Presence of Non-Tuberculous Mycobacteria Including Mycobacterium avium subsp. paratuberculosis Associated with Environmental Amoebae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Cultivation of Microorganisms
2.3. Isolation of Environmental Amoebae
2.4. DNA Extraction and Purification
2.5. Detection and Quantification of Mycobacteria by Quantitative PCR (qPCR)
2.6. Identification of Mycobacteria Isolated from Samples
2.7. Amoebal Identification
| Name | Primers | Sequences | Target |
|---|---|---|---|
| qPCR atpE | Forward | CGGYGCCGGTATCGGYGA | Mycobacterium ssp. |
| Reverse | CGAAGACGAACARSGCCAT | ||
| Probe | FAM-ACSGTGATGAAGAACGGBGTRAA-BHQ1 | ||
| qPCR IS900 | Forward | CCGCTAATTGAGAGATGCGATTGG | |
| Reverse | AATCAACTCCAGCAGCGCGGCCTCG | Map | |
| Probe | FAM-TCCACGCCCGCCCAGACAGG-BHQ1 | ||
| Forward | GGTAGCAGACCTCACCTATGTGT | ||
| qPCR IS6110 | Reverse | AGGCGTCGGTGACAAAGG | MTB complex * |
| Probe | FAM-CACGTAGGCGAACCC-MGB | ||
| qPCR IS1081 | Forward | CCGCCACCGTGATTTCGA | |
| Reverse | GCCAGTCCGGGAAATAGCT | MTB complex * | |
| Probe | FAM-CCGCAACCATCGACGTC-MGB | ||
| qPCR IS1561 | Forward | GATCCAGGCCGAGAGAATCTG | |
| Reverse | GGACAAAAGCTTCGCCAAAA | MTB complex * | |
| Probe | FAM-ACGGCGTTGATCCGATTCCGC-TAMRA | ||
| hsp65 | Forward | CTTGTCGAACCGCATACCCT | |
| Reverse | ACCAACGATGGTGTGTCCAT | Mycobacterium ssp. | |
| 18S rRNA | Forward 566 | CAGCAGCCGCGGTAATTCC | Amoeba |
| Reverse 1200 | CCCGTGTTGAGTCAAATTAAGC |
3. Results
3.1. High Diversity of Non-Tuberculous Mycobacteria from Herd Samples
3.2. Herd Water Samples Rich in Environmental Amoebae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Saez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Toft, N. A review of prevalences of paratuberculosis in farmed animals in Europe. Prev. Vet. Med. 2009, 88, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999, 40, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Barkema, H.W.; Orsel, K.; Nielsen, S.S.; Koets, A.P.; Rutten, V.; Bannantine, J.P.; Keefe, G.P.; Kelton, D.F.; Wells, S.J.; Whittington, R.J.; et al. Knowledge gaps that hamper prevention and control of Mycobacterium avium subspecies paratuberculosis infection. Transbound. Emerg. Dis. 2018, 65 (Suppl. 1), 125–148. [Google Scholar] [CrossRef]
- Byrne, A.W.; Graham, J.; Milne, G.; Guelbenzu-Gonzalo, M.; Strain, S. Is There a Relationship Between Bovine Tuberculosis (bTB) Herd Breakdown Risk and Mycobacterium avium subsp. paratuberculosis Status? An Investigation in bTB Chronically and Non-chronically Infected Herds. Front. Vet. Sci. 2019, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Field, N.L.; McAloon, C.G.; Gavey, L.; Mee, J.F. Mycobacterium avium subspecies paratuberculosis infection in cattle—A review in the context of seasonal pasture-based dairy herds. Ir. Vet. J. 2022, 75, 12. [Google Scholar] [CrossRef]
- Rhodes, G.; Henrys, P.; Thomson, B.C.; Pickup, R.W. Mycobacterium avium subspecies paratuberculosis is widely distributed in British soils and waters: Implications for animal and human health. Environ. Microbiol. 2013, 15, 2761–2774. [Google Scholar] [CrossRef]
- Allen, A.R.; Skuce, R.A.; Byrne, A.W. Bovine Tuberculosis in Britain and Ireland—A Perfect Storm? the Confluence of Potential Ecological and Epidemiological Impediments to Controlling a Chronic Infectious Disease. Front. Vet. Sci. 2018, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Samba-Louaka, A.; Delafont, V.; Rodier, M.H.; Cateau, E.; Hechard, Y. Free-living amoebae and squatters in the wild: Ecological and molecular features. FEMS Microbiol. Rev. 2019, 43, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Molmeret, M.; Horn, M.; Wagner, M.; Santic, M.; Abu Kwaik, Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 2005, 71, 20–28. [Google Scholar] [CrossRef]
- Salah, I.B.; Ghigo, E.; Drancourt, M. Free-living amoebae, a training field for macrophage resistance of mycobacteria. Clin. Microbiol. Infect. 2009, 15, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Drancourt, M. Looking in amoebae as a source of mycobacteria. Microb. Pathog. 2014, 77, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.D.; Falkow, S.; Tompkins, L.S.; Bermudez, L.E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 1997, 65, 3759–3767. [Google Scholar] [CrossRef] [PubMed]
- Whan, L.; Grant, I.R.; Rowe, M.T. Interaction between Mycobacterium avium subsp. paratuberculosis and environmental protozoa. BMC Microbiol. 2006, 6, 63. [Google Scholar] [CrossRef]
- White, C.I.; Birtles, R.J.; Wigley, P.; Jones, P.H. Mycobacterium avium subspecies paratuberculosis in free-living amoebae isolated from fields not used for grazing. Vet. Rec. 2010, 166, 401–402. [Google Scholar] [CrossRef]
- Mura, M.; Bull, T.J.; Evans, H.; Sidi-Boumedine, K.; McMinn, L.; Rhodes, G.; Pickup, R.; Hermon-Taylor, J. Replication and long-term persistence of bovine and human strains of Mycobacterium avium subsp. paratuberculosis within Acanthamoeba polyphaga. Appl. Environ. Microbiol. 2006, 72, 854–859. [Google Scholar] [CrossRef]
- Salgado, M.; Alfaro, M.; Salazar, F.; Badilla, X.; Troncoso, E.; Zambrano, A.; Gonzalez, M.; Mitchell, R.M.; Collins, M.T. Application of cattle slurry containing Mycobacterium avium subsp. paratuberculosis (MAP) to grassland soil and its effect on the relationship between MAP and free-living amoeba. Vet. Microbiol. 2015, 175, 26–34. [Google Scholar] [CrossRef]
- Samba-Louaka, A.; Robino, E.; Cochard, T.; Branger, M.; Delafont, V.; Aucher, W.; Wambeke, W.; Bannantine, J.P.; Biet, F.; Hechard, Y. Environmental Mycobacterium avium subsp. paratuberculosis Hosted by Free-Living Amoebae. Front. Cell Infect. Microbiol. 2018, 8, 28. [Google Scholar] [CrossRef]
- Reveillaud, E.; Desvaux, S.; Boschiroli, M.L.; Hars, J.; Faure, E.; Fediaevsky, A.; Cavalerie, L.; Chevalier, F.; Jabert, P.; Poliak, S.; et al. Infection of Wildlife by Mycobacterium bovis in France Assessment Through a National Surveillance System, Sylvatub. Front. Vet. Sci. 2018, 5, 262. [Google Scholar] [CrossRef]
- Lefrancois, L.H.; Bodier, C.C.; Cochard, T.; Canepa, S.; Raze, D.; Lanotte, P.; Sevilla, I.A.; Stevenson, K.; Behr, M.A.; Locht, C.; et al. Novel feature of Mycobacterium avium subsp. paratuberculosis, highlighted by characterization of the heparin-binding hemagglutinin adhesin. J. Bacteriol. 2013, 195, 4844–4853. [Google Scholar] [CrossRef]
- Lefrancois, L.H.; Cochard, T.; Branger, M.; Peuchant, O.; Conde, C.; Pastuszka, A.; Locht, C.; Lanotte, P.; Biet, F. Feature of Adhesins Produced by Human Clinical Isolates of Mycobacterium intracellulare, Mycobacterium intracellulare subsp. chimaera and Closely Related Species. Microorganisms 2020, 8, 1154. [Google Scholar] [CrossRef] [PubMed]
- Hadziavdic, K.; Lekang, K.; Lanzen, A.; Jonassen, I.; Thompson, E.M.; Troedsson, C. Characterization of the 18S rRNA gene for designing universal eukaryote specific primers. PLoS ONE 2014, 9, e87624. [Google Scholar] [CrossRef] [PubMed]
- Huard, R.C.; Lazzarini, L.C.; Butler, W.R.; van Soolingen, D.; Ho, J.L. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J. Clin. Microbiol. 2003, 41, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Torres, M.J.; Aznar, J.; Gortazar, C.; Vicente, J. DNA Detection Reveals Mycobacterium tuberculosis Complex Shedding Routes in Its Wildlife Reservoir the Eurasian Wild Boar. Transbound. Emerg. Dis. 2017, 64, 906–915. [Google Scholar] [CrossRef]
- Phillips, C.J.; Foster, C.R.; Morris, P.A.; Teverson, R. The transmission of Mycobacterium bovis infection to cattle. Res. Vet. Sci. 2003, 74, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.R.; Ford, T.; Skuce, R.A. Does Mycobacterium tuberculosis var. bovis Survival in the Environment Confound Bovine Tuberculosis Control and Eradication? A Literature Review. Vet. Med. Int. 2021, 2021, 8812898. [Google Scholar] [CrossRef]
- Falkinham, J.O., 3rd. Surrounded by mycobacteria: Nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 2009, 107, 356–367. [Google Scholar] [CrossRef]
- Barbier, E.; Boschiroli, M.L.; Gueneau, E.; Rochelet, M.; Payne, A.; de Cruz, K.; Blieux, A.L.; Fossot, C.; Hartmann, A. First molecular detection of Mycobacterium bovis in environmental samples from a French region with endemic bovine tuberculosis. J. Appl. Microbiol. 2016, 120, 1193–1207. [Google Scholar] [CrossRef]
- Adams, A.P.; Bolin, S.R.; Fine, A.E.; Bolin, C.A.; Kaneene, J.B. Comparison of PCR versus culture for detection of Mycobacterium bovis after experimental inoculation of various matrices held under environmental conditions for extended periods. Appl. Environ. Microbiol. 2013, 79, 6501–6506. [Google Scholar] [CrossRef]
- Opel, K.L.; Chung, D.; McCord, B.R. A study of PCR inhibition mechanisms using real time PCR. J. Forensic Sci. 2010, 55, 25–33. [Google Scholar] [CrossRef]
- Falkinham, J.O., 3rd. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996, 9, 177–215. [Google Scholar] [CrossRef] [PubMed]
- Biet, F.; Boschiroli, M.L. Non-tuberculous mycobacterial infections of veterinary relevance. Res. Vet. Sci. 2014, 97, S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Betelli, L.; Duquenne, P.; Grenouillet, F.; Simon, X.; Scherer, E.; Gehin, E.; Hartmann, A. Development and evaluation of a method for the quantification of airborne Thermoactinomyces vulgaris by real-time PCR. J. Microbiol. Methods. 2013, 92, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.J.; Marsh, I.B.; Reddacliff, L.A. Survival of Mycobacterium avium subsp. paratuberculosis in dam water and sediment. Appl. Environ. Microbiol. 2005, 71, 5304–5308. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.V.; Waters, W.R.; Whipple, D.L. Investigation of the transmission of Mycobacterium bovis from deer to cattle through indirect contact. Am. J. Vet. Res. 2004, 65, 1483–1489. [Google Scholar] [CrossRef]
- Vicente, J.; Hofle, U.; Garrido, J.M.; Fernandez-de-Mera, I.G.; Acevedo, P.; Juste, R.; Barral, M.; Gortazar, C. Risk factors associated with the prevalence of tuberculosis-like lesions in fenced wild boar and red deer in south central Spain. Vet. Res. 2007, 38, 451–464. [Google Scholar] [CrossRef]
- Delafont, V.; Mougari, F.; Cambau, E.; Joyeux, M.; Bouchon, D.; Hechard, Y.; Moulin, L. First evidence of amoebae-mycobacteria association in drinking water network. Environ. Sci. Technol. 2014, 48, 11872–11882. [Google Scholar] [CrossRef]
- Rodriguez-Zaragoza, S. Ecology of free-living amoebae. Crit. Rev. Microbiol. 1994, 20, 225–241. [Google Scholar] [CrossRef]
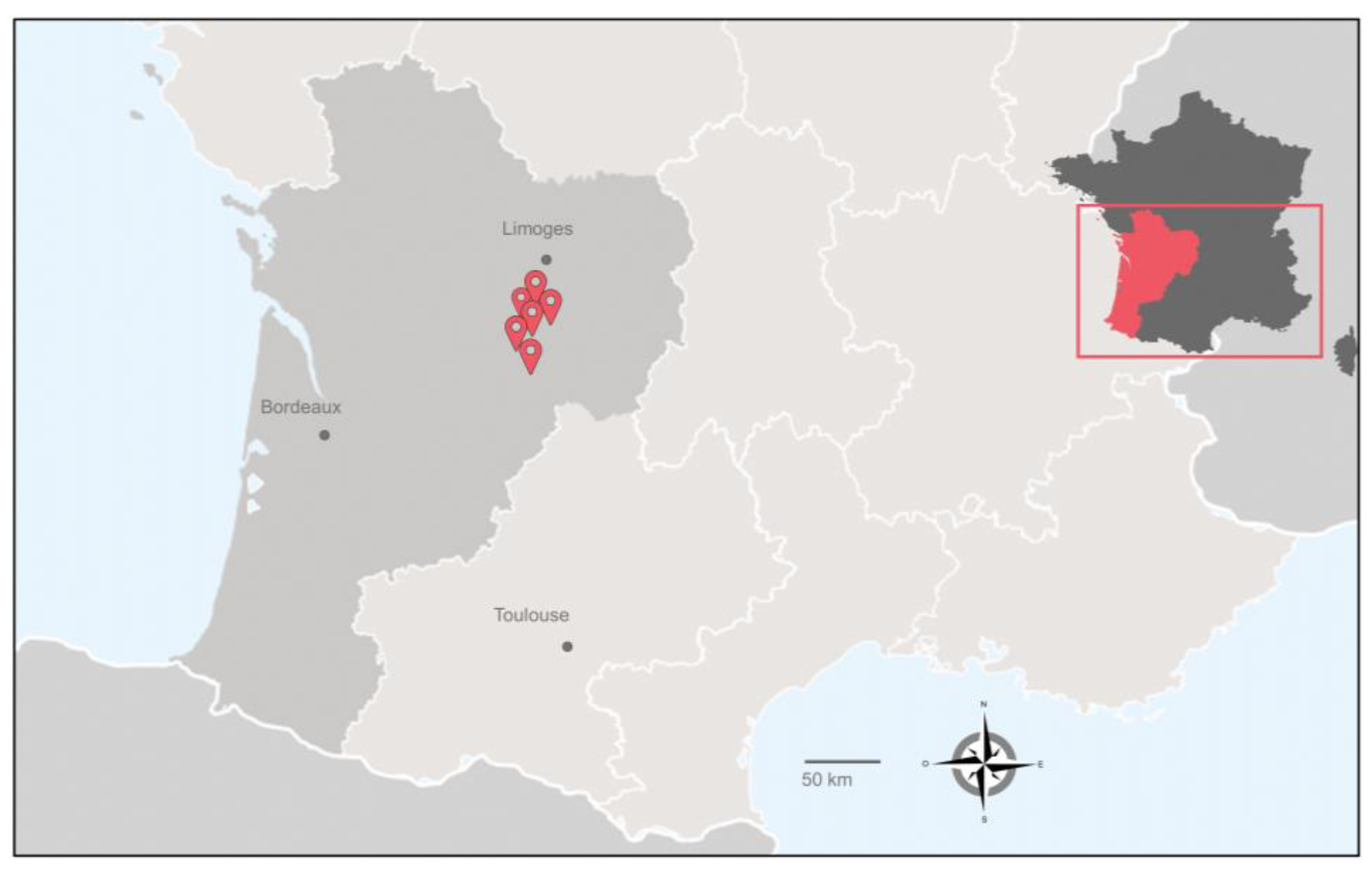



| Herd N° | Number of Cattle | bTB Status | JD Status * | Sample Number | Nature of Water Sampling |
|---|---|---|---|---|---|
| 1 | 68 | positive 6 confirmed cows | ND | 1 | Pond |
| 2 | Pond | ||||
| 3 | Pond | ||||
| 4 | Pond | ||||
| 2 | 82 | positive Two confirmed cows | ND | 5 | Pond |
| 6 | River | ||||
| 7 | Pond | ||||
| 8 | Pond | ||||
| 3 | 87 | positive 1 confirmed cow | negative | 9 | Outdoor drinking trough |
| 10 | Pond | ||||
| 4 | 19 | positive Five confirmed cows | ND | 11 | Outdoor drinking trough |
| 5 | 40 | positive 12 confirmed cows | positive 3.5% | 12 | Outdoor drinking trough |
| 13 | Outdoor drinking trough | ||||
| 14 | Outdoor drinking trough | ||||
| 15 | Outdoor drinking trough | ||||
| 16 | Outdoor drinking trough | ||||
| 17 | Outdoor drinking trough | ||||
| 6 | 235 | positive 6 confirmed cows | positive 0.85% | 18 | Outdoor drinking trough |
| 19 | Outdoor drinking trough | ||||
| 20 | Outdoor drinking trough |
| Herd N° | Sample N° | qPCR on Water Samples * | Culture ID |
|---|---|---|---|
| 1 * | 1 | Ct > 40 | Mycobacterium hiberniae |
| 2 | Ct > 40 | Mycobacterium nonchromogenicum | |
| 3 | Ct > 40 | Mycobacterium icosiumassiliensis | |
| 4 | Ct > 40 | Mycobacterium nonchromogenicum | |
| 2 | 5 | Ct > 40 | Mycobacterium hiberniae |
| 6 | Myco sp. | Mycobacterium avium ssp. paratuberculosis | |
| 7 | Ct > 40 | Mycobacterium arupensis | |
| 8 | Ct > 40 | Mycobacterium parascrofulaceum or genavense | |
| 3 | 9 | Ct > 40 | Contaminated |
| 10 | Ct > 40 | Contaminated | |
| 4 | 11 | Ct > 40 | Contaminated |
| 12 | Myco sp.; Map; MTBC | Mycobacterium hiberniae | |
| 13 | Myco sp.; Map; MTBC | Contaminated | |
| 14 | Myco sp.; Map; MTBC | Contaminated | |
| 5 | 15 | Ct > 40 | Contaminated |
| 16 | Myco sp.; Map; MTBC | Contaminated | |
| 17 | Myco sp.; Map; MTBC | Contaminated | |
| 18 | Ct > 40 | Mycobacterium arupensis | |
| 6 | 19 | Myco sp.; Map; MTBC | Contaminated |
| 20 | Myco sp.; Map; MTBC | Contaminated |
| Herd N° | Sample N° | Myco qPCR Signal * | T° | FLA Identification | Best Score ID | E Value |
|---|---|---|---|---|---|---|
| 1 | 1 | + | 20 | Naegleria | EU377592.1 | 0 |
| 37 | Filamoeba | GU320603.1 | 4 × 10−154 | |||
| 37 | Naegleria | KC164228.1 | 0 | |||
| 3 | + | 20 | Acanthamoeba | GU001160.1 | 0 | |
| 37 | Naegleria | KC164228.1 | 0 | |||
| 2 | 5 | + | 20 | Naegleria | KC164228.1 | 8 × 10−156 |
| 37 | Naegleria | KC164228.1 | 0 | |||
| 6 | + | 20 | Naegleria | KC164228.1 | 0 | |
| 37 | Naegleria | KC164228.1 | 0 | |||
| 7 | + | 37 | Vermamoeba | KX856374.1 | 0 | |
| 8 | + | 20 | Tubulinea group | FN562424.1 | 0 | |
| 37 | Naegleria | KC164228.1 | 6 × 10−132 | |||
| 3 | 9 | + | 37 | Acanthamoeba | AM408796.1 | 0 |
| 10 | + | 20 | Naegleria | OQ034614.1 | 0 | |
| 37 | Naegleria | AY266314.1 | 0 | |||
| 4 | 11 | + | 20 | Acanthamoeba | AY351644.1 | 0 |
| 37 | Naegleria | AY576367.1 | 0 | |||
| 5 | 12 | + | 37 | Vermamoeba | KX856373.1 | 0 |
| 14 | + | 37 | Naegleria | MG699123.1 | 0 | |
| 18 | + | 37 | Naegleria | KC164247.1 | 0 | |
| 6 | 19 | + | 37 | Vermamoeba | LC431240.1 | 0 |
| 20 | + | 20 | Acanthamoeba | HF930501.1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochard, V.; Cochard, T.; Crapart, S.; Delafont, V.; Moyen, J.-L.; Héchard, Y.; Biet, F. Presence of Non-Tuberculous Mycobacteria Including Mycobacterium avium subsp. paratuberculosis Associated with Environmental Amoebae. Animals 2023, 13, 1781. https://doi.org/10.3390/ani13111781
Rochard V, Cochard T, Crapart S, Delafont V, Moyen J-L, Héchard Y, Biet F. Presence of Non-Tuberculous Mycobacteria Including Mycobacterium avium subsp. paratuberculosis Associated with Environmental Amoebae. Animals. 2023; 13(11):1781. https://doi.org/10.3390/ani13111781
Chicago/Turabian StyleRochard, Vincent, Thierry Cochard, Stéphanie Crapart, Vincent Delafont, Jean-Louis Moyen, Yann Héchard, and Franck Biet. 2023. "Presence of Non-Tuberculous Mycobacteria Including Mycobacterium avium subsp. paratuberculosis Associated with Environmental Amoebae" Animals 13, no. 11: 1781. https://doi.org/10.3390/ani13111781
APA StyleRochard, V., Cochard, T., Crapart, S., Delafont, V., Moyen, J.-L., Héchard, Y., & Biet, F. (2023). Presence of Non-Tuberculous Mycobacteria Including Mycobacterium avium subsp. paratuberculosis Associated with Environmental Amoebae. Animals, 13(11), 1781. https://doi.org/10.3390/ani13111781






