Does Dietary Sodium Alginate with Low Molecular Weight Affect Growth, Antioxidant System, and Haemolymph Parameters and Alleviate Cadmium Stress in Whiteleg Shrimp (Litopenaeus vannamei)?
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Ethical Statement
2.2. Experimental Diets
2.3. Shrimp and Husbandry Trial
2.4. Growth Performance
2.5. Biochemical Composition Analysis
2.6. Haemolymph Collection
2.7. Antioxidant Enzyme Activity Malondialdehyde Evaluation and Serological Enzymes
2.8. Statistical Analysis
2.9. Cd Challenge Test
3. Result
3.1. Growth Performance, Survival Rate, and Proximate Composition
3.2. Antioxidant Activities
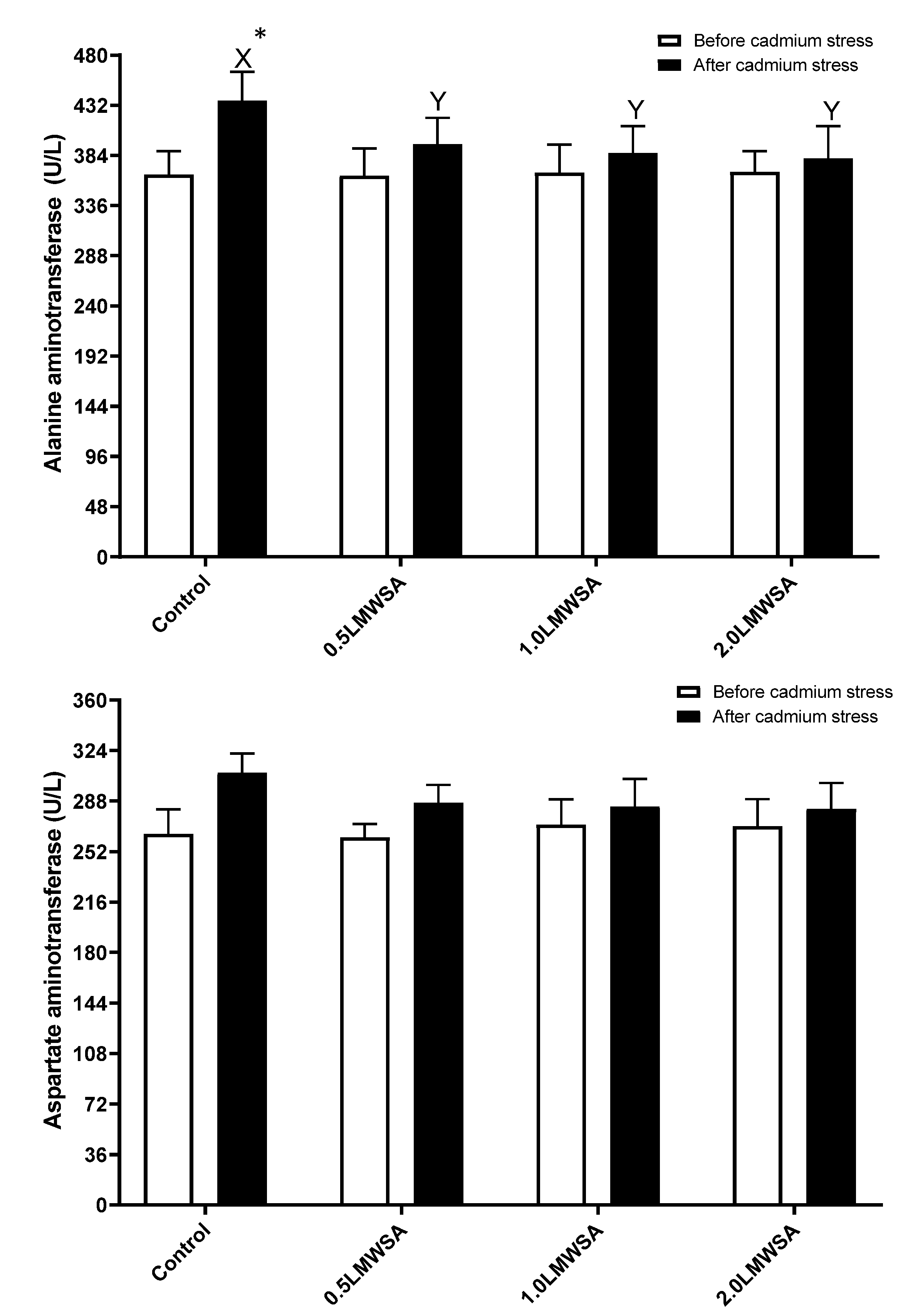
3.3. Haemolymph Enzymes
4. Discussion
4.1. Growth Performance, SurvivalR, and Proximate Composition
4.2. Antioxidant Activities
4.3. Haemolymph Enzymes
4.4. Correlation between Measured Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2020, Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Sotoudeh, E.; Esmaeili, M. Effects of Biotronic® Top3, a feed additive containing organic acids, cinnamaldehyde and a permeabilizing complex on growth, digestive enzyme activities, immunity, antioxidant system and gene expression of barramundi (Lates calcarifer). Aquac. Rep. 2022, 24, 101152. [Google Scholar] [CrossRef]
- Hui, C.-Y.; Guo, Y.; Liu, L.; Yi, J. Recent advances in bacterial biosensing and bioremediation of cadmium pollution: A mini-review. World J. Microbiol. Biotechnol. 2022, 38, 9. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Abkenar, A.M.; Yahyavi, M.; Esmaeili, M.; Rombenso, A. High bioaccumulation factors and ecological risk index of Cd and Hg in Indian white shrimp, hooded oyster, brown algae, and Sediment in northern coasts of the Gulf of Oman before and after a monsoon. Reg. Stud. Mar. Sci. 2021, 41, 101552. [Google Scholar] [CrossRef]
- Pourang, N.; Amini, G. Distribution of Trace Elements in Tissues of Two Shrimp Species from Persian Gulf and Effects of Storage Temperature on Elements Transportation. Water Air Soil Pollut. 2001, 129, 229–243. [Google Scholar] [CrossRef]
- Gokoglu, N.; Yerlikaya, P.; Gokoglu, M. Trace elements in edible tissues of three shrimp species (Penaeus semisulcatus, Parapenaeus longirostris and Paleomon serratus). J. Sci. Food Agric. 2008, 88, 175–178. [Google Scholar] [CrossRef]
- Tu, N.P.C.; Ha, N.N.; Ikemoto, T.; Tuyen, B.C.; Tanabe, S.; Takeuchi, I. Regional variations in trace element concentrations in tissues of black tiger shrimp Penaeus monodon (Decapoda: Penaeidae) from South Vietnam. Mar. Pollut. Bull. 2008, 57, 858–866. [Google Scholar] [CrossRef]
- Costa-Boeddeker, S.; Hoelzmann, P.; de Stigter, H.C.; van Gaever, P.; Huy, H.D.; Smol, J.P.; Schwalb, A. Heavy metal pollution in a reforested mangrove ecosystem (Can Gio Biosphere Reserve, Southern Vietnam): Effects of natural and anthropogenic stressors over a thirty-year history. Sci. Total Environ. 2020, 716, 137035. [Google Scholar] [CrossRef]
- Sarkar, T.; Alam, M.M.; Parvin, N.; Fardous, Z.; Chowdhury, A.Z.; Hossain, S.; Haque, M.; Biswas, N. Assessment of heavy metals contamination and human health risk in shrimp collected from different farms and rivers at Khulna-Satkhira region, Bangladesh. Toxicol. Rep. 2016, 3, 346–350. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Dong, K.F.; Xiao, G.; Ma, D. Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar. Pollut. Bull. 2020, 159, 111505. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, L.; Kong, Z.; Shao, L.; Wang, G.; Ren, X.; Liu, Y. Evaluation of trace metals and rare earth elements in mantis shrimp Oratosquilla oratoria collected from Shandong Province, China, and its potential risks to human health. Mar. Pollut. Bull. 2021, 162, 111815. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Assessment of human health risk for heavy metals in fish and shrimp collected from Subarnarekha river, India. Int. J. Environ. Health Res. 2014, 24, 429–449. [Google Scholar] [CrossRef]
- Mitra, A.; Banerjee, K.; Sinha, S. Shrimp tissue quality in the lower Gangetic delta at the apex of Bay of Bengal. Toxicol. Environ. Chem. 2011, 93, 565–574. [Google Scholar] [CrossRef]
- Núñez-Nogueira, G.; Fernández-Bringas, L.; Ordiano-Flores, A.; Gómez-Ponce, A.; de León-Hill, C.P.; González-Farías, F. Accumulation and regulation effects from the metal mixture of Zn, Pb, and Cd in the tropical shrimp Penaeus vannamei. Biol. Trace Elem. Res. 2012, 150, 208–213. [Google Scholar] [CrossRef]
- Jara-Marini, M.E.; Molina-García, A.; Martínez-Durazo, Á.; Páez-Osuna, F. Trace metal trophic transference and biomagnification in a semiarid coastal lagoon impacted by agriculture and shrimp aquaculture. Environ. Sci. Pollut. Res. 2020, 27, 5323–5336. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Qi, C.L.; Song, J.X.; Deng, H.H.; Ding, Z.L.; Liu, Y.; Wei, S.S.; Ye, J.Y.; Kong, Y.Q. The negative effects of dietary cadmium on antioxidant capacity, immunity and intestine morphology of Macrobrachium nipponense and the alleviation effects of lipoic acid. Aquac. Nutr. 2021, 27, 1212–1220. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Huang, J.; Li, H.; Dong, H.; Zhang, J. Toxic effects of cadmium and lead exposure on intestinal histology, oxidative stress response, and microbial community of Pacific white shrimp Litopenaeus vannamei. Mar. Pollut. Bull. 2021, 167, 112220. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic Effects of Cadmium on Fish. Toxics 2022, 10, 622. [Google Scholar] [CrossRef]
- Zhai, Q.; Yu, L.; Li, T.; Zhu, J.; Zhang, C.; Zhao, J.; Zhang, H.; Chen, W. Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie Van Leeuwenhoek 2017, 110, 501–513. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Badr, H.A. The potential role of turmeric and black pepper powder diet supplements in reversing cadmium-induced growth retardation, ATP depletion, hepatorenal damage, and testicular toxicity in Clarias gariepinus. Aquaculture 2019, 510, 109–121. [Google Scholar] [CrossRef]
- Banaee, M.; Mehrpak, M.; Hagi, B.B.N.; Noori, A. Amelioration of cadmium-induced changes in biochemical parameters of the muscle of Common Carp (Cyprinus carpio) by Vitamin C and Chitosan. Int. J. Aquat. Biol. 2015, 3, 362–371. [Google Scholar]
- Ren, H.; Jia, H.; Kim, S.; Maita, M.; Sato, S.; Yasui, M.; Endo, H.; Hayashi, T. Effect of Chinese parsley Coriandrum sativum and chitosan on inhibiting the accumulation of cadmium in cultured rainbow trout Oncorhynchus mykiss. Fish. Sci. 2006, 72, 263–269. [Google Scholar] [CrossRef]
- Elgendy, M.Y.; Ali, S.E.; Abdelsalam, M.; El-Aziz, T.H.A.; Abo-Aziza, F.; Osman, H.A.; Authman, M.M.N.; Abbas, W.T. Onion (Allium cepa) improves Nile tilapia (Oreochromis niloticus) resistance to saprolegniasis (Saprolegnia parasitica) and reduces immunosuppressive effects of cadmium. Aquac. Int. 2023, 31, 1457–1481. [Google Scholar] [CrossRef]
- Neamat-Allah, A.N.; El-Murr, A.E.I.; Abd El-Hakim, Y. Dietary supplementation with low molecular weight sodium alginate improves growth, haematology, immune reactions and resistance against Aeromonas hydrophila in Clarias gariepinus. Aquac. Res. 2019, 50, 1547–1556. [Google Scholar] [CrossRef]
- Ashouri, G.; Soofiani, N.M.; Hoseinifar, S.H.; Jalali, S.A.H.; Morshedi, V.; Valinassab, T.; Bagheri, D.; Van Doan, H.; Mozanzadeh, M.T.; Carnevali, O. Influence of dietary sodium alginate and Pediococcus acidilactici on liver antioxidant status, intestinal lysozyme gene expression, histomorphology, microbiota, and digestive enzymes activity, in Asian sea bass (Lates calcarifer) juveniles. Aquaculture 2020, 518, 734638. [Google Scholar] [CrossRef]
- Yeh, S.-P.; Chang, C.-A.; Chang, C.-Y.; Liu, C.-H.; Cheng, W. Dietary sodium alginate administration affects fingerling growth and resistance to Streptococcus sp. and iridovirus, and juvenile non-specific immune responses of the orange-spotted grouper, Epinephelus coioides. Fish Shellfish. Immunol. 2008, 25, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Yeh, S.-P.; Kuo, C.-M.; Cheng, W.; Chou, C.-H. The effect of sodium alginate on the immune response of tiger shrimp via dietary administration: Activity and gene transcription. Fish Shellfish. Immunol. 2006, 21, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, C.-H.; Kuo, C.-M.; Chen, J.-C. Dietary administration of sodium alginate enhances the immune ability of white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish. Immunol. 2005, 18, 1–12. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Tu, C.-W.; Chen, Y.-Y.; Nan, F.-H.; Chen, J.-C. The immunostimulatory effects of sodium alginate and iota-carrageenan on orange-spotted grouper Epinephelus coicoides and its resistance against Vibrio alginolyticus. Fish Shellfish. Immunol. 2007, 22, 197–205. [Google Scholar] [CrossRef]
- Chiu, S.-T.; Tsai, R.-T.; Hsu, J.-P.; Liu, C.-H.; Cheng, W. Dietary sodium alginate administration to enhance the non-specific immune responses, and disease resistance of the juvenile grouper Epinephelus fuscoguttatus. Aquaculture 2008, 277, 66–72. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Iehata, S.; Moudud Islam, M.; Kader, M.A.; Ambok Bolong, A.M.; Ikeda, D.; Kinoshita, S. Sodium alginate supplementation modulates gut microbiota, health parameters, growth performance and growth-related gene expression in Malaysian Mahseer Tor tambroides. Aquac. Nutr. 2019, 25, 1300–1317. [Google Scholar] [CrossRef]
- Chung, M.-Y.; Liu, C.-H.; Chen, Y.-N.; Cheng, W. Enhancing the reproductive performance of tiger shrimp, Penaeus monodon, by incorporating sodium alginate in the broodstock and larval diets. Aquaculture 2011, 312, 180–184. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control. 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Cejko, B.I.; Dryl, K.; Sarosiek, B.; Ilgert, J.; Jesiołowski, M.; Kowalski, R.K. Application of sodium alginate solution for short-term storage of different volumes of sex-reversed rainbow trout (Oncorhynchus mykiss) testicular sperm. Aquaculture 2022, 560, 738491. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Tapingkae, W.; Khamtavee, P. The effects of dietary kefir and low molecular weight sodium alginate on serum immune parameters, resistance against Streptococcus agalactiae and growth performance in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2017, 62, 139–146. [Google Scholar] [CrossRef]
- Ashouri, G.; Soofiani, N.M.; Hoseinifar, S.H.; Jalali, S.A.H.; Morshedi, V.; Van Doan, H.; Mozanzadeh, M.T. Combined effects of dietary low molecular weight sodium alginate and Pediococcus acidilactici MA18/5M on growth performance, haematological and innate immune responses of Asian sea bass (Lates calcalifer) juveniles. Fish Shellfish. Immunol. 2018, 79, 34–41. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Tapingkae, W.; Tongsiri, S.; Khamtavee, P. Combined administration of low molecular weight sodium alginate boosted immunomodulatory, disease resistance and growth enhancing effects of Lactobacillus plantarum in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2016, 58, 678–685. [Google Scholar] [CrossRef]
- Ahmadi-Noorbakhsh, S.; Ardakani, E.M.; Sadighi, J.; Aldavood, S.J.; Abbasi, M.F.; Farzad-Mohajeri, S.; Ghasemi, A.; Sharif-Paghaleh, E.; Hatami, Z.; Nikravanfard, N.; et al. Guideline for the Care and Use of Laboratory Animals in Iran. Lab Anim. 2021, 50, 303–305. [Google Scholar] [CrossRef]
- Van Doan, H.; Tapingkae, W.; Moonmanee, T.; Seepai, A. Effects of low molecular weight sodium alginate on growth performance, immunity, and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2016, 55, 186–194. [Google Scholar] [CrossRef]
- Official Methods of Analysis of the AOAC International, The Association; AOAC: Rockville, MD, USA, 2000.
- Abele, D.; Zenteno-Savin, T.; Vazquez-Medina, J.P. Oxidative Stress in Aquatic Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.A.; Aust, S.D. Measurement of Lipid Peroxidation. Curr. Protoc. Toxicol. 1999, 1, 2.4.1–2.4.13. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zaretabar, A.; Ouraji, H.; Kenari, A.A.; Yeganeh, S.; Esmaeili, M.; Amirkolaee, A.K. One step toward aquaculture sustainability of a carnivorous species: Fish meal replacement with barley protein concentrate plus wheat gluten meal in Caspian brown trout (Salmo trutta caspius). Aquac. Rep. 2021, 20, 100714. [Google Scholar] [CrossRef]
- Wu, J.P.; Chen, H.-C. Effects of cadmium and zinc on oxygen consumption, ammonium excretion, and osmoregulation of white shrimp (Litopenaeus vannamei). Chemosphere 2004, 57, 1591–1598. [Google Scholar] [CrossRef]
- Aoe, S.; Mio, K.; Yamanaka, C.; Kuge, T. Low Molecular Weight Barley β-Glucan Affects Glucose and Lipid Metabolism by Prebiotic Effects. Nutrients 2020, 13, 130. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Buathongjan, C.; Sangwan, W.; Rattanaprasert, M.; Weizman, K.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Production of low molecular weight pectins via electron beam irradiation and their potential prebiotic functionality. Food Hydrocoll. 2021, 113, 106551. [Google Scholar] [CrossRef]
- Paesani, C.; Sciarini, L.S.; Moiraghi, M.; Salvucci, E.; Prado, S.B.; Pérez, G.T.; Fabi, J.P. Human colonic in vitro fermentation of water-soluble arabinoxylans from hard and soft wheat alters Bifidobacterium abundance and short-chain fatty acids concentration. LWT 2020, 134, 110253. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Mountzouris, K.C.; Gibson, G.R.; Rastall, R.A. In vitro fermentability of dextran, oligodextran and maltodextrin by human gut bacteria. Br. J. Nutr. 2000, 83, 247–255. [Google Scholar] [CrossRef]
- Van Laere, K.M.J.; Hartemink, R.; Bosveld, M.; Schols, H.A.; Voragen, A.G.J. Fermentation of Plant Cell Wall Derived Polysaccharides and Their Corresponding Oligosaccharides by Intestinal Bacteria. J. Agric. Food Chem. 2000, 48, 1644–1652. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.-H.; Yeh, S.-T.; Chen, J.-C. The immune stimulatory effect of sodium alginate on the white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish. Immunol. 2004, 17, 41–51. [Google Scholar] [CrossRef]
- Santos, H.M.; Tsai, C.Y.; Yanuaria, C.A.S.; Tayo, L.L.; Vo, D.D.; Mariatulqabtiah, A.R.; Chuang, K.P. Effects of sodium alginate-fed Pacific white shrimps, Litopenaeus vannamei, on Toll-like receptors and Vibrio alginolyticus infection. Aquac. Res. 2019, 50, 1384–1392. [Google Scholar] [CrossRef]
- Fujiki, K.; Matsuyama, H.; Yano, T. Protective effect of sodium alginates against bacterial infection in common carp, Cyprinus carpio L. J. Fish Dis. 1994, 17, 349–355. [Google Scholar] [CrossRef]
- Shearer, K.D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 1994, 119, 63–88. [Google Scholar] [CrossRef]
- Romano, N.; Simon, W.; Ebrahimi, M.; Fadel, A.H.; Chong, C.M.; Kamarudin, M.S. Dietary sodium citrate improved oxidative stability in red hybrid tilapia (Oreochromis sp.) but reduced growth, health status, intestinal short chain fatty acids and induced liver damage. Aquaculture 2016, 458, 170–176. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Qu, R.-J.; Wang, X.-H.; Feng, M.-B.; Li, Y.; Liu, H.-X.; Wang, L.-S.; Wang, Z.-Y. The toxicity of cadmium to three aquatic organisms (Photobacterium phosphoreum, Daphnia magna and Carassius auratus) under different pH levels. Ecotoxicol. Environ. Saf. 2013, 95, 83–90. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, H.; Liu, X. Low levels of cadmium exposure induce DNA damage and oxidative stress in the liver of Oujiang colored common carp Cyprinus carpio var. color. Fish Physiol. Biochem. 2011, 37, 97–103. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Z.; Tao, W.; Li, W.; Wang, C.; Wang, M.; Ye, S.; Du, Y.; Wu, X.; Wu, D. Effects of polysaccharides from mycelia of Cordyceps sinensis on growth performance, immunity and antioxidant indicators of the white shrimp Litopenaeus vannamei. Aquac. Nutr. 2015, 21, 173–179. [Google Scholar] [CrossRef]
- Mohan, K.; Padmanaban, M.; Uthayakumar, V. Effects of Ganoderma lucidum crude polysaccharides (GLCP) on growth, survival and biochemical composition of the freshwater prawn Macrobrachium rosenbergii post larvae. Res. J. Chem. Environ. 2015, 19, 9. [Google Scholar]
- Hoseinifar, S.H.; Hoseini, S.M.; Bagheri, D. Effects of Galactooligosaccharide and Pediococcus acidilactici on Antioxidant Defence and Disease Resistance of Rainbow Trout, Oncorhynchus mykiss. Ann. Anim. Sci. 2017, 17, 217–227. [Google Scholar] [CrossRef]
- Rohani, F.; Islam, S.M.; Hossain, K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C. Shahjahan Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish. Immunol. 2021, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.; Dawood, M.A.; Alagawany, M.; Faggio, C.; Nowosad, J.; Kucharczyk, D. Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish Shellfish. Immunol. 2022, 122, 115–130. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, D.K.; Muralisankar, T.; Ganesan, A.R.; Marimuthu, K.; Sathishkumar, P. The potential role of medicinal mushrooms as prebiotics in aquaculture: A review. Rev. Aquac. 2022, 14, 1300–1332. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Celep, A.G.S.; Demirkaya, A.; Solak, E.K. Antioxidant and anticancer activities of gallic acid loaded sodium alginate microspheres on colon cancer. Curr. Appl. Phys. 2020, 40, 30–42. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Wang, J.; Dong, M.; Jia, P.; Bu, T.; Wang, Q.; Wang, L. Sodium alginate-based nanocomposite films with strong antioxidant and antibacterial properties enhanced by polyphenol-rich kiwi peel extracts bio-reduced silver nanoparticles. Food Packag. Shelf Life 2021, 29, 100741. [Google Scholar] [CrossRef]
- Tejpal, C.; Pal, A.; Sahu, N.; Kumar, J.A.; Muthappa, N.; Vidya, S.; Rajan, M. Dietary supplementation of l-tryptophan mitigates crowding stress and augments the growth in Cirrhinus mrigala fingerlings. Aquaculture 2009, 293, 272–277. [Google Scholar] [CrossRef]
- Liu, F.; Shi, H.-Z.; Guo, Q.-S.; Yu, Y.-B.; Wang, A.-M.; Lv, F.; Shen, W.-B. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish. Immunol. 2016, 51, 125–135. [Google Scholar] [CrossRef]
- Dawood, M.A.; Gewaily, M.S.; Monier, M.N.; Younis, E.M.; Van Doan, H.; Sewilam, H. The regulatory roles of yucca extract on the growth rate, hepato-renal function, histopathological alterations, and immune-related genes in common carp exposed with acute ammonia stress. Aquaculture 2021, 534, 736287. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Liu, Q.; Ye, H.; Zou, C.; Xu, M.; Zhang, Y.; Ye, C. Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus coioides) under cold stress. Aquaculture 2019, 498, 545–555. [Google Scholar] [CrossRef]
- Esmaeili, M.; Hosseini, H.; Zare, M.; Akhavan, S.R.; Rombenso, A. Early Mild Stress along with Lipid Improves the Stress Responsiveness of Oscar (Astronotus ocellatus). Aquac. Nutr. 2022, 2022, 8991678. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chen, S.J.; Chen, M.; Tian, L.X.; Niu, J.; Liu, Y.J.; Xu, D.H. Effect of cadmium-polluted diet on growth, salinity stress, hepatotoxicity of juvenile Pacific white shrimp (Litopenaeus vannamei): Protective effect of Zn (II)–curcumin. Ecotoxicol. Environ. Saf. 2016, 125, 176–183. [Google Scholar] [CrossRef]
- Chen, S.; Zhuang, Z.; Yin, P.; Chen, X.; Zhang, Y.; Tian, L.; Niu, J.; Liu, Y. Changes in growth performance, haematological parameters, hepatopancreas histopathology and antioxidant status of pacific white shrimp (Litopenaeus vannamei) fed oxidized fish oil: Regulation by dietary myo-inositol. Fish Shellfish. Immunol. 2019, 88, 53–64. [Google Scholar] [CrossRef]
- Zare, M.; Esmaeili, N.; Hosseini, H.; Choupani, S.M.H.; Akhavan, S.; Rombenso, A. Fish meal replacement and early mild stress improve stress responsiveness of oscar (Astronotus ocellatus) in future stressful events. Animals 2023, 18, 1314. [Google Scholar] [CrossRef]
- Zare, M.; Heidari, E.; Choupani, S.M.H.; Akhavan, S.; Rombenso, A.; Esmaeili, N. The recovery time between early mild stress and final acute stress affects survival rate, growth, immunity, health physiology, and stress response of oscar (Astronotus ocellatus). Animals 2023, 13, 1606. [Google Scholar] [CrossRef]

| Proximal Composition | (%) |
|---|---|
| Crude protein | 40 |
| Crude fat | 9.2 |
| Moisture | 7.5 |
| Crude ash | 10.5 |
| Crude fibre | 3.1 |
| Energy (kJ/g) | 18.59 |
| Nitrogen-free extract | 37.2 |
| Parameters | Control | 0.5 LMWSA | 1.0 LMWSA | 2.0 LMWSA |
|---|---|---|---|---|
| Initial weight (g) | 4.00 ± 0.08 | 3.75 ± 0.38 | 3.81 ± 0.34 | 3.94 ± 0.13 |
| Final weight (g) | 14.75 ± 0.81 | 14.48 ± 0.43 | 13.60 ± 0.26 | 15.16 ± 0.98 |
| Final length (cm) | 11.83 ± 0.52 | 11.98 ± 0.25 | 11.88 ± 0.30 | 11.87 ± 0.46 |
| SGR (%/day) | 2.33 ± 0.06 | 2.42 ± 0.22 | 2.28 ± 0.15 | 2.40 ± 0.08 |
| W.G. (g) | 10.75 ± 0.73 | 10.73 ± 0.73 | 9.79 ± 0.35 | 11.22 ± 0.89 |
| W.G. (%) | 268.66 ± 12.59 | 289.32 ± 47.17 | 258.98 ± 29.24 | 284.20 ± 17.94 |
| Feed consumption (g) | 13.70 ± 0.61 a | 14.49 ± 0.22 a | 13.64 ± 0.27 a | 12.28 ± 0.59 b |
| FCR | 1.28 ± 0.04 a | 1.36 ± 0.11 a | 1.39 ± 0.03 a | 1.10 ± 0.05 b |
| Survival (%) | 91.67 ± 7.64 | 91.67 ± 10.41 | 91.67 ± 7.64 | 90.00 ± 8.66 |
| Survival after Cd stress | 62.05 ± 7.72 | 77.97 ± 6.61 | 85.23 ± 4.16 | 80.20 ± 9.31 |
| Parameters | Control | 0.5 LMWSA | 1.0 LMWSA | 2.0 LMWSA |
|---|---|---|---|---|
| Moisture (%) | 71.01 ± 1.13 | 71.00 ± 1.04 | 71.45 ± 1.33 | 72.16 ± 0.79 |
| Protein (%) | 17.04 ± 0.10 | 16.98 ± 0.12 | 17.04 ± 0.10 | 16.63 ± 0.21 |
| Fat (%) | 1.45 ± 0.11 | 1.46 ± 0.21 | 1.49 ± 0.29 | 1.57 ± 0.18 |
| Ash (%) | 6.98 ± 0.09 | 6.63 ± 0.14 | 6.91 ± 0.11 | 6.86 ± 0.13 |
| Parameters | p-Value | ||
|---|---|---|---|
| ANOVA | Linear | Quadratic | |
| Survival after stress | 0.101 | 0.058 | 0.006 |
| Feed conversion ratio | 0.002 | 0.013 | 0.049 |
| Ash | 0.964 | 0.512 | 0.031 |
| GST | 0.018 | 0.023 | 0.006 |
| MDA-challenege | 0.001 | 0.003 | 0.009 |
| GSH-challenege | NA | 0.018 | 0.020 |
| GST-challenege | 0.002 | 0.008 | 0.001 |
| ALT-challenege | 0.007 | 0.026 | 0.009 |
| AST-challenege | NA | 0.162 | 0.048 |
| Diet Effect | Stress Effect | Interaction | Before Stress | After Stress | Control | 0.5 LMWSA | 1.0 LMWSA | 2.0 LMWSA | |
|---|---|---|---|---|---|---|---|---|---|
| p Value | Main Effects | ||||||||
| GSH | 0.012 | 0.001 | 0.316 | 383.2 | 360.8 | 363.2 b | 367.6 b | 375.2 ab | 382.0 a |
| AST | 0.325 | 0.001 | 0.085 | 266.9 | 290.2 | ||||
| LMWSA Level | Survival-Challenge | FCR | SGR | GST | ALT | MDA-Challenge | GSH-Challenge | GST-Challenge | ALT-Challenge | AST-Challenge | Moisture | Ash | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMWSA level | 1.00 | 0.56 | −0.58 * | 0.11 | 0.64 * | 0.12 | −0.67 * | 0.71 ** | −0.73 ** | −0.67 * | −0.51 | 0.45 | −0.23 |
| Survival-challenge | 0.56 | 1.00 | 0.23 | −0.25 | 0.11 | 0.19 | −0.50 | 0.56 | −0.68 * | −0.59 * | −0.37 | 0.20 | 0.02 |
| FCR | −0.58 * | 0.23 | 1.00 | −0.51 | −0.70 * | 0.15 | 0.24 | −0.24 | 0.16 | 0.17 | 0.19 | −0.48 | 0.03 |
| SGR | 0.11 | −0.25 | −0.51 | 1.00 | −0.05 | −0.59 * | −0.16 | 0.14 | −0.34 | −0.23 | −0.41 | 0.60 * | −0.68 * |
| GST | 0.64 * | 0.11 | −0.70 * | −0.05 | 1.00 | 0.12 | −0.54 | 0.31 | −0.05 | −0.14 | 0.17 | 0.25 | 0.39 |
| ALT | 0.12 | 0.19 | 0.15 | −0.59 * | 0.12 | 1.00 | 0.21 | −0.05 | −0.03 | −0.06 | 0.16 | −0.19 | 0.34 |
| MDA-challenge | −0.67 * | −0.50 | 0.24 | −0.16 | −0.54 | 0.21 | 1.00 | −0.59 * | 0.55 | 0.59 * | 0.23 | −0.25 | 0.22 |
| GSH-challenge | 0.71 ** | 0.56 | −0.24 | 0.14 | 0.31 | −0.05 | −0.59 * | 1.00 | −0.58 * | −0.54 | −0.47 | 0.51 | −0.11 |
| GST-challenge | −0.73 ** | −0.68 * | 0.16 | −0.34 | −0.05 | −0.03 | 0.55 | −0.58 * | 1.00 | 0.84 ** | 0.72 ** | −0.41 | 0.54 |
| ALT-challenge | −0.67 * | −0.59 * | 0.17 | −0.23 | −0.14 | −0.06 | 0.59 * | −0.54 | 0.84 ** | 1.00 | 0.63 * | −0.14 | 0.25 |
| AST-challenge | −0.51 | −0.37 | 0.19 | −0.41 | 0.17 | 0.16 | 0.23 | −0.47 | 0.72 ** | 0.63 * | 1.00 | −0.46 | 0.38 |
| Moisture | 0.45 | 0.20 | −0.48 | 0.60 * | 0.25 | −0.19 | −0.25 | 0.51 | −0.41 | −0.14 | −0.46 | 1.00 | −0.28 |
| Ash | −0.23 | 0.02 | 0.03 | −0.68 * | 0.39 | 0.34 | 0.22 | −0.11 | 0.54 | 0.25 | 0.38 | −0.28 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagheri, D.; Moradi, R.; Zare, M.; Sotoudeh, E.; Hoseinifar, S.H.; Oujifard, A.; Esmaeili, N. Does Dietary Sodium Alginate with Low Molecular Weight Affect Growth, Antioxidant System, and Haemolymph Parameters and Alleviate Cadmium Stress in Whiteleg Shrimp (Litopenaeus vannamei)? Animals 2023, 13, 1805. https://doi.org/10.3390/ani13111805
Bagheri D, Moradi R, Zare M, Sotoudeh E, Hoseinifar SH, Oujifard A, Esmaeili N. Does Dietary Sodium Alginate with Low Molecular Weight Affect Growth, Antioxidant System, and Haemolymph Parameters and Alleviate Cadmium Stress in Whiteleg Shrimp (Litopenaeus vannamei)? Animals. 2023; 13(11):1805. https://doi.org/10.3390/ani13111805
Chicago/Turabian StyleBagheri, Dara, Rohullah Moradi, Mahyar Zare, Ebrahim Sotoudeh, Seyed Hossein Hoseinifar, Amin Oujifard, and Noah Esmaeili. 2023. "Does Dietary Sodium Alginate with Low Molecular Weight Affect Growth, Antioxidant System, and Haemolymph Parameters and Alleviate Cadmium Stress in Whiteleg Shrimp (Litopenaeus vannamei)?" Animals 13, no. 11: 1805. https://doi.org/10.3390/ani13111805
APA StyleBagheri, D., Moradi, R., Zare, M., Sotoudeh, E., Hoseinifar, S. H., Oujifard, A., & Esmaeili, N. (2023). Does Dietary Sodium Alginate with Low Molecular Weight Affect Growth, Antioxidant System, and Haemolymph Parameters and Alleviate Cadmium Stress in Whiteleg Shrimp (Litopenaeus vannamei)? Animals, 13(11), 1805. https://doi.org/10.3390/ani13111805






