid fiber structure of barley and barley hull.
Abstract
The objective of this investigation was to study the effects of different cereal types, barley and wheat, with different particle sizes (PS) on the recovery of ileal digesta and fecal excretion, digestion of nutrients and fiber components, mean transit time (MTT), and short-chain fatty acid content and composition in growing pigs studied in two experiments. Five barrows with ileal cannulas (initial BW 35.9 ± 1.5 kg) in Experiment 1 and thirty-two castrated pigs (30.8 ± 1.3 kg) in Experiment 2 were fed four different diets: barley fine, barley coarse, wheat fine and wheat coarse diets. The cereal type and PS did not influence the relative weight of the small and large intestines and pH of digesta, whereas MTT in the large intestine of pigs fed the coarse barley diet was lower compared to pigs fed other diets (p < 0.05). Pigs fed the coarse barley diet had lower apparent ileal digestibility (AID) and apparent total tract digestibility (ATTD) of nutrients and fiber (p < 0.05), whereas pigs fed the fine barley diet had similar AID and ATTD to pigs fed wheat fine and coarse diets (p < 0.05). In conclusion, the barley diet was more influenced by PS in comparison to wheat, thereby inducing lower AID and ATTD of nutrient.
1. Introduction
The pig is a monogastric animal with endogenous enzymes playing a crucial role in its digestive process [1]. It is therefore essential to provide pigs with high-quality feed that provides nutrients in a readily available form for the enzymes to digest. Apart from the composition of the feed, different feed structures and forms, such as particle size (PS), extrusion, pellet, flake and cooking, significantly affect the efficiency of feed and nutrient utilization. Therefore, they should be optimized for nutrient absorption [2]. Ensuring the provision of adequate essential nutrients to meet the nutritional requirements of pigs presents a challenge due to diverse feed processing techniques and ingredient variations that can significantly impact nutrient utilization [3]. In addition, these effects may vary depending on the growth stage of the pigs [4]. However, finely ground materials can have a negative impact, particularly on gastric ulcers [5].
Barley and wheat are quantitatively the most important components of diets for growing pigs in Denmark and most other European countries. Both cereals have a high concentration of carbohydrates, predominantly as polysaccharides, including starch and non-starch polysaccharides (NSP). The principal polysaccharides in cereal NSP are arabinoxylans (AX), cellulose and mixed linked (1- > 3; 1- > 4)-β-D-glucan (β-glucan), which, together with lignin, make up most of the cell wall and are referred to as dietary fiber (DF). The composition of the cell walls varies between the cellular tissues within the cereal grain and among similar tissues of different grains [6]. Barley has a husk layer that may remain even after the threshing process, whereas wheat husk is lost during the threshing of wheat, thus the DF content is approximately 50% higher in barley than in wheat [7]. These differences in DF content, which are counteracted by a higher starch concentration in wheat compared to barley [7], are responsible for the higher apparent total tract digestibility (ATTD) of wheat diets compared to barley diets in pigs [8].
Grinding is a physical process that reduces PS and increases the surface area, allowing better contact with digestive enzyme, which improves apparent ileal digestibility (AID) and ATTD [9]. This enables optimal nutrient utilization and enhances animal performance. Furthermore, particle size has been associated with changes in the microbial population, and coarse particles stimulate microbial fermentation of DF, which contributes to improved intestinal health by reducing ulceration and E. coli adhesion to the mucosa in the small intestine [10]. The hypothesis of the present study was that finely ground feed improves the digestibility of nutrients and the concentration of short-chain fatty acids (SCFA) in the digesta.
The objective of this study was to investigate the influence of PS of barley and wheat in diets on AID, ATTD, recovery of nutrients and DF, mean transit time (MTT) in the small and large intestines, and SCFA concentration and composition in growing pigs.
2. Materials and Methods
The study complied with the guidelines of the Danish Ministry of Justice, Act no. 474 of 15 May 2014, concerning experiments with animals and the care of experimental animals, as stipulated in the executive order no. 12 of 7 January 2016.
2.1. Diets
Four experimental diets that differed in cereal type (barley or wheat) and PS (fine or coarse) were used (Table 1). The grain components were ground for diets with a fine PS. To achieve approximately the same PS in the ground diets irrespective of grain type, it was found that barley should be ground using a 3 mm sieve and wheat should be ground using a 4.5 mm sieve in a hammer mill. The barley and wheat used in the coarse diets were rolled before inclusion. It was possible to produce rolled feed without the risk of whole grains in the feed. Chromic oxide (2 g/kg diet) was included in the diets as a marker for the determination of the AID and ATTD of nutrients and energy, and MTT.

Table 1.
Ingredients and chemical composition of the experimental diets (g/kg dry matter).
2.2. Nimals and Experimental Designs
Two animal experiments were conducted to perform distinct analyses. The pigs (DanBred Genetics, Ballerup, Denmark) used in both experiments were from the pig herd of Aarhus University, Denmark.
2.3. Experiment 1
The experiment was conducted according to a 5 × 5 Latin square design, using five crossbred barrows [initial BW 35.9 ± 1.5 kg; (Danish Landrace × Yorkshire) × Duroc]. The pigs were fed five different diets, including the four experimental diets and a standard diet, over five periods, each with a duration of two weeks. However, the standard diet was not part of this study, and the results from the standard diet were not included in the statistical analyses. The pigs were fitted with a simple T-cannula at the ileum, approximately 15 cm anterior to the ileocecal junction, following previously outlined procedures [11]. The pigs were fed the same amount of daily net energy, and the amount of feed was adjusted throughout the experiment to match the body weight of the pigs. The feed was provided in three meals of equal size at 07:00, 15:00 and 22:00 h, and the meal size was gradually increased following feeding units for growing pigs [12]. Each experimental period consisted of 14 days: 8 days of adaptation to the experimental diets, followed by 3 days of feces collection and 3 days of ileal digesta collection. The pigs were placed in stainless steel metabolic crates on the last day of the adaptation period. Feces were collected from 07:00 to 15:00 on days 9 to 11. Digesta were collected from 07:00 to 15:00 on days 12 to 14. This approach has been shown to provide a representative sample of digesta that encompasses postprandial changes in nutrient flow [13]. During the collection period, digesta were collected every hour, weighed and immediately frozen (−20 °C). After 6 day collection, period the pigs were returned to their pens for 8 days of adaptation to the next experimental diet.
2.4. Experiment 2
This study involved 32 castrated male pigs with an initial weight of 30.8 ± 1.3 kg [(Danish Landrace × Yorkshire) × Duroc]. The experimental design was a randomized block design with eight blocks, each consisting of four pigs fed one of the four diets. The animals were fed equal amounts of feed on an energy basis at approximately 10% below ad libitum feed intake. The pigs were fed twice a day, and the meal size was gradually increased from 6.16 MJ to 7.70 MJ net energy per day as the animals grew [14]. The pigs were individually housed on a concrete floor with no bedding material.
The pigs were fed one of the four experimental diets for a period of four weeks, after which they were euthanized, and samples were collected. The animals were stunned followed by exsanguinations. The digestive tract was rapidly removed and divided into the following sections: the stomach, the small intestine, the cecum and four equal sections of the colon (Colon1, Colon2, Colon3 and Colon4). The total digesta of the small intestine, the cecum and the four colonic sections (Colon1–4) were collected and weighed. The samples were frozen and stored at −20 °C until needed for further analysis.
2.5. Chemical Analyses
All analyses were made in duplicate. Cr2O3, nitrogen and starch determinations were performed on wet material, while all other analyses were carried out on freeze-dried materials. SCFA was performed on freeze-dried material in Experiment 1 and on wet material in Experiment 2. The dry matter content of feed, digesta and feces was determined by drying at 105 °C until a constant weight was achieved. Protein (N × 6.25) was determined using the Kjeldahl method with a Kjell-Foss 16,200 autoanalyzer. Gross energy was determined using bomb calorimetry with a LECO AC 300 automated calorimeter system 789–500 (LECO, St Joseph, MI, USA). Fat was extracted with diethyl ether after acid hydrolysis [15]. Cr2O3 content was determined using the method described by Schurch et al. [16]. Digesta samples were analyzed for SCFA by gas chromatography as described in detail by Jensen et al. [17].
Sugars (glucose, fructose and sucrose) and fructans in feed, ileal digesta and fecal samples were analyzed using the enzymatic-colorimetric method of Larsson and Bengtsson [18], and the sucrose present as part of fructans was corrected as described by Bach Knudsen and Hessov [19]. Starch was analyzed using a modified enzymatic method as described by Bach Knudsen [7]. In feed, starch determination was also conducted without further milling preceding the analysis. In digesta and feces, starch was determined in wet and freeze-dried ground samples. Total β-glucan was determined using an enzymatic-colorimetric method [20]. Total non-starch polysaccharides (T-NSP) and their constituent sugars were determined as alditol acetates by gas-liquid chromatography for neutral sugars and by a colorimetric method for uronic acids, as described by Bach Knudsen [7]. Soluble NSP (S-SNP) in the starch-free residue was extracted using a phosphate buffer at neutral pH (0.2 mol/L, 100 °C, pH 7.0) [21], and the neutral and acidic sugars in insoluble NSP (I-NSP) were analyzed as previously described [7]. The content of cellulose was calculated as follows:
non-cellulosic polysaccharides (NCP) as:
arabinoxylan (AX) as:
and S-NSP as:
Cellulose = NSPglucose − β-glucan,
NCP = rhamnose + fucose + arabinose + xylose + mannose + galactose + (glucose- β-glucan) + uronic acids,
AX = arabinose + xylose,
S-NSP = Total-NSP − I-NSP.
Klason lignin was measured gravimetrically as the residue resistant to 12 mol/L H2SO4 [22,23].
2.6. Calculations and Statistical Analyses
The apparent digestibility of nutrients at the terminal ileum and total tract were calculated relative to the indigestible marker (Cr2O3) content:
where X is the nutrient in question. X(diet) and X(digesta) are concentrations of specific nutrients in the diet and digesta from the terminal ileum or feces.
The quantitative flow (recovery) of nutrient X was calculated as follows:
the mean transit time in the intestinal segments was calculated as follows:
where Cr2O3(GI) and Cr2O3day are the amounts of Cr2O3 in the specific GI segment and the daily intake of Cr2O3.
Before the animal experiment, a power analysis was performed using SAS JMP based on previous experience with digestibility and SCFA concentration. Based on a power level of 80% and a significance level (α) of 0.05, the minimum required sample size for the study was determined to be 5 pigs. All data were analyzed as least squares means on the Fit Model platform of SAS JMP version 15. 0. 0 (SAS Inst. Inc., Cary, NC, USA). Statistical significance was determined at p < 0.05, and trends are considered for 0.05 ≤ p < 0.10. The least square means were calculated using a post-hoc Tukey test.
The data from Experiment 1 were analyzed as a Latin square design using two-way ANOVA:
where Yijkl is the measured dependent variable, μ is the overall mean, pi is the random effect of period, αj is the effect of animal, ck is the main effect of cereal types (k = barley and wheat), pl is the main effect of PS (l = fine or coarse), cpkl is the interaction between cereal types and PS, and εijkl is the residual component.
Yijkl = μ + pi + αj + ck + sl + cskl + εijkl,
The data from Experiment 2 were analyzed as a randomized block design using two-way ANOVA:
where Y is the measured variable, is the overall mean, bj is the random effect of block, ck is the main effect of cereal types (k = barley or wheat), sl is the main effect of PS (l = fine or coarse), cpkl is the interaction between cereal types and PS, and εjkl is the residual component.
Yjkl = μ + bj + ck + sl + cskl + εjkl
3. Results
3.1. Diets
The gross energy concentration of the diets was similar. However, a few differences between the diets, reflecting the differences between the cereal types and the PS of the feed, were observed (Table 1). The protein content was higher in the coarse diets when compared to their fine counterparts.
The starch analysis was performed both with and without milling preceding the analysis to evaluate how much starch was bound in the particles of the diets (Table 1). When the analysis was performed on the diets without milling, a difference between cereal type and PS of the feed was observed. The content of starch was highest in the fine wheat diet and lowest in the coarse barley diet. Milling the diets prior to analysis resulted in a higher starch content of the barley diets and the difference between the fine and coarse diet equaled out. In the wheat diets, the difference between the fine and coarse diets persisted after milling, and a higher starch content was observed for both diets when compared to the analysis done without milling.
The content of DF differed between the barley and wheat diets, being highest in the barley diets, whereas no difference due to PS was observed. The barley diets had a higher total and soluble NSP content than the wheat diets caused by a higher content of cellulose and β-glucan, whereas no difference was observed for AX and Klason lignin (Table 1).
Determination of the PS distribution showed that the coarse diets had the highest percentage of particles greater than 1 mm, and the fine diets were almost devoid of particles greater than 2 mm (Table 1). When comparing the coarse diets, the barley diet had the largest proportion of both particles greater than 1 and 2 mm.
3.2. Recovery of Ileal and Fecal Materials
The recovery of total ileal wet and solid digesta, organic matter (OM), total carbohydrates, nitrogen (g/d), fat (g/d), and residue (g/d) was higher when feeding the barley coarse diet compared with the other diets (Table 2; p < 0.05). In the recovery of fecal materials, the cereal and PS effects were interactive with respect to total solid fecal materials, OM, total carbohydrates (g/d), nitrogen (g/d), fat (g/d), and organic acids (g/d; p < 0.05). The recovery of nutrients in fecal material of pigs fed the barley coarse diet was higher than when feeding the wheat diets. PS and the type of cereal grain tended to have an interactive effect on fat (g/kg) solid, total solid in ileal material (g/d), and ash (g/d) in fecal material (0.05 ≤ p < 0.10).

Table 2.
Recovery of ileal and fecal materials of pigs fed the experimental diets (Experiment 1; n = 5).
3.3. Apparent Ileal and Total Tract Digestibility
Cereal and PS had an interactive effect on AID of most nutrients as well as DF and some of its components. The AID of OM, energy, starch, fat, total carbohydrates, NSP, cellulose, xylose and DF was lower in pigs fed the coarse barley diet compared to the fine barley diet and the wheat diets (p < 0.05; Table 3). No interaction between cereal and PS was observed for the AID of β-glucan, AX and arabinose (p > 0.05). Instead, the effect of PS was found for these components (p < 0.05), and an effect of cereal was seen for the AID of fructan (p = 0.027). Using different sample preparations prior to starch analyses showed that milling the diets and digesta prior to analyses resulted in a higher digestibility of starch at the ileal level for all diets compared to analyses done on the raw samples. The difference between the sample preparations was especially pronounced for the coarse barley diet, where the starch digestibility was increased from 89.1 to 92.2% at the terminal ileum (Table 3). Interactive tendencies were observed in the AID of fructans and AX, and ATTD of total carbohydrates (0.05 ≤ p < 0.10).

Table 3.
Apparent digestibility of nutrients and fiber at the terminal ileum and feces in pigs fed experimental diets (Experiment 1; n = 5).
The apparent digestibilities of total NSP, cellulose, AX and β-glucan from the ileum to feces across different intestinal segments are shown in Figure 1. Overall, there was an interaction between cereal type and PS for the cecum and colon segments, with lower values found for the coarse barley diet compared to the other diets. There were also significant the degradation of NSP components along the large intestine; almost all β-glucan was degraded in the cecum, whereas cellulose was degraded at more distal locations, and with AX in-between. The negative digestibility values for cellulose in cecum are most likely caused by the separation of cellulose relative to the marker at this site in the small and large intestines during the pigs euthanization process. At the fecal level, a cereal effect was found for the ATTD of total carbohydrates, DF, NSP, AX, and cellulose (p < 0.05). The PS effect was observed for the ATTD of total carbohydrates, DF, NSP, AX and xylose. These differences also translated into lowest ATTD of OM, energy and fat for the pigs fed barley coarse diet and the highest ATTD values for the other diets (p < 0.05; Table 3).
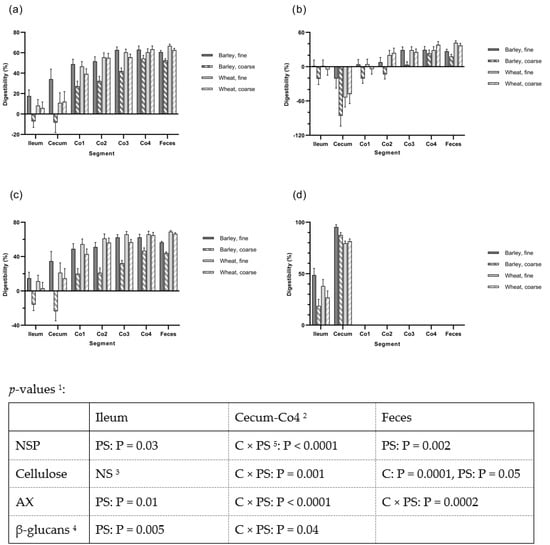
Figure 1.
The digestibility of (a) Non-starch polysaccharides (NSP), (b) Cellulose, (c) Arabinoxylan (AX), and (d) β-glucan in ileal and fecal material from ileal cannulated pigs in Experiment 1(Experiment 1; n = 5) and in digesta from cecum and four segments of the colon from pigs slaughtered in experiment 2 (Experiment 2; n = 8). 1 PS: Particle size; C: Cereal. 2 The digestibilities in the segment’s cecum through Co4 were analyzed as repeated measurements with diet as the between-animal effect and segment as the within-animal effect. The effect of segment was significant in all cases (p < 0.0001) but no interactions between segment and particle size and cereal were observed. 3 NS: Not significant. 4 The content of β-glucan was only measured in ileal and cecal digesta. 5 C × P, the interaction between cereal type and particle size.
3.4. The Relative Intestinal Weight, Digesta Weight, pH and Mean Transit Time in Digesta of the Small and Large Intestines
The experimental diets had no effect on the relative weight of the segments of the small and large intestines (Table 4). An interaction between cereal and PS was found for digesta weight in colon4 and for pH in colon3. The digesta weight in colon4 of pigs fed wheat coarse diet was higher than other diets, and barley and wheat fine diets were positioned between barley and wheat coarse diets. The pH in colon3 of pigs fed barley coarse diet was lower compared to other diets (Table 4; p < 0.05). A cereal effect was observed for digesta weight in colon1 and total colon, and pH in colon2 (p < 0.05), whereas no PS effect was found on digesta weight and pH of digesta.

Table 4.
The relative weight, digesta weight and pH in different intestinal segments of pigs fed experimental diets (Experiment 2; n = 8).
Mean transit time in the cecum, colon segments, total colon, and overall (the small and large intestine) of pigs fed barley coarse diet was lower than for pigs fed barley fine or the wheat diets (Table 5; p < 0.05). No interaction was found for MTT in the small intestine (p > 0.05).

Table 5.
Mean transit time of digesta in the small and large intestines of pigs fed the experimental diets (Experiment 2; n = 8).
3.5. Concentration of Short Chain Fatty Acids in the Large Intestine
No interaction between cereal type and PS, and no PS effect on the concentration of SCFAs in digesta, was found (Table 6). Cereal type affected the total SCFA in the cecum, the proportion of acetic acid in the cecum, colon1, and colon2, and the proportion of branched-chain fatty acids (BCFA) in colon2 and colon3 (p < 0.05). Cereal tended to have an effect on the total SCFA in colon1 and colon2, the proportion of acetic acid in colon3, and the proportion of propionic acid in cecum (0.05 < p < 0.1).

Table 6.
The concentration of short chain fatty acids in digesta of the small and large intestines in growing pigs (Experiment 2; n = 8).
4. Discussion
Grinding feed materials to reduce their PS is a conventional way to increase the surface area of the feed particles for improved nutrient digestibility and utilization. These aspects have been studied with different ingredients such as distillers’ dried grains with solubles, corn, soybean meal and soybean hulls. These studies have generally demonstrated improved AID and ATTD in growing pigs and improved feed efficiency without affecting gastric ulceration in growing pigs fed a corn-wheat-soybean meal-based diet [24]. However, PS distribution at low and high DF levels may influence gut health in different ways [25], and knowledge on how finely and coarsely ground European cereal feedstuffs such as wheat and barley with contrasting DF content influence AID and ATTD of nutrients and the degradation through the large intestine is lacking. In the current study, we found that pigs fed a coarse barley diet had lower AID and ATTD of nutrients compared with other diets. For the DF and its components, the ATTD of wheat and the fine PS diets were higher compared with coarse PS diets. The main reason for the difference in NSP and DF between barley and wheat is the presence of the husk layer in barley, which accounts for 10–15% of the whole grain [26]. In barley, the husk is tightly attached to the pericarp layer, whereas wheat loses its husk layer during threshing and therefore only has the pericarp and testa layers left as part of the grain [27,28]. Total wet and solid materials and other nutrients in ileal and fecal materials of pigs fed a barley coarse diet were higher compared with other diets. Furthermore, the dry matter content of the ileal digesta after feeding the coarse barley diet was higher, indicating that it was primarily the undigested residues induced by the coarse structure that caused the higher ileal digesta flow rather than differences in the physicochemical properties. The weight of digesta in the colon, however, was only influenced by cereal type. Furthermore, the MTT in pigs fed a barley coarse diet was lower than that of pigs fed other diets, suggesting that the digesta of barley coarse diet did not have enough time to be fermented in the large intestine. This phenomenon has previously been seen when the diet contained high insoluble fiber such as cellulose and insoluble NCP [29].
In the present study, the digestibility of NSP and its the main components—cellulose, AX and β-glucan—clearly increased during passage of the large intestine but at various rates according to the property of DF components and cereal type. The cellulose digestibility in ileum was not influenced by either cereal type or PS due to the insolubility of cellulose [30]. However, the digestibility of total NSP, AX and β-glucan was influenced by PS at the ileal level. β-glucan was already extensively degraded at the terminal ileum, as found in other studies with barley and oats [31], and almost completely degraded in the cecum, as also found with oats [32]. The degradation of cellulose and AX occurred more slowly and with a significant influence of PS for cellulose and for both cereal type and PS for AX. The degradation of AX was consistently lower for barley than for wheat, which is most likely caused by the structure of the AX in the husk layer and ferulic acid cross-linkages. The ferulic acid content, 731 µg/g in whole grain barley compared to 689 µg/g in whole grain wheat is known to hinder fermentation and degradation of the cell wall in the large intestine [33,34]. Generally, ferulic acid cross-linkages profoundly affect the degradation and fermentation of cell walls, decreasing digestibility in the small and large intestines [35].
The cereal type, PS, and level of DF did not influence the relative weight of the small intestine, cecum, and colon and digesta weight. The final body weight of the experimental pigs was approximately 52 ± 1.5 kg. Generally, smaller pigs have lower fermentation ability in the large intestine than larger pigs [36], and although there was a larger inflow of potentially fermentable carbohydrates to the large intestine with the barley diets, the total degradation of carbohydrates in the large intestine was only higher for the barley coarse diet. However, this had no influence on the relative weight of the large intestine. In contrast, in pigs exceeding a body weight of 100 kg, it has been shown that the fermentation of DF could lead to an increase in the weight of the small and large intestines [36,37].
The NSP content in the barley diets was higher compared to the wheat diets, which was expected to induce more fermentation in the large intestine, thereby increasing SCFA concentration. However, the SCFA concentration in pigs fed the wheat diets was higher than in pigs fed barley diets. This is probably related to the generally higher digesta weight in the colon of pigs fed barley diets [38]. The husk of barley has a rigid structure, and although the DF intake was higher for the barley diets compared to the wheat diets, it was only in the case of the barley coarse diet that the total degradation of carbohydrates in the large intestine was higher (245 g/d) compared to the other diets (151–158 g/d). In addition, the mean transit time, which was significantly lower for the barley coarse compared to the other diets, also seems to have limited importance for total SCFA in the large intestine. Unlike our results, Stewart and Slavin [38] reported that a finely ground aleurone by-product of wheat and small particle size of wheat bran showed higher SCFA concentrations in vitro compared to large particle size or coarsely grounded by-products probably due to increased accessible surface area. This difference may be caused by different microbial fermentation between wheat and barley diets, as barley β-glucan can decrease the abundance of Bacteroides, Porphyromonas, and Prevotella spp, which are related to DF fermentation [39]. In the current study, the β-glucan level in the barley diet (2.1–2.4 g/kg) is 4–5 times higher than in the wheat diets (0.4–0.5 g/kg), and thus there is a potential for higher β-glucan content in barley-based diets to impede the fermentation process with specific microbiota, thereby the PS effect became blurred.
5. Conclusions
In conclusion, the outcomes of our investigation revealed that the variation in PS within wheat-based diets did not significantly impact the digestibility and transit time of digesta within the small and large intestines. However, feeding a coarse barley diet resulted in substantially reduced digestibility and a faster MTT in comparison to both the fine barley diet and the wheat-based diets in growing pigs. This response is likely due to the structural difference between the barley hull and the outer layer of wheat. Therefore, when formulating diets for pigs, it is advisable to consider not only the PS but also the structural dissimilarities between the cereals being included.
Author Contributions
Conceptualization, M.S.H. and K.E.B.K.; Formal analysis, M.S.H., K.E.B.K. and G.-I.L.; Funding acquisition, M.S.H. and K.E.B.K.; Investigation, M.S.H., K.E.B.K. and G.-I.L.; Methodology, M.S.H.; Project administration, M.S.H. and K.E.B.K.; Supervision, M.S.H. and K.E.B.K.; Validation, M.S.H., K.E.B.K. and G.-I.L.; Visualization, M.S.H.; Writing—original draft, G.-I.L.; Writing—review & editing, M.S.H., K.E.B.K. and G.-I.L. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by the Danish Agricultural and Veterinary Research Council (file no. 9400568).
Institutional Review Board Statement
This study was performed according to the procedures approved by the Danish Ministry of Justice, Act no. 474 of 15 May 2014, concerning experiments with animals and care of experimental animals as stipulated in the executive order no. 12 of 7 January 2016.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Winnie Østergaard Thomsen for their excellent technical assistance.
Conflicts of Interest
There is no conflict of interest.
References
- Fothergill, L.J.; Galiazzo, G.; Hunne, B.; Stebbing, M.J.; Fakhry, J.; Weissenborn, F.; Coles, T.E.F.; Furness, J.B. Distribution and co-expression patterns of specific cell markers of enteroendocrine cells in pig gastric epithelium. Cell Tissue Res. 2019, 378, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Vukmirović, Đ.; Čolović, R.; Rakita, S.; Brlek, T.; Đuragić, O.; Solà-Oriol, D. Importance of Feed Structure (Particle Size) and Feed Form (Mash Vs. Pellets) in Pig Nutrition—A Review. Anim. Feed Sci. Technol. 2017, 233, 133–144. [Google Scholar] [CrossRef]
- Patience, J.F.; Rossoni-Serão, M.C.; Gutiérrez, N.A. A review of feed efficiency in swine: Biology and application. J. Anim. Sci. Biotechnol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.A.; Petry, A.L.; Gould, S.A.; Jones, C.K.; Stark, C.R.; Fahrenholz, A.C.; Patience, J.F. Enhancing digestibility of corn fed to pigs at two stages of growth through management of particle size using a hammermill or a roller mill. Transl. Anim. Sci. 2019, 4, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Regina, D.C.; Eisemann, J.H.; Lang, J.A.; Argenzio, R.A. Changes in gastric contents in pigs fed a finely ground and pelleted or coarsely ground meal diet. J. Anim. Sci. 1999, 77, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.; Fincher, G.B. Evolution and development of cell walls in cereal grains. Front. Plant Sci. 2014, 5, 456. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E. Carbohydrate and Lignin Contents of Plant Materials Used in Animal Feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Zhou, X.; Beltranena, E.; Zijlstra, R. Effect of feeding wheat- or barley-based diets with low or and high nutrient density on nutrient digestibility and growth performance in weaned pigs. Anim. Feed Sci. Technol. 2016, 218, 93–99. [Google Scholar] [CrossRef]
- Fan, Y.; Guo, P.; Yang, Y.; Xia, T.; Liu, L.; Ma, Y. Effects of particle size and adaptation duration on the digestible and metabolizable energy contents and digestibility of various chemical constituents in wheat for finishing pigs determined by the direct or indirect method. Asian-Australas. J. Anim. Sci. 2017, 30, 554–561. [Google Scholar] [CrossRef]
- Huting, A.M.; Middelkoop, A.; Guan, X.; Molist, F. Using Nutritional Strategies to Shape the Gastro-Intestinal Tracts of Suckling and Weaned Piglets. Animals 2021, 11, 402. [Google Scholar] [CrossRef]
- Jørgensen, H.; Jakobsen, K.; Eggum, B.O. The Influence of Different Protein, Fat and Mineral Levels on the Digestibility of Fat and Fatty Acids Measured at the Terminal Ileum and in Faeces of Growing Pigs. Acta Agric. Scand. Sect. A Anim. Sci. 1992, 42, 177–184. [Google Scholar] [CrossRef]
- Just, A. The net energy value of balanced diets for growing pigs. Livest. Prod. Sci. 1982, 8, 541–555. [Google Scholar] [CrossRef]
- Jørgensen, H.; Lindberg, J.E.; Andersson, C. Diurnal Variation in the Composition of Ileal Digesta and the Ileal Digestibilities of Nutrients in Growing Pigs. J. Sci. Food Agric. 1997, 74, 244–250. [Google Scholar] [CrossRef]
- Jørgensen, L.; Tybirk, P. Normer for Næringsstoffer (Recommendation for Nutrients); Danish Pig Research Centre: Copenhagen, Denmark, 2005; Volume 12. [Google Scholar]
- Stoldt, W. Vorschlag zur Vereinheitlichung der Fettbestimmung in Lebensmittlen. Fette Seifen 1952, 54, 146–164. [Google Scholar] [CrossRef]
- Schürch, A.F.; Lloyd, L.E.; Crampton, E.W. The Use of Chromic Oxide as an Index for Determining the Digestibility of a Diet. J. Nutr. 1950, 41, 629–636. [Google Scholar] [CrossRef]
- Jensen, M.T.; Cox, R.P.; Jensen, B.B. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. Anim. Sci. J. 1995, 61, 293–304. [Google Scholar] [CrossRef]
- Larsson, K.; Bengtsson, S. Bestämning Av Lättilgängelig Kolhydrater I Växtmaterial (Determination of Readily Available Carbohydrates in Plant Material); Chemistry Methods Report no. 22; Statens Lantbrukskemiska Laboratorium: Uppsala, Sweden, 1983. [Google Scholar]
- Knudsen, B.K.; Hessov, I. Recovery of Inulin from Jerusalem Artichoke (Helianthus Tuberosus L.) in the Small Intestine of Man. Br. J. Nutr. 1995, 74, 101–113. [Google Scholar] [CrossRef]
- McCleary, B.V.; Codd, R. Measurement of (1-3), (1-4)-Β-D-Glucan in Barley and Oats: A Streamlined Enzymatic Procedure. J. Sci. Food Agric. 2016, 55, 3003–3012. [Google Scholar] [CrossRef]
- Englyst, H.; Wiggins, H.S.; Cummings, J.H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [Google Scholar] [CrossRef]
- Theander, O.; Aaman, P. Studies on Dietary Fibres, 1: Analysis and Chemical Characterization of Water-Soluble and Water-Insoluble Dietary Fibres. Swed. J. Agric. Res. 1979, 9, 99–106. [Google Scholar]
- Theander, O.; Westerlund, E.A. Studies on dietary fiber. 3. Improved procedures for analysis of dietary fiber. J. Agric. Food Chem. 1986, 34, 330–336. [Google Scholar] [CrossRef]
- Saqui-Salces, M.; Luo, Z.; Urriola, P.E.; Kerr, B.J.; Shurson, G.C. Effect of dietary fiber and diet particle size on nutrient digestibility and gastrointestinal secretory function in growing pigs. J. Anim. Sci. 2017, 95, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Millet, S.; Kumar, S.; De Boever, J.; Meyns, T.; Aluwé, M.; De Brabander, D.; Ducatelle, R. Effect of Particle Size Distribution and Dietary Crude Fibre Content on Growth Performance and Gastric Mucosa Integrity of Growing–Finishing Pigs. Vet. J. 2012, 192, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Evers, T.; Millar, S. Cereal Grain Structure and Development: Some Implications for Quality. J. Cereal Sci. 2002, 36, 261–284. [Google Scholar] [CrossRef]
- Antoine, C.; Peyron, S.; Mabille, F.; Lapierre, C.; Bouchet, B.; Abecassis, J.; Rouau, X. Individual Contribution of Grain Outer Layers and Their Cell Wall Structure to the Mechanical Properties of Wheat Bran. J. Agric. Food Chem. 2003, 51, 2026–2033. [Google Scholar] [CrossRef]
- Olkku, J.; Kotaviita, E.; Salmenkallio-Marttila, M.; Sweins, H.; Home, S. Connection between Structure and Quality of Barley Husk. J. Am. Soc. Brew. Chem. 2005, 63, 17–22. [Google Scholar] [CrossRef]
- Wilfart, A.; Montagne, L.; Simmins, H.; Noblet, J.; van Milgen, J. Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. Br. J. Nutr. 2007, 98, 54–62. [Google Scholar] [CrossRef]
- Röhe, I.; Zentek, J. Lignocellulose as an Insoluble Fiber Source in Poultry Nutrition: A Review. J. Anim. Sci. Biotechnol. 2021, 12, 82. [Google Scholar] [CrossRef]
- Jha, R.; Rossnagel, B.; Pieper, R.; Van Kessel, A.; Leterme, P. Barley and Oat Cultivars with Diverse Carbohydrate Composition Alter Ileal and Total Tract Nutrient Digestibility and Fermentation Metabolites in Weaned Piglets. Animal 2010, 4, 724–731. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Jensen, B.B.; Hansen, I. Digestion of polysaccharides and other major components in the small and large intestine of pigs fed on diets consisting of oat fractions rich in β-D-glucan. Br. J. Nutr. 1993, 70, 537–556. [Google Scholar] [CrossRef]
- Boz, H. Ferulic acid in cereals—A review. Czech J. Food Sci. 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Ndolo, V.U.; Beta, T. Comparative Studies on Composition and Distribution of Phenolic Acids in Cereal Grain Botanical Fractions. Cereal Chem. 2014, 91, 522–530. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, W.-K.; Wu, Q.-C.; Yang, H.-J. The release and catabolism of ferulic acid in plant cell wall by rumen microbes: A review. Anim. Nutr. 2022, 9, 335–344. [Google Scholar] [CrossRef]
- Le Sciellour, M.; Labussière, E.; Zemb, O.; Renaudeau, D. Effect of Dietary Fiber Content on Nutrient Digestibility and Fecal Microbiota Composition in Growing-Finishing Pigs. PLoS ONE 2018, 13, e0206159. [Google Scholar] [CrossRef]
- Fernandez, J.A.; Jørgensen, H.; Just, A. Comparative Digestibility Experiments with Growing Pigs and Adult Sows. Anim. Prod. Sci. 1986, 43, 127–132. [Google Scholar] [CrossRef]
- Stewart, M.L.; Slavin, J.L. Particle Size and Fraction of Wheat Bran Influence Short-Chain Fatty Acid Production In Vitro. Br. J. Nutr. 2009, 102, 1404–1407. [Google Scholar] [CrossRef]
- De Angelis, M.; Montemurno, E.; Vannini, L.; Cosola, C.; Cavallo, N.; Gozzi, G.; Maranzano, V.; Di Cagno, R.; Gobbetti, M.; Gesualdo, L. Effect of Whole-Grain Barley on the Human Fecal Microbiota and Metabolome. Appl. Environ. Microbiol. 2015, 81, 7945–7956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).