Simple Summary
Enterococci are among the most responsible agents for nosocomial infections and are globally prevalent antibiotic-resistant microorganisms. The risk of calves being fed colostrum contaminated with these bacteria or antimicrobial-resistant bacteria, leading to the colonization of their gastrointestinal tract, is a concern of public health. The objective of this study was to investigate whether bovine colostrum can act as a reservoir and vehicle for the dissemination of antibiotic-resistant Enterococcus spp. via the food chain. The sensitivity to 14 antibiotics using the disk diffusion method, as well as the presence of antibiotic-resistant genes and virulence genes, were analyzed in calf colostrum samples. E. faecalis, E. faecium and E. gallinarum were identified in the colostrum samples. The results demonstrated a very high percentage (92.1%) of isolates classified as multidrug-resistant (≥3 antimicrobial classes). Additionally, 52% of the isolates showed the presence of ≥4 virulence genes. E. faecium was most likely to carry erythromycin and tetracycline resistance genes, as well as virulence genes. This study revealed that colostrum serves as a reservoir and/or vehicle for the spread of antibiotic resistance and virulence genes. These results have to be analyzed in a One Health perspective to better combat this spread to humans, other animals and the environment.
Abstract
Enterococci are considered among the most prevalent global multidrug-resistant microorganisms globally. Their dissemination is a global concern, particularly by food-producing animals for both animals and humans. The aim of this study was to identify the species and investigate the antibiotic resistance and virulence profile of Enterococcus in bovine colostrum. Out of 88 presumptive Enterococcus isolates, species identification and susceptibility to 14 antimicrobials were tested using the disk diffusion method. An analysis of the antibiotic resistance and virulence genes was performed on the most prevalent species, using specific PCR assays. Enterococcus faecalis (54.5%), E. faecium (14.8%) and E. gallinarum (6.8%) were the identified species. To the best of our knowledge, this is the first report of E. gallinarum in bovine colostrum. The majority of the isolates showed resistance to quinupristin-dalfopristin (95.9%), erythromycin (80.7%), tetracycline (80.7%) and streptomycin (58%). Ninety-two percent of isolates were classified as multidrug-resistant. The most frequently detected resistance genes were tet(K) (61.1%), tet(M) (75.9%), tet(L) (90.7%), erm(B) (55.6%) and ant(6)-Ia (46.3%). The most prevalent virulence factors were cpd, esp, agg and cylLL. Enterococcus faecium showed a higher probability of carrying the erm(C), tet(M), ace and gel(E) genes (p < 0.05). These results demonstrated that colostrum can constitute an important reservoir and vehicle for the dissemination of antibiotic resistance and virulence genes to the three niches included in a One Health perspective (humans, animals and the environment), highlighting the importance of hygiene sanitary measures to mitigate colostrum microbial contamination.
1. Introduction
Agriculture and livestock are responsible for more than half of the annual consumption of antibiotics, greatly exceeding hospital consumption [1], which contributes to the development of antibiotic-resistant bacteria in intensive animal production [2,3]. These bacteria can increase the incidence of infectious diseases that are difficult to treat and, consequently, animal mortality, which leads to productivity losses in the sector [4,5]. These pathogens also pose a threat to public health when they colonize the gastrointestinal tracts of young animals and are transmitted to humans as foodborne contaminants, a problem that has been aggravated by the globalization of the food industry and the international trade of live animals [2,4].
Bovine colostrum is a liquid secreted in the first 3–5 days after calving and administered to newborn calves [6,7]. It is characterized by a unique composition, with immunological, nutritional and growth functions, and is extremely important for the health and development of animals [7,8]. A wide variety of bacterial species have been described in colostrum, colonizing the gastrointestinal tract of animals early in life [9,10]. Among these are Enterococcus, a bacterial genus that was initially seen as harmless but that is currently considered one of the most important agents of nosocomial infections. In particular, the species E. faecalis and E. faecium, cause bacteremia, urinary tract infections and endocarditis [11].
Enterococci exhibit an intrinsic resistance to numerous antimicrobial classes, as well as the ability to acquire resistance to many others [12,13]. Acquired resistance by enterococci to vancomycin is one of the most relevant. It is considered that the livestock industry, due to the use of avoparcin (an antibiotic analogous to vancomycin) as a food additive, played an important role in the emergence and dissemination of vancomycin resistance outside the hospital environment [14,15]. Thus, it is hypothesized that colostrum may be responsible for the colonization of calves by Enterococcus spp. resistant to antibiotics, contributing to the spread of bacteria carrying antibiotic-resistant genes.
The aim of this study was to identify the species found in colostrum and investigate the antibiotic resistance and virulence profile of Enterococcus from colostrum to understand whether bovine colostrum can act as a reservoir and vehicle for the dissemination of antibiotic-resistant Enterococcus spp. for animals, humans and the environment.
2. Materials and Methods
2.1. Isolates
A total of 88 Enterococcus spp. isolates were previously obtained from 29 bovine colostrum samples of the Holstein Friesian and Angus breeds. These were collected from 13 dairy farms during the first milking after calving in the Portuguese region of Entre Douro e Minho between December 2019 and January 2021 (Table S1). All procedures and methods were carried out in accordance with the approved guidelines by the Portuguese Veterinary Authority of the Ministry for Agriculture, Sea, Environment and Spatial Planning (Decree law No. 113/2013 of 7 August 2013), for which the current European Communities Council Directive of September 2010 (2010/63/UE) is present.
Ten microliters of each sample was inoculated in selective media for the growth and isolation of Enterococcus spp., such as Slanetz–Bartley Agar (Liofilchem® s.r.l., Roseto d. Abruzzi, Italy) at 37 °C for 24–48 h. After the period of incubation, when compatible Enterococcus growth was observed in each plaque, 1 to 4 isolates were collected and incubated/isolated in Kanamycin Aesculin Azide Agar (Liofilchem® s.r.l., Roseto d. Abruzzi, Italy). The identification of the isolates was confirmed by routine biochemical methods such as Gram staining, catalase test and growth in the presence of 6.5% NaCl. Subsequently, these were stored in Medical Microbiology Laboratory of the University of Trás-os-Montes and Alto Douro at −20 °C until further analyses.
2.2. DNA Extraction
For DNA extraction, the GRS Genomic DNA Kit—Bacteria (GRiSP Research Solutions, Porto, Portugal) was used, according to the manufacturer’s instructions. The concentration and purity of the extracted DNA were evaluated using the ND-100 Spectrophotometer, NanoDrop®. In addition, DNA integrity analysis was performed by an agarose gel 1.2%, using the BioRAD’s ChemiDoc™ XRS equipment and BioRAD’s Image LabTM.
2.3. Species Identification
Enterococcus species identification of the 88 isolates was performed by PCR assay, using the ProFlexTM PCR System thermal cycler (Applied Biosystems, Waltham, MA, USA). Specific primers were tested for 6 possible species: E. faecalis (ddl E. faecalis), E. faecium (ddl E. faecium), E. gallinarum (vanC1), E. casseliflavus/E. falavescens (vanC2/vanC3) and E. durans (mur2). The primer sequences, PCR reaction conditions and amplicon size are shown in Table S2 [16,17]. The followed protocol for DNA amplification was used: a final volume of 25 µL contained 17.67 µL of ultra-pure water, 2.5 µL complete buffer (Bioron, Römerberg, Germany), 0.38 µL of 100 mM MgCl2, 0.5 µL of 10 mM deoxynucleotides triphosphate, 0.4 µL of 50 µM of forward primer, 0.4 µL of 50 µM of primer reverse, 0.15 µL of U/µL DFS-Taq DNA polymerase (BIORON®, Römerberg, Germany) and 3 µL of DNA (10 ng) sample. Positive and negative controls used in all the experiments belonged to the strain collection of the University of Trás-os-Montes e Alto Douro.
2.4. Phenotypic Characterization
The antimicrobial susceptibility test of all isolates (n = 88) was performed by the disk diffusion method or the Kirby–Bauer method in Mueller-Hinton II agar (Oxoid®, Basingstoke, UK). A total of 14 antibiotics (Liofilchem® s.r.l., Italy) were tested: quinupristin-dalfopristin (15 μg), tetracycline (30 μg), erythromycin (15 μg), streptomycin (300 μg), rifampicin (5 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), vancomycin (30 μg), linezolid (30 μg), fosfomycin (200 μg), nitrofurantoin (300 μg), teicoplanin (30 μg), ampicillin (10 μg) and gentamicin (120 μg). The concentrations used in each antibiotic complied with the indications of the Clinical and Laboratory Standards Institute (CLSI) [18]. The diameter of the zone of inhibition formed around each disc was measured, and the isolates were classified as sensitive and resistant (intermediate zone of inhibition was classified as resistant) for each antibiotic, according to CLSI recommendations [18].
2.5. Genotypic Characterization
The presence of antibiotic resistance genes and virulence factors was investigated in 54 representative isolates belonging to the species E. faecium and E. faecalis, based on the farm origin and observation of different macroscopic morphologies of colonies. The PCR protocol was performed as described for species identification. The primer sequences, PCR reaction conditions and amplicon size are shown in Tables S3 and S4 [16,19,20,21,22,23,24,25,26].
2.5.1. Detection of Antibiotic Resistance Genes
Fifteen primers were tested for genes encoding resistance to 6 different antibiotics: erythromycin (erm(A), erm(B), erm(C) and erm(T)), tetracycline (tet(K), tet(L), tet(M) and tet(O)), gentamicin (aac(6′)-aph(2″)), chloramphenicol (cat(A)), quinupristin-dalfopristin (vat(D) and vat(E)), vancomycin (van(A) and van(B)) and streptomycin (ant(6)-Ia).
2.5.2. Detection of Virulence Genes
Ten specific primers were used for testing genes that encode or are involved in the expression of the following 6 virulence factors: genes encoding collagen-binding protein (ace); genes encoding aggregation substance (agg); genes encoding sex pheromones (cpd); genes encoding gelatinase (gel(E)); genes involved in regulating expression of the genes encoding gelatinase (fsr); genes encoding enterococcal surface protein (esp); and genes encoding cytolysin (cylA, cylB, cylLL and cylM).
2.6. Statistical Analysis
Statistical analysis was strictly descriptive via the chi-square (χ2) independence test and Fisher’s exact test, using the SPSS 15® program (SPSS Inc., Chicago, IL, USA). A probability level lower than 0.05 (p < 0.05) was considered statistically significant in the association of variables. It was performed based on the association of Enterococcus species with the detected resistance and virulence genes.
3. Results
3.1. Identification of Enterococcus Species
Three different species were identified in 67 of the isolates: 48 were identified as E. faecalis, 13 as E. faecium and 6 as E. gallinarum (Table 1). No identification of Enterococcus species was obtained in 21 of the isolates, which were classified as Enterococcus spp.

Table 1.
Enterococcus species identified from bovine colostrum in this study.
3.2. Phenotypic Characterization
Of the 88 isolates, the majority showed resistance to the following antibiotics: quinupristin-dalfopristin, tetracycline, erythromycin and streptomycin. This was followed by rifampicin, chloramphenicol and ciprofloxacin. Resistance to the remaining antibiotics was below 10%. None of the isolates showed resistance to ampicillin or gentamicin (Table 2).

Table 2.
Phenotypic profile of antibiotic-resistant Enterococcus isolates (n = 88) obtained from bovine colostrum by the disk diffusion method performed in this study.
In total, 39 different antibiotic-resistant phenotypes were found (Table S5), with all isolates showing resistance to at least one antibiotic. Only 7 isolates (8%) exhibited resistance to fewer than three different antimicrobial classes, while the remaining 81 isolates (92%) displayed resistance to three or more different antimicrobial classes, demonstrating a multidrug-resistant profile (Table 2).
3.3. Genotypic Characterization
3.3.1. Detection of Antibiotic-Resistant Genes
Among the 54 selected enterococcal isolates of the most prevalent species identified, the most frequently detected antibiotic-resistant genes were three tetracycline resistance genes, including tet(K), tet(M) and tet(L); one erythromycin resistance gene, erm(B); and the gene encoding streptomycin resistance, ant(6)-Ia. No resistance genes for vancomycin (van(A) and van(B)) were found (Table 3).

Table 3.
Antibiotic-resistant genes in E. faecalis and E. faecium obtained from bovine colostrum in this study (n = 54).
A comparison between the obtained phenotypic and genotypic resistance profiles showed that several isolates (n = 30) that did not display phenotypic resistance to a particular antibiotic were found to carry genes that encode resistance to that antibiotic upon analysis using the PCR assay (Table 4 and Table S6).

Table 4.
Antibiotic resistance genes identified in enterococci isolates that did not exhibit the corresponding resistance phenotype.
3.3.2. Detection of Virulence Genes
The most identified virulence factors were cpd, esp, agg, cylLL and ace (Table 5). E. feacalis was shown to have a greater range of virulence factors than E. faecium.

Table 5.
Virulence factors detected in E. feacalis (n = 43) and E. faecium (n = 11) isolated from bovine colostrum.
Only one genotype was detected for the presence of the gel(E) gene and its regulator, the fsr locus (Table 5). Fourteen (25.9%) isolates, eight E. faecalis and six E. faecium, carried the gel(E) gene but did not show the fsr locus, resulting in the gel(E)+fsr− genotype.
Furthermore, five different genotypes were identified for the tested cyl operon genes (Table 6). Out of the 54 isolates, only 4 (7.4%), all belonging to E. faecalis, presented all the four tested genes. In contrast, only one gene (cylLL) was identified in E. faecium.

Table 6.
Detection of cyl operon isolated in E. faecalis and E. faecium analyzed in this study.
Of the 54 analyzed enterococci isolates, only 4 (7%) did not show amplification for any virulence factor. The remaining isolates were identified with two (n = 6; 11%), three (n = 14; 26%), four (n = 11; 20%), five (n = 10; 19%) or more (n = 7; 13%) virulence factors (Figure 1).
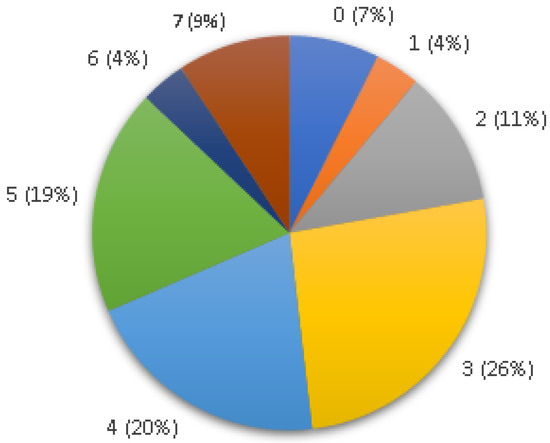
Figure 1.
Number of virulence genes identified per Enterococcus isolate in this study.
The statistical analysis showed significant results (p < 0.05) for the resistance genes including erm(C) and tet(M), with E. faecium having a greater probability of having both genes. For the virulence genes, significant results were observed for the ace and gel(E) genes, which were also more prevalent in E. faecium (Table 7).

Table 7.
Statistical results based on the detected Enterococcus species in relation to the resistance and virulence genes analyzed in this study.
4. Discussion
Bovine colostrum is a rich feed that transfers passive immunity to calves. Moreover, the microbiological quality of colostrum is considered one of the key factors of successful calf management and health [6]. E. faecalis and E. faecium were the Enterococcus species predominantly isolated from colostrum, which was consistent with previous findings for bovine and human colostrum [9,27]. Our obtained results are in concordance with these findings, although a small percentage of the isolates were classified as E. gallinarum. To the best of our knowledge, this is the first report of their presence in bovine colostrum; however, they have already been reported in raw cow’s milk [28]. Unfortunately, the species was not identified in 21 isolates, making it necessary to extend the analysis with specific primers for other species.
There are a variety of reasons for microbial contamination in colostrum. The presence of enterococci in colostrum may originate from mammary gland infection or be indicative of inadequate hygienic and sanitary conditions during milking, such as the fecal contamination of the animal’s skin, milking instruments or workers’ hands [27,29]. Some of the samples had been previously pasteurized, indicating that the bacteria were not eliminated due to inadequate heat treatment or that the colostrum may have suffered subsequent contamination [30]. These reasons may explain why, in our study, four analyzed samples that were also pasteurized (Table S1) showed enterococci growth.
Most of the isolates under study showed antibiotic resistance to quinupristin-dalfopristin (95.5%), as expected, since Enterococcus isolates are intrinsically resistant to quinupristin-dalfopristin [18]. However, a slightly lower percentage (60.8%) of Enterococcus spp. resistant to quinupristin-dalfopristin was detected in mastitis cow milk [31]. Tetracycline and erythromycin also showed high levels of resistance (80.7%), in contrast to what was observed in E. faecalis and E. faecium obtained from cheeses [32]. A little more than half (58.0%) of the isolates showed resistance to streptomycin, in contrast to what was observed in dairy products, processed meats and chicken carcasses [33].
Less than half of the isolates were resistant to rifampicin (47.8%), chloramphenicol (26.1%) and ciprofloxacin (11.4%). In obtained E. faecalis and E. faecium isolates from cheese and chicken, a high resistance to rifampicin and ciprofloxacin and a low resistance to chloramphenicol was reported [32,34]. Less than 10% of the colostrum isolates showed resistance to antimicrobials such as linezolid, fosfomycin and nitrofurantoin. Similar results have been reported from ready-to-eat dairy products in isolates of E. faecium, E. faecalis, E. gallinarum and E. casseliflavius [35]. None of the isolates showed resistance to ampicillin or gentamicin, in contrast to what was observed in E. faecalis and E. faecium isolated from raw cow’s milk [36].
One of the most important resistances to evaluate in Enterococcus spp. is resistance to glycopeptides (vancomycin and teicoplanin). In this study, the percentage of resistance was low. Studies conducted on raw cow’s milk with E. faecium and E. faecalis revealed a higher resistance to both antibiotics, in which 37% were resistant to vancomycin and 44% were resistant to teicoplanin [36].
The species that showed resistance to a greater number of antibiotics in the phenotypic analysis was E. faecalis with a total of 11 antibiotics, followed by E. gallinarum with 8 antibiotics and, finally, E. faecium with 7 antibiotics. Several studies have also reported that E. faecalis is the species with the most resistance to the greatest number of antibiotics [33,37]. All isolates under study showed resistance to at least one antimicrobial class, and a very high percentage (92%) of isolates were classified as multidrug-resistant. In agreement with these findings, another study described the presence of multidrug-resistant E. faecalis and E. faecium isolates in chickens, fresh and fermented meat, raw and fermented milk, and cheese [34].
From the genotypic characterization, tet(L) was the most detected of the screened tetracycline-resistant genes. While in another study, the most frequently detected gene was tet(M) [35]. In the case of erythromycin resistance, the erm(B) gene has been identified as the most prevalent worldwide [37], which is in line with the results obtained in our study. The ant(6)-Ia gene was detected in half of the isolates that showed phenotypic resistance to streptomycin. Similarly, samples of game meat, from which five isolates of E. faecium and E. faecalis are resistant to this antibiotic, did not show this gene [38].
The van(A) and van(B) genes, individually or in combination, are the most commonly found genotypes among Enterococcus spp. with acquired resistance mechanisms to vancomycin in humans and animals [39]. Although one of the isolates of E. faecalis, from bovine colostrum, was phenotypically resistant to vancomycin, the van(A) and van(B) genes were not amplified, which agrees with results obtained in studies of human colostrum and milk [40].
Several isolates did not show phenotypic resistance to certain antibiotics (tetracycline, streptomycin, chloramphenicol, erythromycin and gentamicin), but genes that encode resistance to corresponding antibiotics were detected. This can be explained by the negative regulation of the resistance gene, low levels of gene expression or inactive gene product expression. Since environmental factors can interfere with gene expression, this may happen when an isolate that is not resistant to an antibiotic in vitro, under conditions found in humans or animals, proves to be resistant [24].
The presence of antibiotic-resistant genes does not make a bacterium pathogenic; to be able to colonize and subsequently cause disease, it requires the presence and expression of several virulence factors. The cpd and esp virulence factors were the most detected in E. faecalis and E. faecium colostrum isolates, as previously observed in ready-to-eat shrimp [41]. Alternatively, the agg and ace genes were found in lower proportions in E. faecalis isolates from dairy and meat products than in bovine colostrum isolates [33].
The gel(E) gene was detected in isolates from this study; however, its regulator, fsr locus, was not detected in any of the isolates. Previous studies observed that isolates with gelatinase activity present amplification for gel(E) and its regulator fsr [42,43]. According to these data, the isolates of this study do not present gelatinase activity (gel(E)+ fsr− genotype) since, even if they have the gene, it may be silenced [32]. In our study, the presence of the four tested cyl operon genes were observed in four isolates. The expression of this bacteriocin requires the presence of the entire cyl operon, which means that only four of the studied isolates (all E. faecalis) have the possibility of expressing it [44].
E. feacalis has been shown to have a greater range of virulence factors than E. faecium. E. faecium was more likely to carry the ace and gel(E) virulence genes than E. faecalis. However, other studies have reported that the prevalence of these virulence genes and others (esp and agg) was significantly higher in E. faecalis isolates [45,46]. Only 4 of the 54 analyzed Enterococcus showed no virulence factors. The number of virulence factors in obtained enterococci from bovine colostrum contradicts the hypothesis that these determinants are more prevalent in clinical isolates than in isolates recovered from food, food-producing animals or wild animals [42,47,48].
5. Conclusions
The present study demonstrated that bovine colostrum can be a reservoir for Enterococcus spp. resistant to antibiotics, which possess significant pathogenic potential. As colostrum is an essential source of immunity and nutrition for calves, when present, these multidrug-resistant bacteria are likely to be transmitted to them. Consequently, it is likely that these microorganisms persist in the food or products derived from these calves, such as milk or their carcasses, that enter the human food chain. This cycle of dissemination must be stopped as it culminates in a food safety and public health problem. In order to prevent colostrum from being a reservoir and vehicle for the spread of antibiotic-resistant bacteria, it is important to make a more prudent use of antimicrobials and to apply good hygiene and manufacturing practices during the collection and management of colostrum.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani13121900/s1. Table S1: Characteristics of the 29 colostrum samples from 13 dairy farms used in this study, as well as the number of Enterococcus isolates obtained in each farm; Table S2: Nucleotide sequence of the primers used in the identification of Enterococccus species, as well as amplification conditions and size of the band obtained; Table S3: Nucleotide sequence of primers for amplification of antibiotic-resistant genes in Enterococcus spp., as well as amplification conditions and size of the band obtained; Table S4: Nucleotide sequence of primers for amplification of virulence factors in Enterococcus spp., as well as amplification conditions and size of the band obtained; Table S5: Resistance phenotypes obtained in E. faecalis, E. faecium, E. gallinarum and Enterococcus spp. from bovine colostrum (n = 88); Table S6: Phenotype and genotype characterization of E. faecalis (n = 43) and E. faecium (n = 11) isolates.
Author Contributions
Conceptualization, S.C. and C.M.; methodology, S.C., C.M., R.S., Â.M. and M.M.; software, Â.M.; validation, G.I., F.S. and P.P.; writing—original draft preparation, S.C. and C.M.; supervision, G.I., F.S. and P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Associate Laboratory for Green Chemistry—LAQV, which is financed by national funds from FCT/MCTES (UIDB/50006/2020 and UIDP/50006/2020). This was also supported by the projects UIDP/CVT/00772/2020 and LA/P/0059/2020 funded by the Portuguese Foundation for Science and Technology (FCT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2018, 84, 41–44. [Google Scholar] [CrossRef]
- McDermott, P.; Zhao, S.; Wagner, D.; Simjee, S.; Walker, R.; White, D. The food safety perspective of antibiotic resistance. Anim. Biotechnol. 2002, 13, 71–84. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance in the Food Chain. Food Safety. 2017. Available online: https://www.who.int/foodsafety/areas_work/antimicrobial-resistance/amrfoodchain/en/ (accessed on 22 January 2021).
- FAO. Antimicrobial Resistance and Foods of Plant Origin. Summary Report of an FAO Meeting of Experts. 2018. Available online: http://www.fao.org/3/BU657en/bu657en.pdf (accessed on 25 February 2021).
- FAO. Is Antimicrobial Resistance a Food Safety Issue? Food Safety and Quality. 2020. Available online: http://www.fao.org/food-safety/news/news-details/en/c/1331603/ (accessed on 23 January 2021).
- McGrath, B.; Fox, P.; McSweeney, P.; Kelly, A. Composition and properties of bovine colostrum: A review. Dairy Sci. Technol. 2015, 96, 133–158. [Google Scholar] [CrossRef]
- Preeti, N.; Priyadarishini, S.; Priya, R.I.; Amritkumar, P. Nutritive Analysis and Microbial Characterization of Bovine Colostrum. IOSR J. Biotechnol. Biochem. 2018, 4, 55–60. [Google Scholar]
- Dzik, S.; Miciński, B.; Aitzhanova, I.; Miciński, J.; Pogorzelska, J.; Beisenov, A.; Kowalski, I. Properties of bovine colostrum and the possibilities of use. Pol. Ann. Med. 2017, 24, 295–299. [Google Scholar] [CrossRef]
- Taweerodjanakarn, S.; Haertlé, T.; Chobert, J. Functional properties of Enterococcus faecalis isolated from colostrum drawn from Thai mothers. Int. Food Res. J. 2019, 26, 141–151. [Google Scholar]
- Baltrukova, S.; Zagorska, J.; Eihvalde, I. Preliminary study of bovine colostrum quality in Latvia. Res. Rural Dev. 2019, 1, 234–240. [Google Scholar] [CrossRef]
- Nowakiewicz, A.; Ziółkowska, G.; Zięba, P.; Gnat, S.; Trościańczyk, A.; Adaszek, Ł. Characterization of Multidrug Resistant E. faecalis Strains from Pigs of Local Origin by ADSRRS-Fingerprinting and MALDI-TOF MS; Evaluation of the Compatibility of Methods Employed for Multidrug Resistance Analysis. PLoS ONE 2017, 12, e171160. [Google Scholar] [CrossRef]
- Araújo, A.; Grassotti, T.; Frazzon, A. Characterization of Enterococcus spp. isolated from a fish farming environment in southern Brazil. Braz. J. Biol. 2020, 81, 954–961. [Google Scholar] [CrossRef]
- Růžičková, M.; Vítězová, M.; Kushkevych, I. The Characterization of Enterococcus Genus: Resistance Mechanisms and Inflammatory Bowel Disease. Open Med. 2020, 15, 211–224. [Google Scholar] [CrossRef]
- Giraffa, G. Enterococci from foods. FEMS Microbiol. Rev. 2002, 26, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Falci, D.; Dalarosa, M. Enterococcus resistente à Vancomicina: Um problema no Rio Grande do Sul. Rev. Epidemiol. Control. Infect. 2012, 2, 73–79. [Google Scholar] [CrossRef]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of Glycopeptide Resistance Genotypes and Identification to the Species Level of Clinically Relevant Enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Robredo, B.; Singh, K.V.; Torres, C.; Panesso, D.; Murray, B.E. Rapid identification of Enterococcus hirae and Enterococcus durans by PCR and detection of a homologue of the E. hirae mur-2 Gene in E. durans. J. Clin. Microbiol. 2006, 44, 1567–1570. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Sutcliffe, J.; Grebe, T.; Tait-Kamradt, A.; Wondrack, L. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 1996, 40, 2562–2566. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Agerso, Y.; Gerner-Smidt, P.; Madsen, M.; Jensen, L.B. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 2000, 37, 127–137. [Google Scholar] [CrossRef]
- Aminov, R.I.; Garrigues-Jeanjean, N.; Mackie, R.I. Molecular ecology of tetracycline resistance: Development and validation of primers for detection of tetracycline resistance genes encoding ribosomal protection proteins. Appl. Environ. Microbiol. 2001, 67, 22–32. [Google Scholar] [CrossRef]
- Del Campo, R.; Tenorio, C.; Rubio, C.; Castillo, J.; Torres, C.; Gómez-Lus, R. Aminoglycoside-modifying enzymes in high-level streptomycin and gentamicin resistant Enterococcus spp. in Spain. Int. J. Antimicrob. Agents 2000, 15, 221–226. [Google Scholar] [CrossRef]
- Robredo, B.; Singh, K.V.; Baquero, F.; Murray, B.E.; Torres, C. Vancomycin-resistant enterococci isolated from animals and food. Int. J. Food. Microbiol. 2000, 54, 197–204. [Google Scholar] [CrossRef]
- Eaton, T.J.; Gasson, M.J. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 2001, 67, 1628–1635. [Google Scholar] [CrossRef]
- Mannu, L.; Paba, A.; Daga, E.; Comunian, R.; Zanetti, S.; Duprè, I.; Sechi, L.A. Comparison of the incidence of virulence determinants and antibiotic resistance between Enterococcus faecium strains of dairy, animal and clinical origin. Int. J. Food Microbiol. 2003, 88, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Semedo, T.; Santos, M.A.; Lopes, M.F.; Figueiredo Marques, J.J.; Barreto Crespo, M.T.; Tenreiro, R. Virulence factors in food, clinical and reference Enterococci: A common trait in the genus? Syst. Appl. Microbiol. 2003, 26, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Delgado, S.; Fernández, L.; García, N.; Albújar, M.; Papi, A.; Rodríguez, J. Assessment of the bacterial diversity of human colostrum and screening of staphylococcal and enterococcal populations for potential virulence factors. Res. Microbiol. 2008, 159, 595–601. [Google Scholar] [CrossRef]
- Bouymajane, A.; Rhazi Filali, F.; Oulghazi, S.; Ed-Dra, A.; Benhallam, F.; El Allaoui, A.; Anissi, J.; Sendide, K.; Ouhmidou, B.; Moumni, M. Occurrence, molecular and antimicrobial resistance of Enterococcus spp. isolated from raw cow’s milk trade by street trading in Meknes city, Morocco. Germs 2018, 8, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, M.; Önen, A.; Ektİk, N.; Kara, R.; Torlak, E.; Metli, M. Detection of prevalence, antibiotic resistance and virulence factors of Enterococcus spp. isolated from ready to eat foods. Kocatepe Vet. J. 2017, 10, 76–82. [Google Scholar]
- Jamet, E.; Akary, E.; Poisson, M.A.; Chamba, J.F.; Bertrand, X.; Serror, P. Prevalence and characterization of antibiotic resistant Enterococcus faecalis in French cheeses. Food Microbiol. 2012, 31, 191–198. [Google Scholar] [CrossRef]
- Różańska, H.; Lewtak-Piłat, A.; Kubajka, M.; Weiner, M. Occurrence of Enterococci in Mastitic Cow’s Milk and their Antimicrobial Resistance. J. Vet. Res. 2019, 63, 93–97. [Google Scholar] [CrossRef]
- Sanlibaba, P.; Şentürk, E. Prevalence, Characterization and Antibiotic Resistance of Enterococci from Traditional Cheeses in Turkey. Int. J. Food Prop. 2018, 21, 1955–1963. [Google Scholar] [CrossRef]
- Elal Mus, T.; Cetinkaya, F.; Cibik, R.; Soyutemiz, G.; Simsek, H.; Coplu, N. Pathogenicity determinants and antibiotic resistance profiles of enterococci from foods of animal origin in Turkey. Acta Vet. Hung. 2017, 65, 461–474. [Google Scholar] [CrossRef]
- Sanlibaba, P.; Uymaz Tezel, B.; Şentürk, E. Antimicrobial Resistance of Enterococcus Species Isolated from Chicken in Turkey. Korean J. Food Sci. Anim. Resour. 2018, 38, 391–402. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; García-Solache, M. Ready-to-eat dairy products as a source of multidrug-resistant Enterococcus strains: Phenotypic and genotypic characteristics. J. Dairy Sci. 2018, 103, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Citak, S.; Yucel, N.; Mendi, A. Antibiotic resistance of Enterococcal isolates in raw milk. J. Food Process. Preserv. 2005, 29, 183–195. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Łaniewska-Trokenheim, Ł. Diversity of Antibiotic Resistance Genes in Enterococcus Strains Isolated from Ready-to-Eat Meat Products. J. Food Sci. 2016, 81, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Ramos, E.; Molina González, D.; Blanco-Morán, S.; Igrejas, G.; Poeta, P.; Alonso-Calleja, C.; Capita, R. Prevalence, Antimicrobial Resistance, and Genotypic Characterization of Vancomycin-Resistant Enterococci in Meat Preparations. J. Food Prot. 2016, 79, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Campo, R.; Coque, T. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 185–227. [Google Scholar] [CrossRef]
- Özmen Toḡay, S.; Temiz, A.; Çelebi, A.; Açık, L.; Yalçın, S. Investigation of potential virulence genes and antibiotic resistance characteristics of Enterococcus faecalis isolates from human milk and colostrum samples. Turk. J. Biol. 2014, 38, 357–364. [Google Scholar] [CrossRef]
- Igbinosa, E.; Beshiru, A. Antimicrobial Resistance, Virulence Determinants, and Biofilm Formation of Enterococcus Species From Ready-to-Eat Seafood. Front. Microbiol. 2019, 10, 728. [Google Scholar] [CrossRef]
- Silva, N.; Igrejas, G.; Vaz, J.; Araújo, C.; Cardoso, L.; Rodrigues, J.; Torres, C.; Poeta, P. Virulence Factors in Enterococci from Partridges (Alectoris rufa) Representing a Food Safety Problem. Foodborne Pathog. Dis. 2011, 8, 831–833. [Google Scholar] [CrossRef]
- Poeta, P.; Costa, D.; Naouel, K.; Rodrigues, J.; Torres, C. Phenotypic and Genotypic Study of Gelatinase and β-Haemolysis Activities in Faecal Enterococci of Poultry in Portugal. J. Vet. Med. 2006, 53, 203–208. [Google Scholar] [CrossRef]
- Gilmore, M.; Coburn, P.; Nallapareddy, S.; Murray, B. Enterococcal virulence. In The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance; Gilmore, M.S., Ed.; ASM Press: Washington, DC, USA, 2002; pp. 301–354. [Google Scholar] [CrossRef]
- Strateva, T.; Atanasova, D.; Savov, E.; Petrova, G. Incidence of virulence determinants in clinical Enterococcus faecalis and Enterococcus faecium isolates collected in Bulgaria. Braz. J. Infect. D 2016, 20, 127–133. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, Y.J. Molecular Characteristics of Enterococcus faecalis and Enterococcus faecium from Bulk Tank Milk in Korea. Animals 2021, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Klare, I.; Konstabel, C.; Mueller-Bertling, S.; Werner, G.; Strommenger, B.; Kettlitz, C.; Borgmann, S.; Schulte, B.; Jonas, D.; Serr, A.; et al. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur. J. Clin. Microbiol. Infect. 2006, 24, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Igrejas, G.; Rodrigues, J.; Capelo, J.; Poeta, P. Genetic characterisation of antibiotic resistance and virulence factors in vanA-containing enterococci from cattle, sheep and pigs subsequent to the discontinuation of the use of avoparcin. Vet. J. 2012, 193, 301–313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).