Can an Enrichment Programme with Novel Manipulative and Scent Stimuli Change the Behaviour of Zoo-Housed European Wildcats? A Case Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Housing
2.2. Apparatuses and Experimental Conditions
- -
- Baseline from 11 to 15 September 2017—we did not provide any semiochemical or novel objects to the wildcats.
- -
- Rags from 18 to 22 September 2017—we provided the wildcats with plain cloth rags.
- -
- F3 rags from 25 to 29 September 2017—we kept cloth rags and sprayed them with F3.
- -
- Blocks from 2 to 6 October 2017—we provided the wildcats with plain white blocks.
2.3. Data Collection and Analysis
3. Results
3.1. Time Budget
3.2. Solitary Behaviours
3.3. Social Behaviours
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell-Palmer, R.; Rosell, F. The Importance of Chemical Communication Studies to Mammalian Conservation Biology: A Review. Biol. Cons. 2011, 144, 1919–1930. [Google Scholar] [CrossRef]
- Wells, D. Sensory Stimulation as Environmental Enrichment for Captive Animals: A review. Appl. Anim. Behav. Sci. 2009, 118, 1–11. [Google Scholar] [CrossRef]
- Hosey, G.R. How Does the Zoo Environment Affect the Behaviour of Captive Primates? Appl. Anim. Behav. Sci. 2005, 90, 107–129. [Google Scholar] [CrossRef]
- Christensen, J.W.; Rundgren, M. Predator Odour per se Does not Frighten Domestic Horses. Appl. Anim. Behav. Sci. 2008, 112, 136–145. [Google Scholar] [CrossRef]
- Price, L.J. A Preliminary Study of the Effects of Environmental Enrichment on the Behaviour of Captive African Wild Dogs (Lycaon pictus). Biosci. Horiz. 2010, 3, 132–140. [Google Scholar] [CrossRef]
- Schuett, E.B.; Frase, B.A. Making Scents: Using the Olfactory Senses for Lion Enrichment. Shape Enrich. 2001, 10, 1–3. [Google Scholar]
- Baker, B.; Taylor, S.; Montrose, V.T. The Effects of Olfactory Stimulation on the Behavior of Captive Ring-tailed Lemurs (Lemur catta). Zoo Biol. 2018, 37, 16–22. [Google Scholar] [CrossRef]
- Binks, J.; Taylor, S.; Wills, A.; Montrose, V.T. The Behavioural Effects of Olfactory Stimulation on Dogs at a Rescue Shelter. Appl. Anim. Behav. Sci. 2018, 202, 69–76. [Google Scholar] [CrossRef]
- Fay, C.; Miller, L.J. Utilizing Scents as Environmental Enrichment: Preference Assessment and Application with Rothschild Giraffe. Anim. Behav. Cogn. 2015, 2, 285–291. [Google Scholar] [CrossRef]
- Samuelson, M.M.; Lauderdale, L.K.; Pulis, K.; Solangi, M.; Hoffland, T.; Lyn, H. Olfactory Enrichment in California Sea Lions (Zalophus californianus): An Effective Tool for Captive Welfare? J. Appl. Anim. Welf. Sci. 2017, 20, 75–85. [Google Scholar] [CrossRef]
- Vaglio, S.; Kaburu, S.; Pearce, R.; Bryant, L.; McAuley, A.; Lott, A.; Sheppard, D.J.; Smith, S.; Tompkins, B.E.; Elwell, E.; et al. Effects of Scent Enrichment on Behavioural and Physiological Indicators of Stress in Zoo Primates. Am. J. Primatol. 2021, 83, e23247. [Google Scholar] [CrossRef]
- Quirke, T.; O’Riordan, R.M. The Effect of Randomised Enrichment Treatment Schedule on the Behvaiour of Cheetahs (Acinonyx jubatus). Appl. Anim. Behav. Sci. 2011, 135, 103–109. [Google Scholar] [CrossRef]
- Rafacz, M.L.; Santymire, R.M. Using Odor Cues to Elicit a Behavioral and Hormonal Response in Zoo-housed African Wild Dogs. Zoo Biol. 2014, 33, 144–149. [Google Scholar] [CrossRef]
- Heitman, K.; Rabquer, B.; Heitman, E.; Streu, C.; Anderson, P. The Use of Lavender Aromatherapy to Relieve Stress in Trailered Horses. J. Eq. Vet. Sci. 2018, 63, 8–12. [Google Scholar] [CrossRef]
- Blackie, N.; de Sousa, M. The Use of Garlic Oil for Olfactory Enrichment Increases the Use of Ropes in Weaned Pigs. Animals 2019, 9, 148. [Google Scholar] [CrossRef]
- Uccheddu, S.; Mariti, C.; Sannen, A.; Vervaecke, H.; Arnout, H.; Rufo, J.G.; Gazzano, A.; Haverbeke, A. Behavioral and Cortisol Responses of Shelter Dogs to a Cognitive Bias Test after Olfactory Enrichment with Essential Oils. Dog Behav. 2018, 2, 1–14. [Google Scholar]
- Bol, S.; Caspers, J.; Buckingham, L.; Anderson-Shelton, G.D.; Ridgwat, C.; Buffington, C.A.T.; Schulz, S.; Bunnik, E.M. Responsiveness of Cats (Felidae) to Silver Vine (Actinidia polygama), Tatarian Honeysuckle (Lonicera tatarica), Valerian (Valeriana officinalis) and Catnip (Nepeta cataria). BMC Vet. Res. 2017, 13, 70. [Google Scholar] [CrossRef]
- Wells, D.L.; Hepper, P.G.; Coleman, D.; Challis, M.G. A Note on the Effect of Olfactory Stimulation on the Behaviour and Welfare of Zoo-housed Gorillas. Appl. Anim. Behav. Sci. 2007, 106, 155–160. [Google Scholar] [CrossRef]
- Wells, D.L.; Egli, J.M. The Influence of Olfactory Enrichment on the Behaviour of Captive Black-footed Cats, Felis nigripes. Appl. Anim. Behav. Sci. 2004, 85, 107–119. [Google Scholar] [CrossRef]
- Myles, S.; Montrose, V.T. The Effects of Olfactory Stimulation on the Behaviour of Captive Meerkats (Suricata suricatta). J. Zoo Aq. Res. 2015, 3, 37–42. [Google Scholar]
- Clark, F.; King, A.J. A Critical Review of Zoo-based Olfactory Enrichment. Chem. Signals Vertebr. 2008, 11, 391–398. [Google Scholar]
- Gronqvist, G.; Kingston-Jones, M.; May, A.; Lehmann, J. The Effects of Three Types of Environmental Enrichment on the Behaviour of Captive Javan Gibbons (Hylobates moloch). Appl. Anim. Behav. Sci. 2013, 147, 214–223. [Google Scholar] [CrossRef]
- Elwell, E.J.; Vaglio, S. The Scent Enriched Primate. Animals 2023, 13, 1617. [Google Scholar] [CrossRef]
- Pageat, P.; Mengoli, M.; Cozzi, A. Using Feline Pheromones: From Pet Cats to Wild Felids. In Proceedings of the International Society of Chemical Ecology Annual Congress, Urbana Champaign, IL, USA, 8–12 July 2014. [Google Scholar]
- Pageat, P.; Gaultier, E. Current Research in Canine and Feline Pheromones. Vet. Clin. Small Anim. Pract. 2003, 33, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.R. Tools for Managing Feline Problem Behaviors: Pheromone Therapy. J. Fel. Med. Surg. 2018, 20, 1024–1032. [Google Scholar] [CrossRef]
- Berteselli, G.V.; Regaiolli, B.; Normando, S.; De Mori, B.; Zaborra, C.A.; Spiezio, C. European Wildcat and Domestic Cat: Do They Really Differ? J. Vet. Behav. 2017, 22, 35–40. [Google Scholar] [CrossRef]
- Macri, A.M.; Patterson-Kane, E. Behavioural Analysis of Solitary versus Socially Housed Snow Leopards (Panthera uncia), with the Provision of Simulated Social Contact. Appl. Anim. Behav. Sci. 2011, 130, 115–123. [Google Scholar] [CrossRef]
- Martínez-Macipe, M.; Lafont-Lecuelle, C.; Manteca, X.; Pageat, P.; Cozzi, A. Evaluation of an Innovative Approach for Sensory Enrichment in Zoos: Semiochemical Stimulation for Captive Lions (Panthera leo). Anim. Welf. 2015, 24, 455–461. [Google Scholar] [CrossRef]
- Gaultier, E.; Falewee, C.; Bougrat, L.; Pageat, P. The Introduction of a Female Tiger (Panthera tigris) in a Pre-established Group of two Neutered Males: A Case Study. In Current Issues and Research in Veterinary Behavioural Medicine; Purdue University Press: West Lafayette, IN, USA, 2005. [Google Scholar]
- Nace, A.; Swanson, W.F.; Graham, L.H. Assessing the Ability of the Synthetic Cat Pheromone ‘Feliway’ to Mitigate Post-operative Stress in Tigers (Panthera tigris). In Campbell Centre for the Study of Animal Welfare Research Symposium; University of Guelph: Guelph, ON, Canada, 2015. [Google Scholar]
- Costa, A.L.M.D.; Teixeira, R.H.F.; Ribeiro, V.L.; Kokubun, H.S.; Riva, H.G. Uso do Feromônio Facial Felino Fração F3 no Tratamento de Dermatite Psicogênica em Gatos-maracajá (Leopardus wiedii) Cativos-relato de Casos. Clin. Vet. 2016, 21, 60–64. [Google Scholar] [CrossRef]
- Bertoni, V.; Spiezio, C.; Regaiolli, B.; Cozzi, A.; Valsecchi, P.; Normando, S. Group Reunion in Zoo European Wildcats Using Cat Appeasing Pheromone (CAP) and Gradual Release of the Animals in the Exhibit—A Case Study. Animals 2022, 12, 1302. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behaviour: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Nemenyi, P. Distribution-Free Multiple Comparisons. Ph.D. Thesis, Princeton University, Princeton, NJ, USA, 1963. [Google Scholar]
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). R Package. 2014. Available online: http://CRAN.R-project.org/package=PMCMR (accessed on 27 April 2023).
- Podturkin, A.A. In search of the Optimal Enrichment Program for Zoo-housed Animals. Zoo Biol. 2021, 40, 527–540. [Google Scholar] [CrossRef]
- Dawkins, M.S. Using Behaviour to Assess Animal Welfare. Anim. Welf. 2004, 13, S3–S7. [Google Scholar] [CrossRef]
- Szokalski, M.S.; Litchfield, C.A.; Foster, W.K. Enrichment for Captive Tigers (Panthera tigris): Current Knowledge and Future Directions. Appl. Anim. Behav. Sci. 2012, 139, 1–9. [Google Scholar] [CrossRef]
- Biolatti, C.; Modesto, P.; Dezzutto, D.; Pera, F.; Tarantola, M.; Gennero, M.S.; Maurella, C.; Acutis, P.L. A Behavioural Analysis of Captive Tigers (Panthera tigris): A Water Pool Makes the Difference. Appl. Anim. Behav. Sci. 2016, 174, 173–180. [Google Scholar] [CrossRef]
- Spiezio, C.; Valsecchi, V.; Sandri, C.; Regaiolli, B. Investigating Individual and Social Behaviour of the Northern Bald Ibis (Geronticus eremita): Behavioural Variety and Welfare. PeerJ 2018, 6, e5436. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.J.; Martin, A.L. Applied Behavior Analysis and the Zoo: Forthman and Ogden (1992) Thirty Years Later. J. Appl. Behav. Anal. 2023, 56, 29–54. [Google Scholar] [CrossRef]
- Migli, D.; Astaras, C.; Boutsis, G.; Diakou, A.; Karantanis, N.E.; Youlatos, D. Spatial Ecology and Diel Activity of European Wildcat (Felis silvestris) in a Protected Lowland Area in Northern Greece. Animals 2021, 11, 3030. [Google Scholar] [CrossRef]
- Karanth, K.U.; Sunquist, M.E. Behavioural Correlates of Predation by Tiger (Panthera tigris), Leopard (Panthera pardus) and Dhole (Cuon alpinus) in Nagarahole, India. J. Zool. 2000, 250, 255–265. [Google Scholar] [CrossRef]
- Bashaw, M.J.; Bloomsmith, M.A.; Marr, M.; Maple, T.L. To Hunt or not to Hunt? A Feeding Enrichment Experiment with Captive Large Felids. Zoo Biol. 2003, 22, 189–198. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of Positive Emotions in Animals to Improve their Welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Green, T.; Mellor, D. Extending Ideas about Animal Welfare Assessment to Include ‘Quality of Life’ and Related Concepts. N. Z. Vet. J. 2011, 59, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Mellor, D.J.; Cronin, G.M.; Tilbrook, A.J. Scientific Assessment of Animal Welfare. N. Z. Vet. J. 2015, 63, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Regaiolli, B.; Rizzo, A.; Ottolini, G.; Miletto Petrazzini, M.E.; Spiezio, C.; Agrillo, C. Motion Illusions as Environmental Enrichment for Zoo Animals: A Preliminary Investigation in Lions (Panthera leo). Front. Psychol. 2019, 10, 2220. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.J.; Sherwen, S.L.; Rault, J.L. Number of Nearby Visitors and Noise Level Affect Vigilance in Captive Koalas. Appl. Anim. Behav. Sci. 2014, 154, 76–82. [Google Scholar] [CrossRef]
- Quadros, S.; Goulart, V.D.L.; Passos, L.; Vecci, M.A.M.; Young, R.J. Zoo Visitor Effect on Mammal Behaviour: Does Noise Matter? Appl. Anim. Behav. Sci. 2014, 156, 78–84. [Google Scholar] [CrossRef]
- Bernachon, N.; Beata, C.; Crastes, N.; Monginoux, P.; Gatto, H.; McGahie, D. Response to Acute Stress in Domestic Cats using Synthetic Analogues of Natural Appeasing Pheromones with Nepeta Cataria Extract Rich in Nepetalactone: A Double-blinded, Randomized, Positive Controlled Cross-over Study. Int. J. Appl. Res. Vet. Med. 2015, 13, 2. [Google Scholar]
- Zhang, L.; Bian, Z.; Liu, Q.; Deng, B. Dealing with Stress in Cats: What is New about the Olfactory Strategy? Front. Vet. Sci. 2022, 9, 928943. [Google Scholar] [CrossRef]

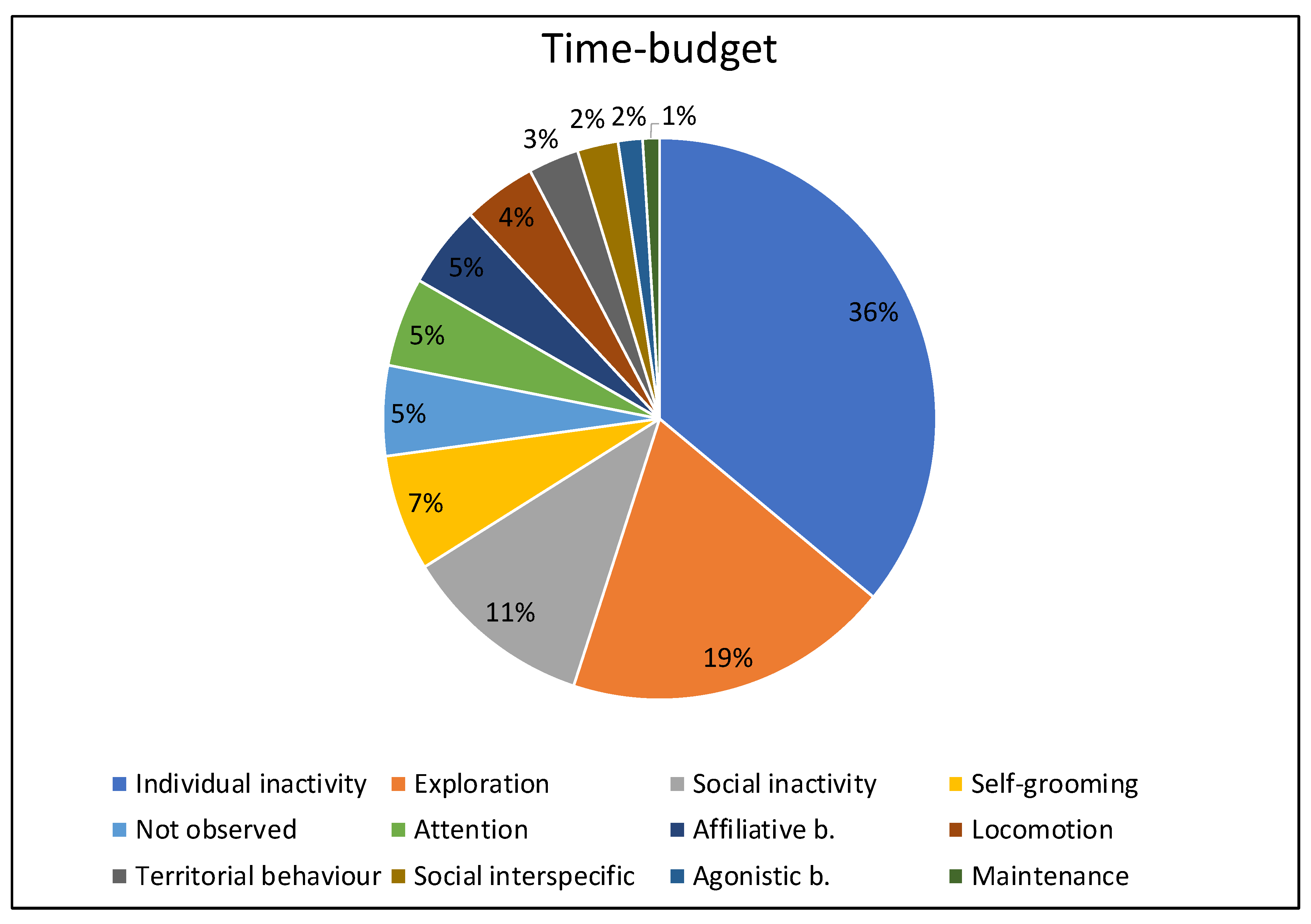
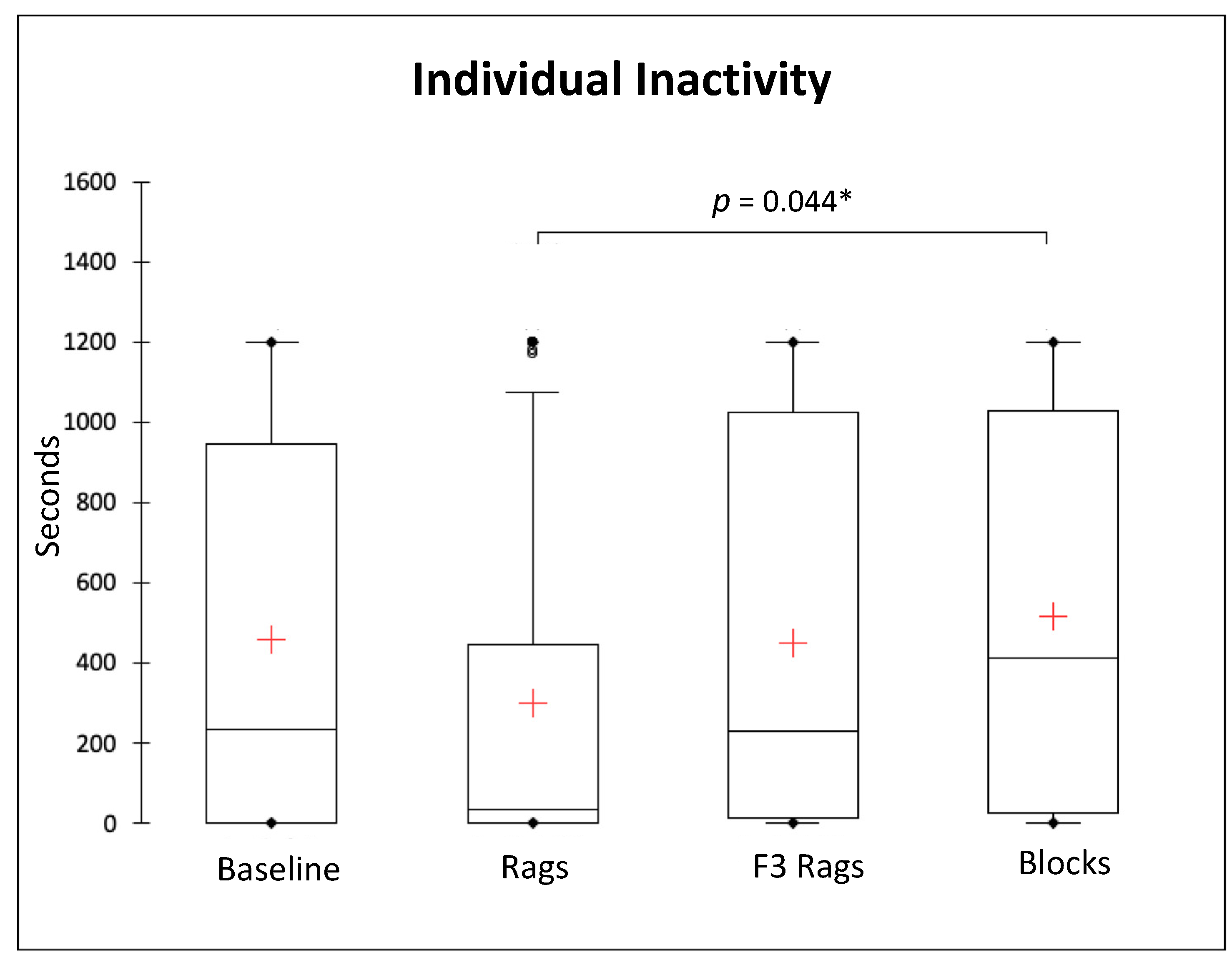
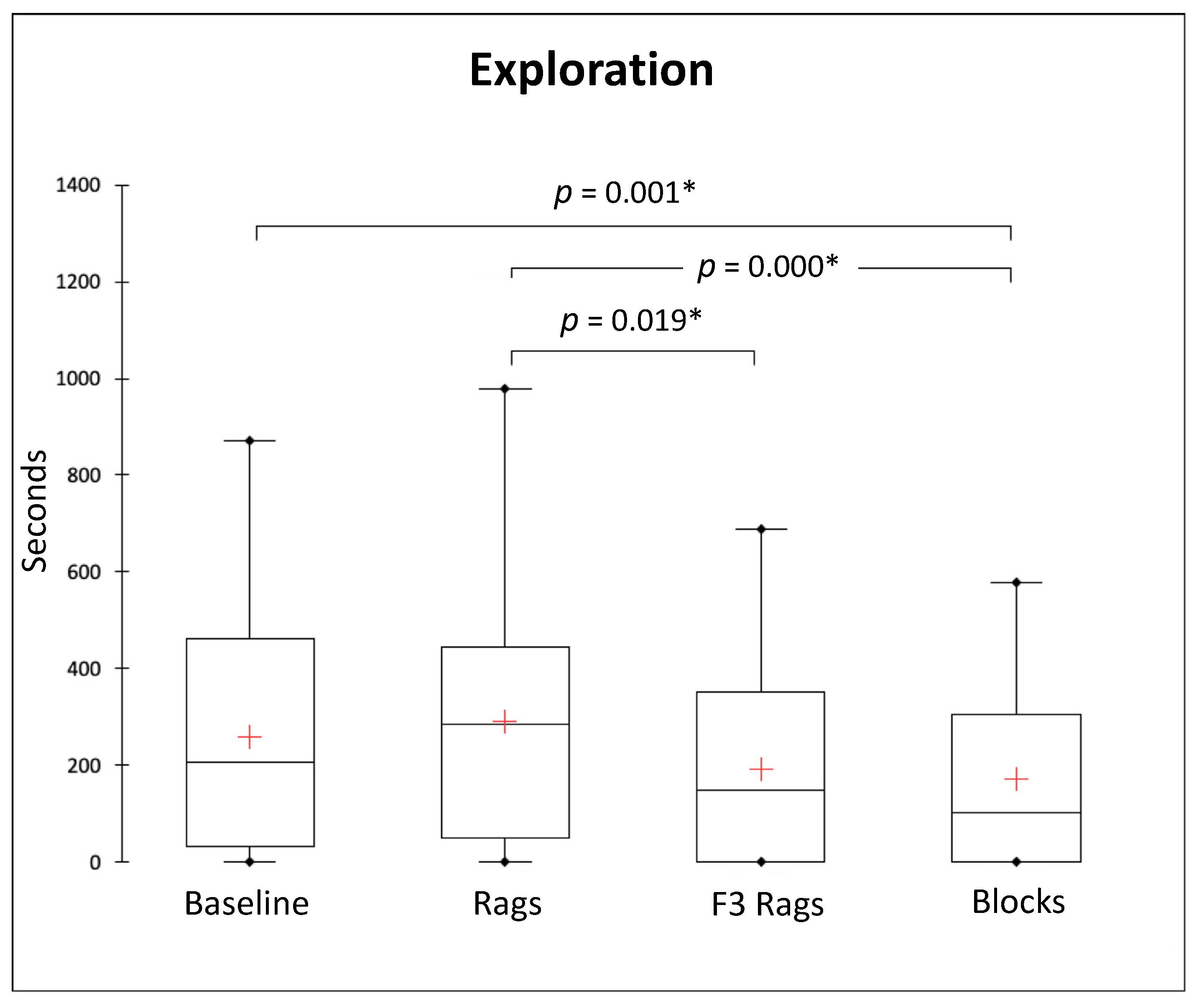
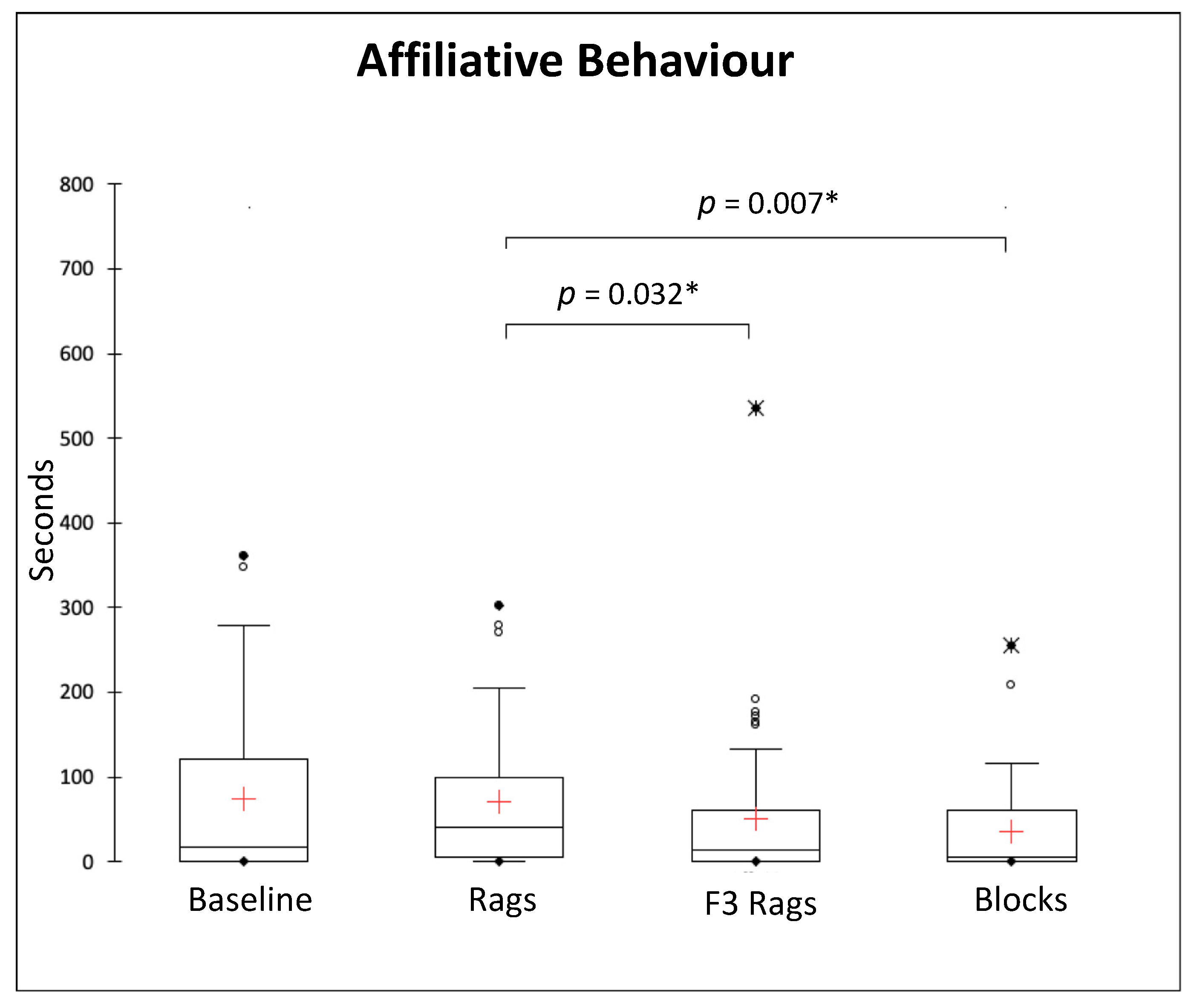
| Behavioural Categories | Definition |
|---|---|
| Individual behaviours | |
| Attention | A wildcat is alert and stares at a specific point with straight ears or with ears backwards. |
| Maintenance | A wildcat defecates and then covers faeces, urinates, eats, and drinks. Include stretching, body shake, individual play, manipulation of plants and other objects, and yawning after waking up. |
| Exploration | A wildcat visually or olfactorily explores the environment (sniffing the ground or any object). |
| Self-grooming | A wildcat cleans its fur by licking, scratching, biting, or chewing, or by licking a paw and swiping it on the head with the apparent intent to clean the head. |
| Locomotion | A wildcat walks, runs, or jumps inside the enclosure. |
| Territorial behaviours | A wildcat marks the environment by urine spray with a vertical tail and a horizontal urine jet, clawing, rubbing the head against an object, defecating without covering the faces, and patrolling. |
| Social behaviours | |
| Affiliative | A wildcat observes, sniffs, or licks another subject or rubs the head and nose against the body of another wildcat. |
| Agonistic | A wildcat stares at another subject, with ears forward on the head. It can move the tail with fast movements or can have ears flat. Includes agonistic displays such as yawning toward conspecifics, piloerection, raising a paw, and baring teeth. |
| Social interspecific | A wildcat observes zookeepers, visitors, or animals belonging to species other than its own. |
| Inactivity | |
| Individual inactivity | A wildcat sleeps or rests alone. |
| Social inactivity | A wildcat sleeps or rests in contact with another subject. |
| Not observed | A wildcat is hiding or is not distinctly visible. |
| Baseline | Rags | F3 Rags | Blocks | |
|---|---|---|---|---|
| Individual behaviours | ||||
| Attention | 29.5 (91.5) s | 26.5 (53.5) s | 42 (127.5) s | 35.5 (149) s |
| Exploration | 205.5 (429.8) s | 285 (393) s | 149.5 (352.25) s | 102.5 (305.25) s |
| Inactivity | 235 (945.5) s | 32 (444.25) s | 231 (1010.5) s | 411.5 (1006.25) s |
| Locomotion | 5.5 (83.25) s | 12 (111.75) s | 3.5 (77.75) s | 0 (68.75) s |
| Maintenance | 7.5 (13.75) s | 5 (18) s | 3.5 (14) s | 3 (11.25) s |
| Not observed | 0 (59.75) s | 0 (33) s | 0 (69.25) s | 7 (54.5) s |
| Territorial behaviours | 0 (5) s | 0 (10.5) s | 0 (10) s | 0 (4) s |
| Self-grooming | 6.5 (55.25) s | 19.5 (190.5) s | 0 (81.75) s | 0 (38.75) s |
| Social behaviours | ||||
| Agonistic behaviours | 1 (5.5) s | 3 (34.75) s | 0 (3.5) s | 0 (2) s |
| Affiliative behaviours | 17 (120.3) s | 40 (94) s | 14 (60.75) s | 5 (61) s |
| Social inactivity | 0 (0) s | 0 (0) s | 0 (0) s | 0 (0) s |
| Interspecific | 29 (52) s | 13 (34.75) s | 8 (39) s | 17 (51.25) s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoni, V.; Regaiolli, B.; Cozzi, A.; Vaglio, S.; Spiezio, C. Can an Enrichment Programme with Novel Manipulative and Scent Stimuli Change the Behaviour of Zoo-Housed European Wildcats? A Case Study. Animals 2023, 13, 1762. https://doi.org/10.3390/ani13111762
Bertoni V, Regaiolli B, Cozzi A, Vaglio S, Spiezio C. Can an Enrichment Programme with Novel Manipulative and Scent Stimuli Change the Behaviour of Zoo-Housed European Wildcats? A Case Study. Animals. 2023; 13(11):1762. https://doi.org/10.3390/ani13111762
Chicago/Turabian StyleBertoni, Valentina, Barbara Regaiolli, Alessandro Cozzi, Stefano Vaglio, and Caterina Spiezio. 2023. "Can an Enrichment Programme with Novel Manipulative and Scent Stimuli Change the Behaviour of Zoo-Housed European Wildcats? A Case Study" Animals 13, no. 11: 1762. https://doi.org/10.3390/ani13111762
APA StyleBertoni, V., Regaiolli, B., Cozzi, A., Vaglio, S., & Spiezio, C. (2023). Can an Enrichment Programme with Novel Manipulative and Scent Stimuli Change the Behaviour of Zoo-Housed European Wildcats? A Case Study. Animals, 13(11), 1762. https://doi.org/10.3390/ani13111762






