Morphologic and Genetic Characterization of Protospirura canariensis n. sp. (Nematoda, Spiruridae), a Parasite of the Black Rat Rattus rattus (Rodentia, Muridae) from El Hierro Island (Canary Archipelago, Spain) †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
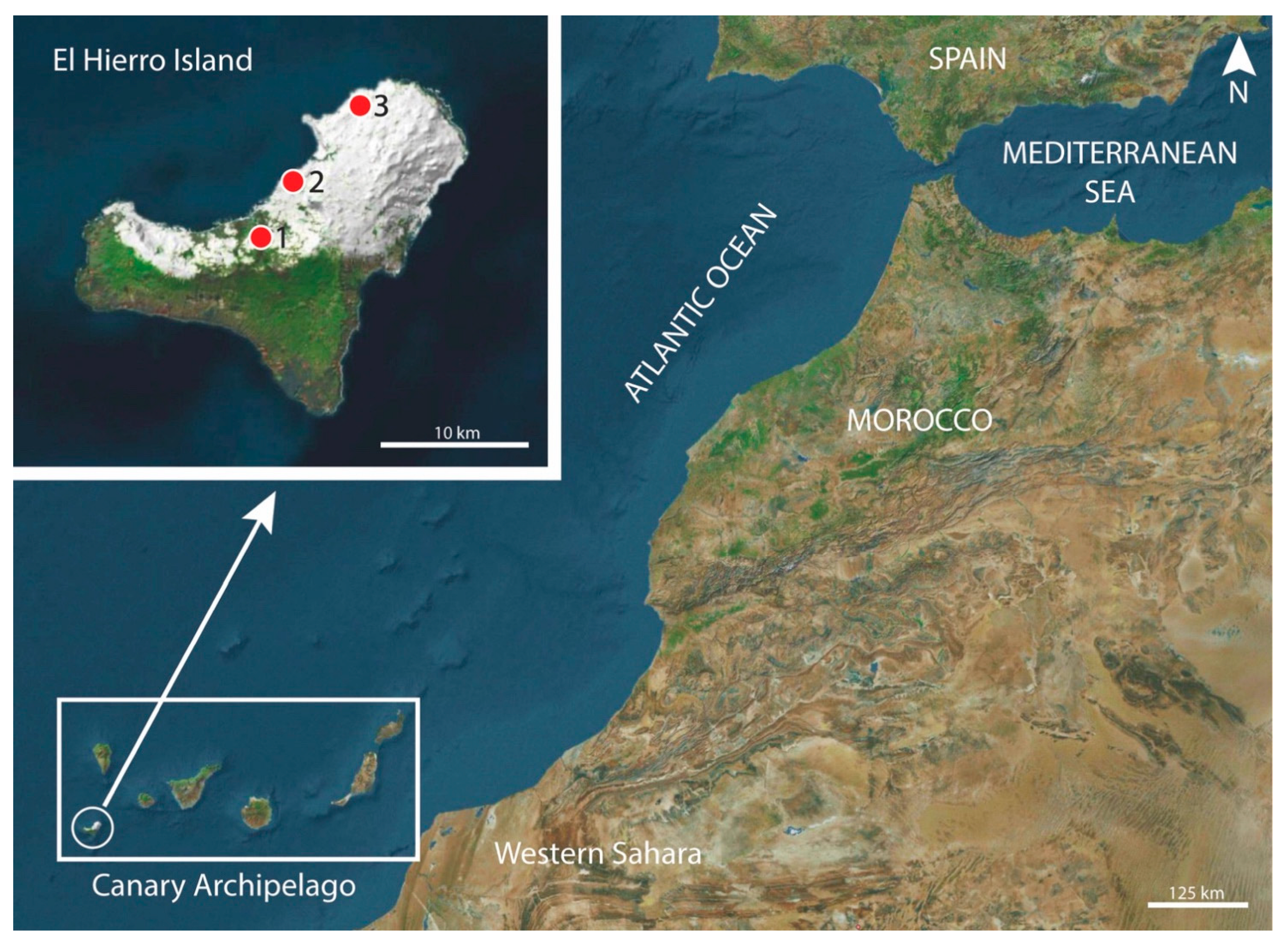
2.1. Specimens
2.2. Light Microscopy Study
2.3. Scanning Electron Microscopy Study
2.4. Molecular Analyses and Phylogenetic Tree
3. Results
3.1. Taxonomic Summary
- -
- holotype, ♂No. 1, MNHN HEL1905.
- -
- allotype, ♀No. 1, MNHN HEL1906.
- -
- Nineteen paratypes:
- -
- Seven males: ♂No. 2, MNHN HEL1907; ♂No. 4, MNHN HEL1908; ♂No. 5, MNHN HEL1909; ♂No. 7, MNHN HEL1910; ♂No. 8, MNHN HEL1911; ♂No. 10, MNHN HEL1912 and ♂No. 11, MNHN HEL1913.
- -
- Twelve females: ♀No. 2, MNHN HEL1914; ♀No. 3, MNHN HEL1915; ♀No. 4, MNHN HEL1916; ♀No. 5, MNHN HEL1917; ♀No. 6, MNHN HEL1918; ♀No. 7, MNHN HEL1919; ♀No. 8, MNHN HEL1920; ♀No. 9, MNHN HEL1921; ♀No. 10, MNHN HEL1922; ♀No. 11, MNHN HEL1923; ♀No. 12, MNHN HEL1924 and ♀No. 13, MNHN HEL1925.
3.2. Description
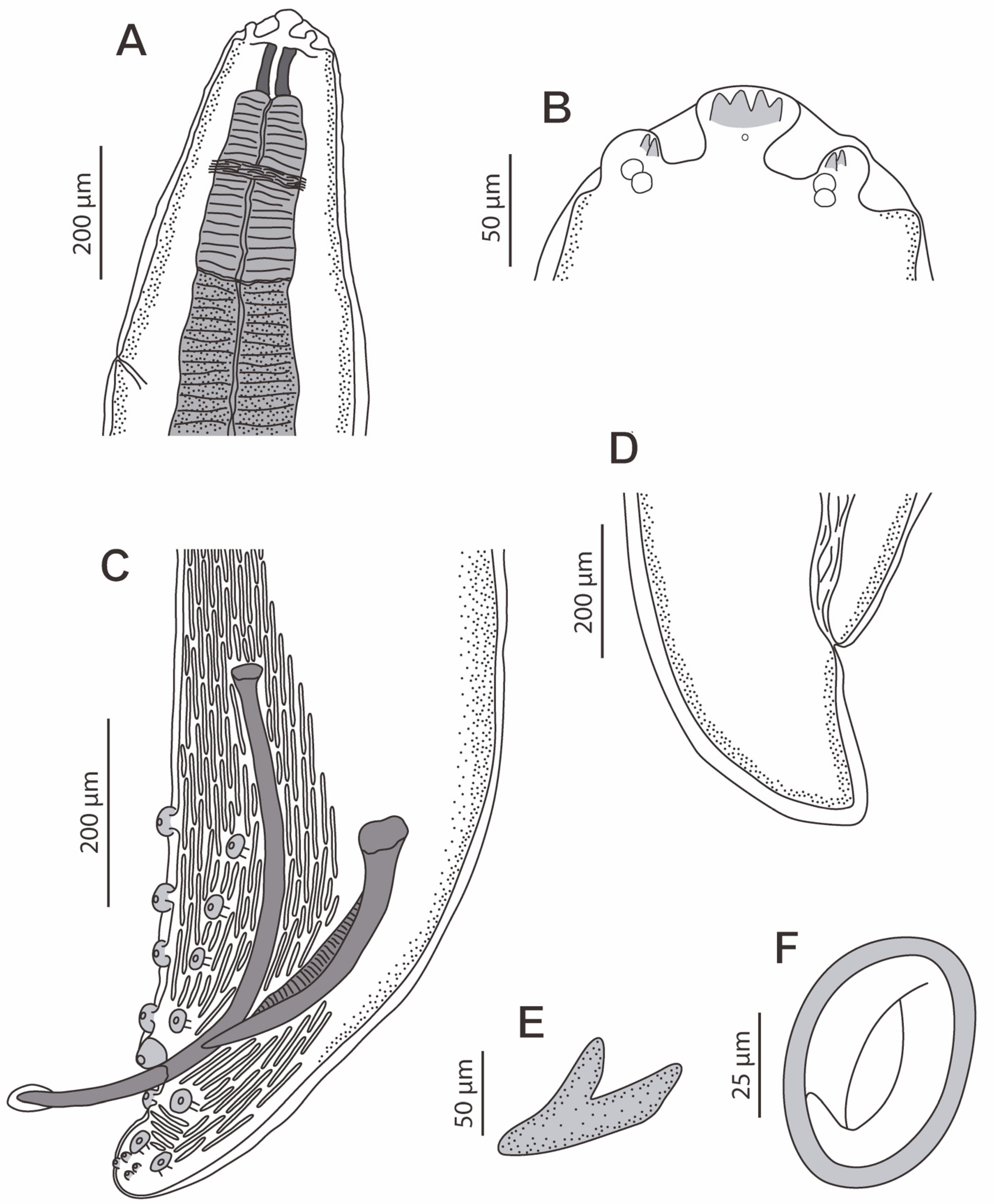

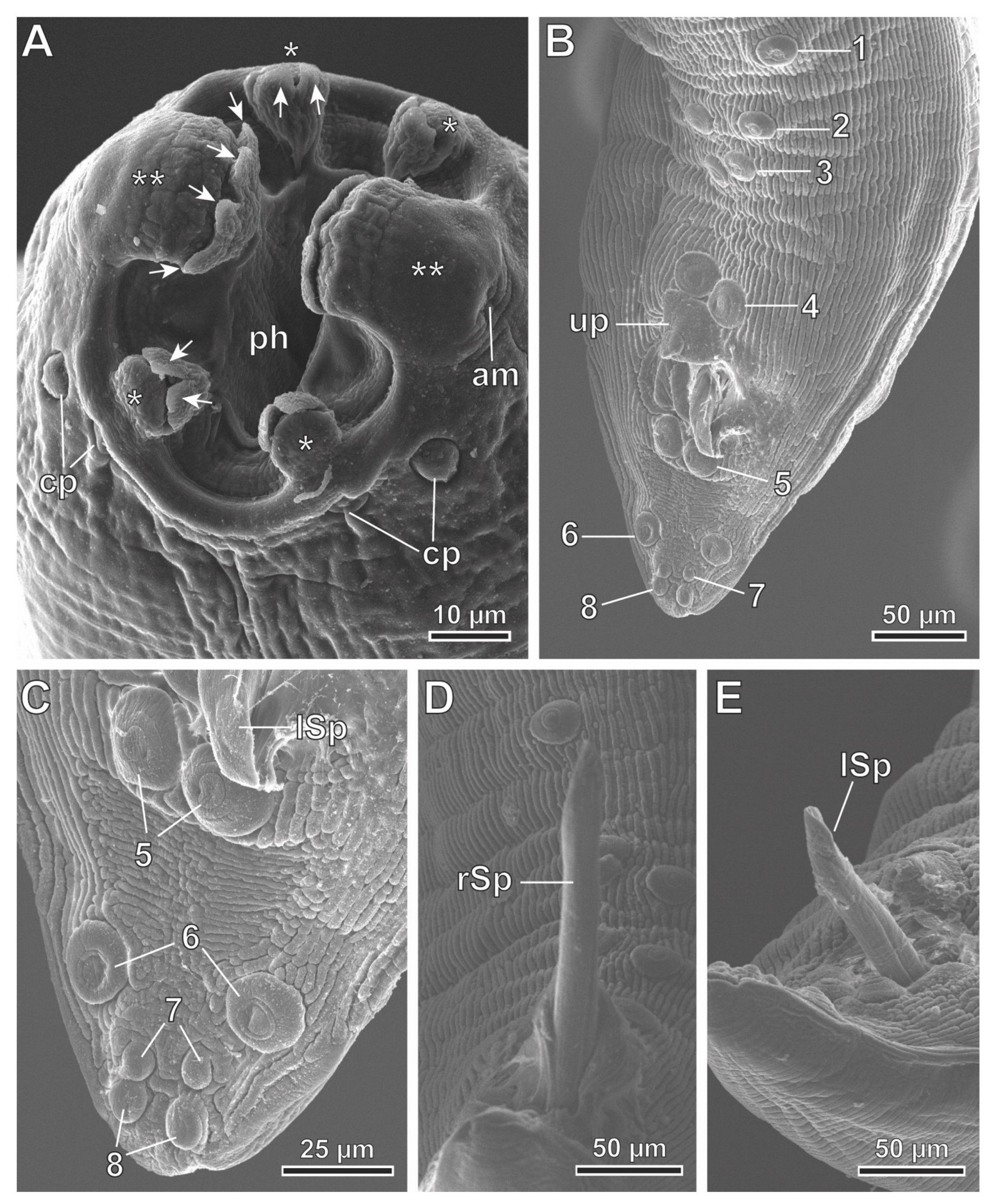
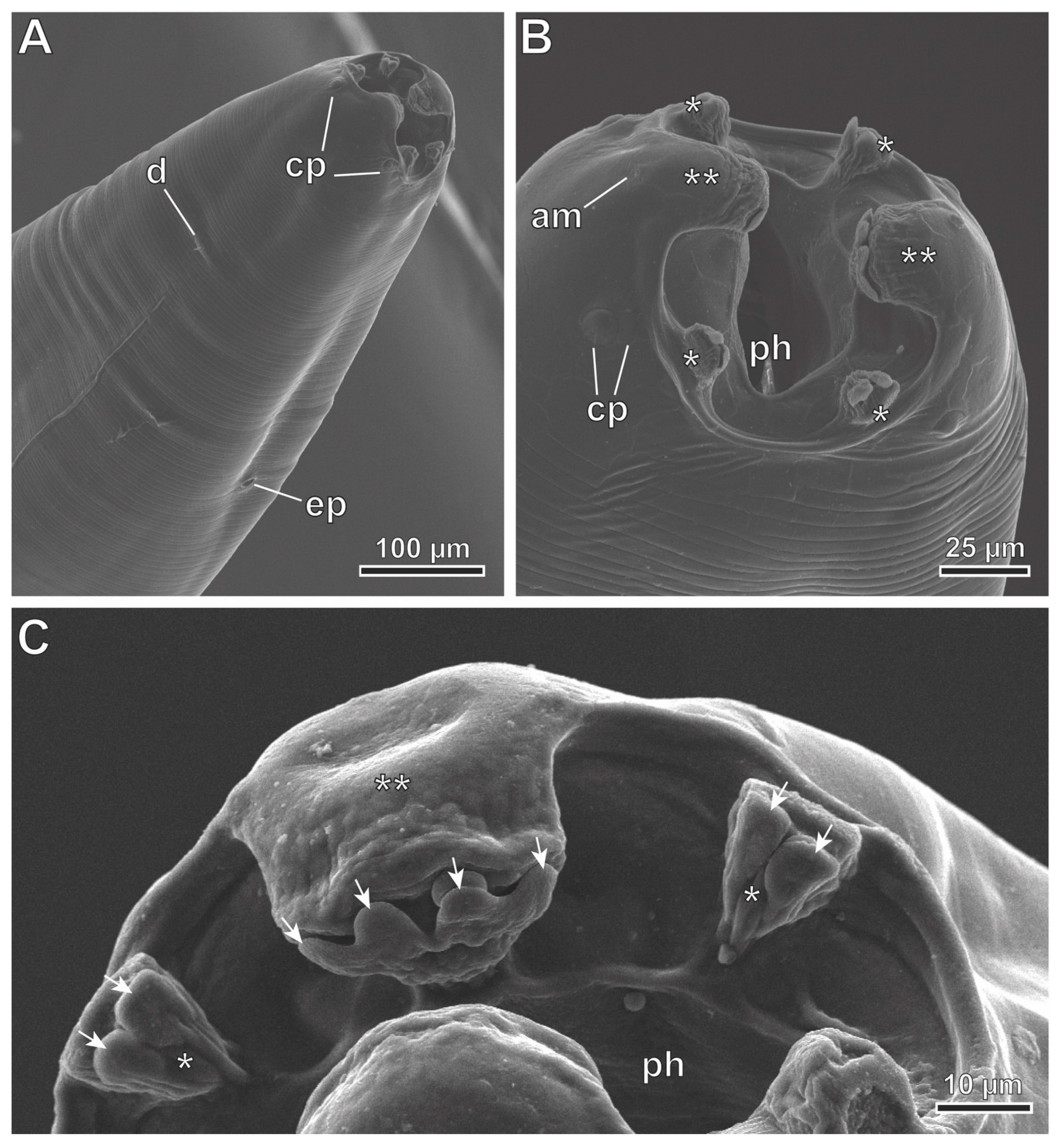
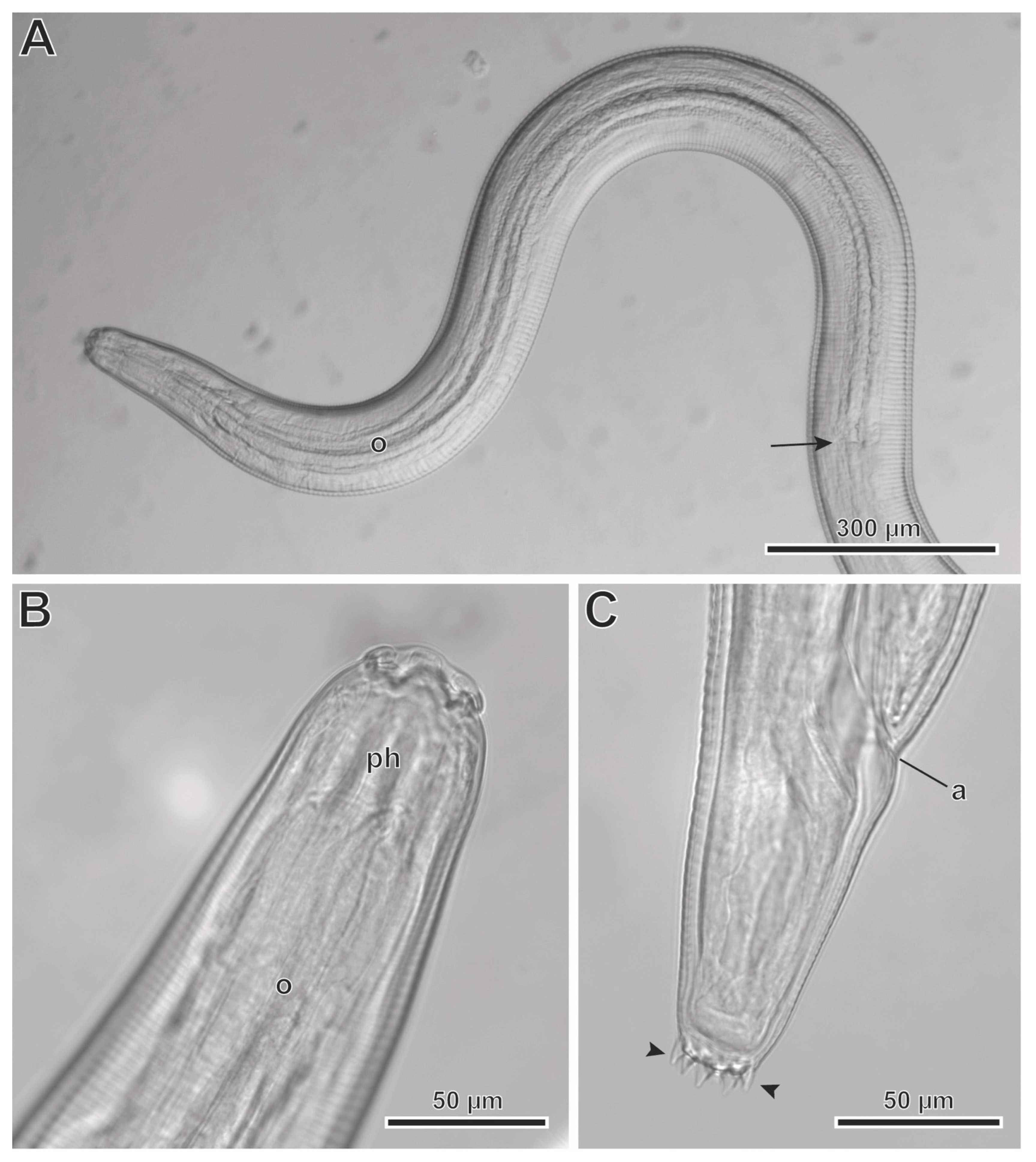
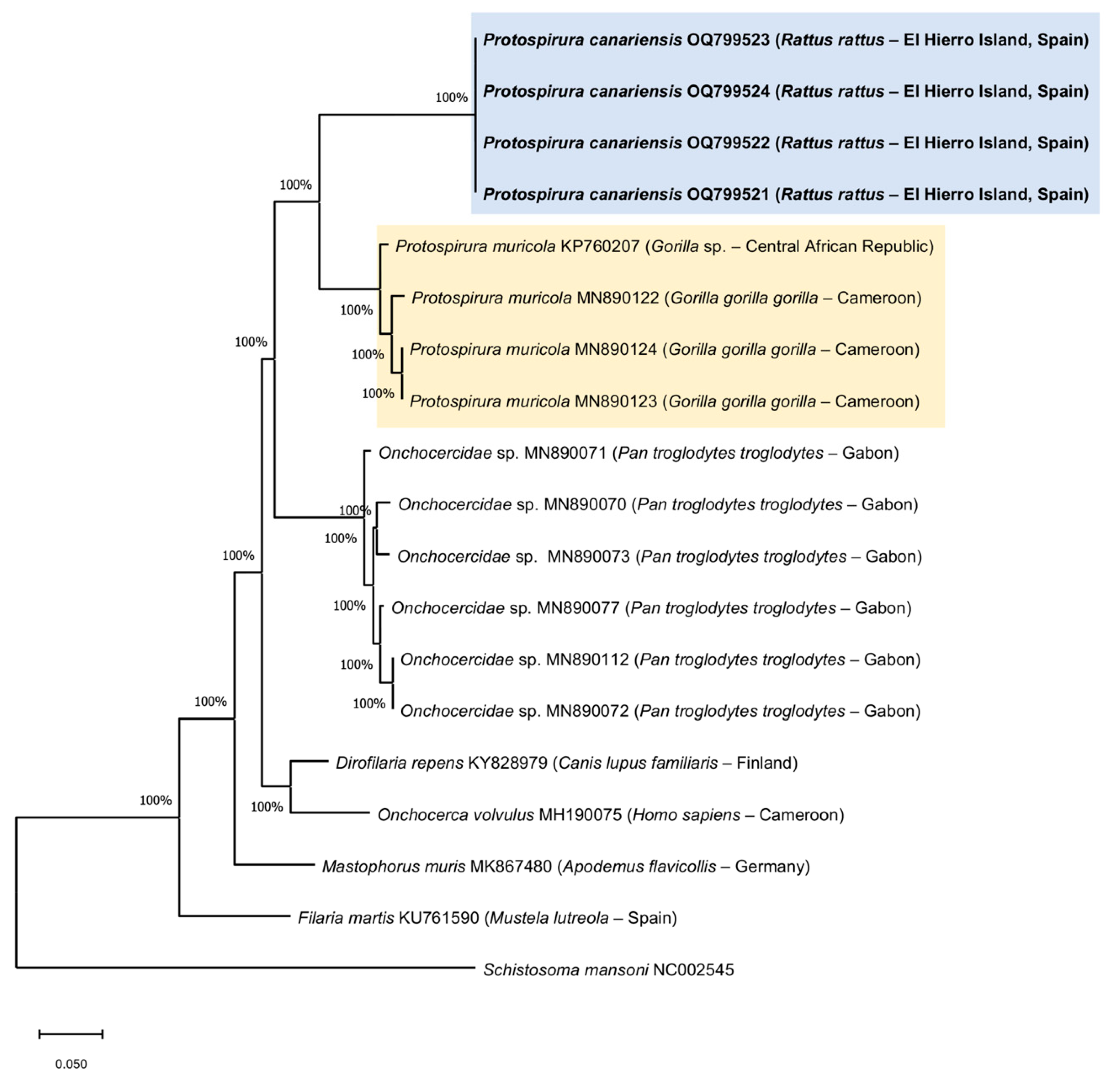
3.3. Molecular Phylogeny
4. Discussion
| Species | Pseudolabia | Spicules | Cloacal Papillae | Vulva | References | ||
|---|---|---|---|---|---|---|---|
| Subm | Lat | Right | Left | ||||
| Protospirura anopla Kreis, 1938 | 0 [6] 4 [9] | 0 [6] 4 [9] | 287 | 632 | 4 pre, 5 post (18) | posteq | [6,9] |
| Protospirura armeniana Alojan, 1951 | 2 [6] 4 [9] | 2 [6] 4 [9] | 620–639 | 370–411 | 4 pre, upre, 7 post, upost (24) | posteq | [6,9] |
| Protospirura canariensis n. sp. | 2 | 4 | 643–715 (675) | 309–412 (368) | 4 pre, upre, 4 post (17) | preeq | Present study |
| Protospirura chabaudi Vuylsteke, 1964 | 4 [9] 2 [18] | 4 [9] 0 [18] | 980 | 420 | 4 pre, 5 post (18) | posteq | [9,18] |
| Protospirura kaindiensis Smales, 2001 | 2 | 2 | 450–480 | 310–330 | 4 pre, upre, 5 post (19) | preeq | [9] |
| Protospirura mexicana Falcón and Sanabria, 1995 | 1 | 0 | 340–465 (404) | 420–527 (481) | 4 pre, upre, 4 post (17) | posteq | [4] |
| Protospirura munimuniensis Smales, 2021 | 2 | 2 | 602–603 | 430–455 | 4 pre, upre, 6 post (21) | ? | [16] |
| Protospirura muricola Gedoelst, 1916 | 2 | 2 | 268–430 (352) | 290–501 (411) | 4 pre, upre, 6 post (21) | posteq | [15,25] |
| Protospirura numidica Seurat, 1914 | 3 [5] 4 [9] | 3 [5] 4 [9] 4 [13] | 830 | 420 | 4 pre, upre, 5 post (19) | posteq | [5,9,13] |
| Protospirura numidica criceticola Quentin, Karimi and Rodriguez de Almeida, 1968 | ? | 4 | 1250 | 470 | 4 pre, upre, 7 post (23) | posteq | [13] |
| Protospirura okinavensis Hasegawa, 1990 | 4 | 4 | 600–650 (620) | 320–350 (320) | 5–6 pre, upre, 4 post (19 or 21) | preeq | [8] |
| Protospirura peromysci Babero and Matthias, 1967 | 2 [3] 4 [9] | 4 [3] 4 [9] | 820–1200 | 330–380 | 4 pre, upre, 7 post (23) | posteq | [3,9] |
| Protospirura pseudomuris Yokohata and Abe, 1989 | 1 [7] 2 [9] | 1 [7] 2 [9] | 540–790 (680) | 270–380 (330) | 4 pre, upre, 6 post (21) | preeq | [7,8,9] |
| Protospirura siamensis Ribas, Veciana, Chaisiri and Morand, 2012 | 4 | 4 | 469–701 (572.6) | 348–380 (368.7) | 4–5 pre, upre, 4 post (17 or 19) | posteq | [14] |
| Protospirura suslica Schulz, 1927 | 2 [6] 4 [9] | 2 [6] 4 [9] | 315 | 873 | 5 pre, upre, 6 post (23) | posteq | [6,9] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chitwood, B.G. The status of Protospirura vs. Mastophorus with a consideration of the species of these genera. In Livro Jubilar do Professor Lauro Travassos; Silva, B., de Almeida, B.J., Ferreira, N., Gonçalves, A., Strommer, S., Gonçalves, O., de Almeida, C.A., Cantizano dos Santos, A., Eds.; Typographia do Instituto Oswaldo Cruz: Rio de Janeiro, Brazil, 1938; pp. 115–118. [Google Scholar]
- Wertheim, G. A study of Mastophorus muris (Gmelin, 1790) (Nematoda: Spirurida). Trans. Am. Microsc. Soc. 1962, 81, 274–279. [Google Scholar] [CrossRef]
- Babero, B.B.; Matthias, D. Protospirura peromysci n. sp. (Nematoda: Spiruridea) and other helminths from Peromyscus spp. in Nevada. Proc. Helminthol Soc. Wash. 1967, 34, 255–261. [Google Scholar]
- Falcón Ordaz, J.; Sanabria Espinoza, M.A. Especie nueva del género Protospirura (Nemata: Spiruridae) de Peromyscus difficilis (Rodentia: Cricetidae) de Hidalgo, México. An. Inst. Biol. UNAM Ser. Zool. 1995, 66, 17–26. [Google Scholar]
- Seurat, L.-G. Sur un nouveau spiroptère du Chat ganté. C. R. Soc. Biol. Paris 1914, 77, 344–347. [Google Scholar]
- Skryabin, K.I.; Sobolev, A.A. Spirurata of Animals and Man and the Diseases Caused by Them. Spirurata, Part 5, Spiruroidea. In Osnovy Nematologii; Skryabin, K.I., Ed.; Nauka: Moscow, Russia, 1963; Volume 11. (In Russian) [Google Scholar]
- Yokohata, Y.; Abe, H. Two new Spirurid Nematodes in Japanese Moles, Mogera spp. Jpn. J. Parasitol. 1989, 38, 92–99. [Google Scholar]
- Hasegawa, H. Protospirura okinavensis sp. n. (Nematoda: Spiruridae) from Mus caroli on Okinawa Island, Japan. J. Helminthol. Soc. Wash. 1990, 57, 153–156. [Google Scholar]
- Smales, L.R. Protospirura kaindiensis n. sp. (Spirura: Spiruridae) and other helminths from Pseudohydromys (Muridae: Hydromyinae) from Papua New Guinea. J. Parasitol. 2001, 87, 169–172. [Google Scholar] [CrossRef]
- Chabaud, A.G. No. 3 Keys to genera of the order Spirurida. Part 2. Spiruroidea, Habronematoidea and Acuarioidea. In CIH Keys to the Nematode Parasites of Vertebrates; Anderson, R.C., Chabaud, A.G., Willmott, S., Eds.; CAB: Farnham Royal, UK, 1975; pp. 29–58. [Google Scholar]
- Kreis, H.A. Beitrage zur Kenntnis parasitischer nematoden. VII. Parasitische Nematoden der schweizerischen wissenschaftlichen Expedition nach Angola (Afrika) in Jahre 1932. Ctbl. Bakt. I. Orig. 1938, 142, 90–105. [Google Scholar]
- Pence, D.B.; Windberg, L.A. Population dynamics across selected habitat variables of the helminth community in coyotes, Canis latrans, from South Texas. J. Parasitol. 1984, 70, 735–746. [Google Scholar] [CrossRef]
- Quentin, J.-C.; Karimi, Y.; Rodríguez de Almeida, C. Protospirura numidica criceticola, n. subsp. parasite de Rongeurs Cricetidae du Brésil. Cycle évolutif. Ann. Parasitol. 1968, 43, 583–596. [Google Scholar] [CrossRef]
- Ribas, A.; Veciana, M.; Chaisiri, K.; Morand, S. Protospirura siamensis n. sp. (Nematoda: Spiruridae) from rodents in Thailand. Syst. Parasitol. 2012, 82, 21–27. [Google Scholar] [CrossRef]
- Smales, L.R.; Harris, P.D.; Behnke, J.M. A redescription of Protospirura muricola Gedoelst, 1916 (Nematoda: Spiruridae), a parasite of murid rodents. Syst. Parasitol. 2009, 72, 15–26. [Google Scholar] [CrossRef]
- Smales, L.R. The gastrointestinal nematodes of Chiruromys forbsei Thomas and C. lamia (Thomas) (Rodentia: Muridae) with the description of a new species of Helgenema (Heligmonellidae) and a new species of Protospirura (Spiruridae) from Papua New Guinea. Trans. R. Soc. S. Aust. 2021, 145, 60–76. [Google Scholar] [CrossRef]
- Tinnin, D.S.; Ganzorig, S.; Gardner, S.L. Helminths of small mammals (Erinaceomorpha, Soricomorpha, Chiroptera, Rodentia, and Lagomorpha) of Mongolia; Museum of Texas Tech University: Lubbock, TX, USA, 2011; Volume 59, pp. 1–50. [Google Scholar] [CrossRef]
- Vuylsteke, C. Mission de Zoologie médicale au Maniema (Congo, Léopoldville) (P.L.G. Bennoit, 1959). 3. Vermes–Nematoda. Ann. Mus. R. Afr. Centr. Ser. 8 Zool. 1964, 132, 41–66. [Google Scholar]
- Movsesyan, S.O.; Nikoghosian, M.A.; Petrosian, R.A.; Vlasov, E.A.; Kuznetsov, D.N. Nematodes of rodents of Armenia. Ann. Parasitol. 2018, 64, 173–180. [Google Scholar] [PubMed]
- Quentin, J.-C. Cycle biologique de Protospirura muricola Gedoelst, 1916 Nematoda Spiruridae. Ann. Parasitol. 1969, 44, 485–504. [Google Scholar] [CrossRef]
- López, C.; Clemente, S.; Almeida, C.; Brito, A.; Hernández, M. A genetic approach to the origin of Millepora sp. in the eastern Atlantic. Coral Reefs 2015, 34, 631–638. [Google Scholar] [CrossRef]
- Gaillard, C.M.; Pion, S.D.; Hamou, H.; Sirima, C.; Bizet, C.; Lemarcis, T.; Locatelli, S. Detection of DNA of filariae closely related to Mansonella perstans in faecal samples from wild non-human primates from Cameroon and Gabon. Parasites Vectors 2020, 13, 313. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Baylis, H.A. On a collection of nematodes from Nigerian mammals (chiefly rodents). Parasitology 1928, 20, 280–304. [Google Scholar] [CrossRef]
- Anderson, R.C. Nematode Parasites of Vertebrates. Their Development and Transmission, 2nd ed.; CABI Publishing: Wallingford, UK, 2000; pp. 413–427. [Google Scholar]
- Brouat, C.; Kane, M.; Diouf, M.; Bâ, K.; Sall-Dramé, R.; Duplantier, J.M. Host ecology and variation in helminth community structure in Mastomys rodents from Senegal. Parasitology 2007, 134, 437–450. [Google Scholar] [CrossRef]
- Chen, H.T. A preliminary report on a survey of animal parasites of Canton, China, rats. Lignan Sci. J. 1933, 12, 65–74. [Google Scholar]
- Hasegawa, H.; Kobayashi, J.; Otsuru, M. Helminth parasites collected from Rattus rattus on Lanyu, Taiwan. J. Helminthol. Soc. Wash. 1994, 61, 95–102. [Google Scholar]
- Smales, L.R. The gastrointestinal helminths of Rattus niobe (Rodentia: Muridae) with descriptions of two new genera and three new species (Nematoda) from Papua New Guinea and Papua Indonesia. Zootaxa 2016, 4117, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Temme, M. Stomach nematodes of the Polynesian rat Rattus exulans in the Northern Marshall Islands, Pacific Ocean. Z. Angew. Zool. 1983, 70, 463–472. [Google Scholar]
- Rajper, M.; Birmani, N.A. New record of Protospirura siamensis Ribas, 2012 (Nematoda: Spiruridae) recovered from Rat and Mice of district Hyderabad, Sindh, Pakistan. In Proceedings of the 40th Pakistan Congress of Zoology, Virtual, 14–19 December 2021; p. 74. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miquel, J.; Martín-Carrillo, N.; Ribas, A.; Sánchez-Vicente, S.; Feliu, C.; Foronda, P. Morphologic and Genetic Characterization of Protospirura canariensis n. sp. (Nematoda, Spiruridae), a Parasite of the Black Rat Rattus rattus (Rodentia, Muridae) from El Hierro Island (Canary Archipelago, Spain). Animals 2023, 13, 1806. https://doi.org/10.3390/ani13111806
Miquel J, Martín-Carrillo N, Ribas A, Sánchez-Vicente S, Feliu C, Foronda P. Morphologic and Genetic Characterization of Protospirura canariensis n. sp. (Nematoda, Spiruridae), a Parasite of the Black Rat Rattus rattus (Rodentia, Muridae) from El Hierro Island (Canary Archipelago, Spain). Animals. 2023; 13(11):1806. https://doi.org/10.3390/ani13111806
Chicago/Turabian StyleMiquel, Jordi, Natalia Martín-Carrillo, Alexis Ribas, Santiago Sánchez-Vicente, Carlos Feliu, and Pilar Foronda. 2023. "Morphologic and Genetic Characterization of Protospirura canariensis n. sp. (Nematoda, Spiruridae), a Parasite of the Black Rat Rattus rattus (Rodentia, Muridae) from El Hierro Island (Canary Archipelago, Spain)" Animals 13, no. 11: 1806. https://doi.org/10.3390/ani13111806
APA StyleMiquel, J., Martín-Carrillo, N., Ribas, A., Sánchez-Vicente, S., Feliu, C., & Foronda, P. (2023). Morphologic and Genetic Characterization of Protospirura canariensis n. sp. (Nematoda, Spiruridae), a Parasite of the Black Rat Rattus rattus (Rodentia, Muridae) from El Hierro Island (Canary Archipelago, Spain). Animals, 13(11), 1806. https://doi.org/10.3390/ani13111806






