Social Network Changes in Cotton-Top Tamarins (Saguinus oedipus) after the Birth of New Infants
(This article belongs to the Section Human-Animal Interactions, Animal Behaviour and Emotion)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Installations
2.2. Data Collection
2.3. Data Analysis
3. Results
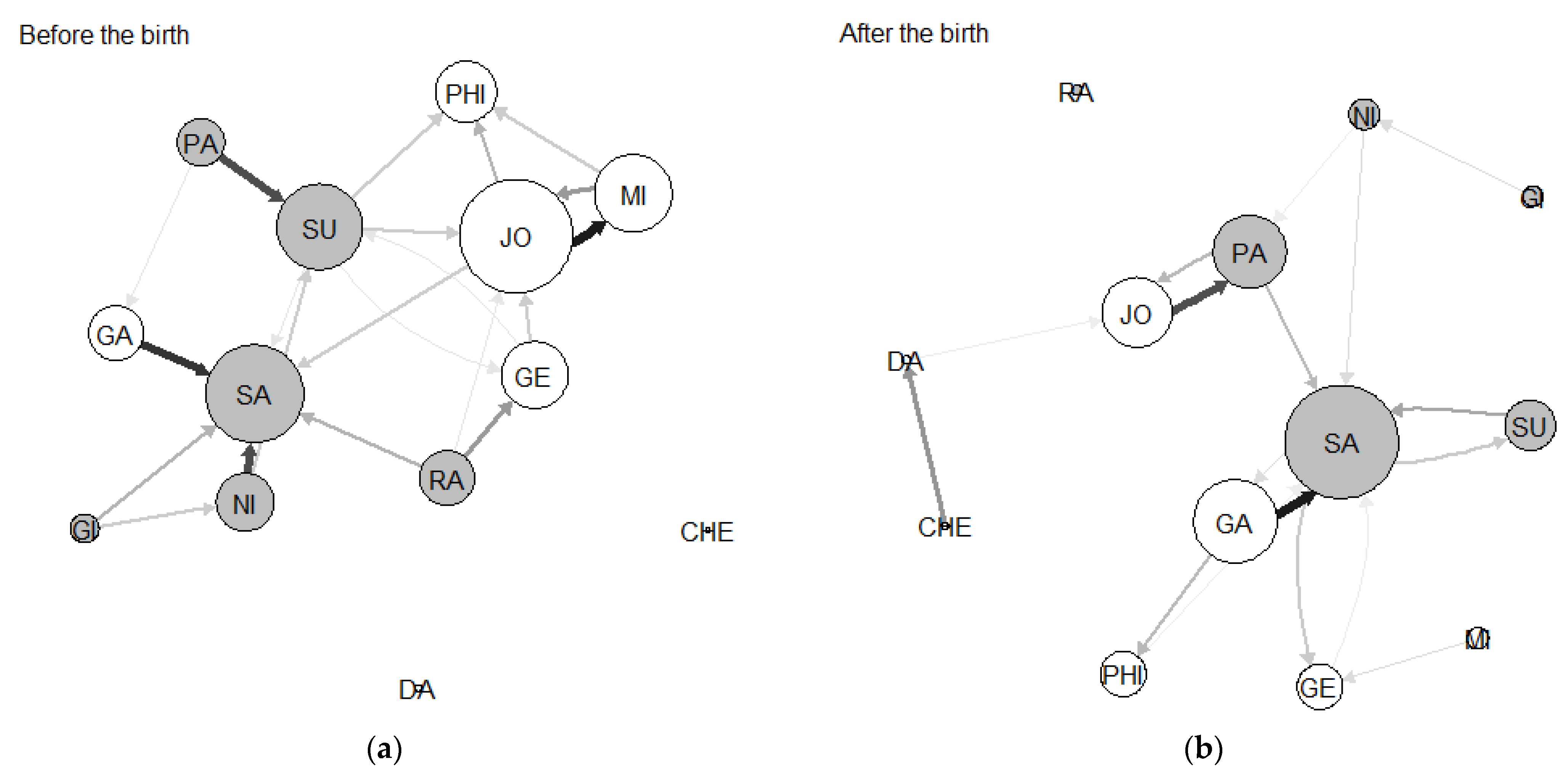
3.1. Network Sociograms
3.2. Density
3.3. MRQAP Regression
3.4. Permutation-Based Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, C. Park or Ride? Evolution of Infant Carrying in Primates. Int. J. Primatol. 2001, 22, 749–771. [Google Scholar] [CrossRef]
- Achenbach, G.G.; Snowdon, C.T. Costs of Caregiving: Weight Loss in Captive Adult Male Cotton-Top Tamarins (Saguinus oedipus) Following the Birth of Infants. Int. J. Primatol. 2002, 23, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, S.; Peláez, F.; Gil-Bürmann, C.; Kaumanns, W. Costs of Infant-Carrying in the Cotton-Top Tamarin (Saguinus oedipus). Am. J. Primatol. 1999, 48, 99–111. [Google Scholar] [CrossRef]
- Sánchez, S.; Peláez, F.; Morcillo, A.; Gil-Bürmann, C. Effect of the Enclosure on Carriers’ Body Weight Loss in the Cotton-Top Tamarin (Saguinus oedipus). Am. J. Primatol. 2005, 66, 279–284. [Google Scholar] [CrossRef]
- Bales, K.; French, J.A.; Dietz, J.M. Explaining Variation in Maternal Care in a Cooperatively Breeding Mammal. Anim. Behav. 2002, 63, 453–461. [Google Scholar] [CrossRef]
- Price, E.C. The Benefits of Helpers: Effects of Group and Litter Size on Infant Care in Tamarins (Saguinus oedipus). Am. J. Primatol. 1992, 26, 179–190. [Google Scholar] [CrossRef]
- Snowdon, C.T.; Ziegler, T.E. Growing Up Cooperatively: Family Processes and Infant Care in Marmosets. J. Dev. Process. 2007, 2, 40–66. [Google Scholar]
- Zahed, S.R.; Kurian, A.V.; Snowdon, C.T. Social Dynamics and Individual Plasticity of Infant Care Behavior in Cooperatively Breeding Cotton-Top Tamarins. Am. J. Primatol. 2010, 72, 296–306. [Google Scholar] [CrossRef]
- Yamamoto, M.E.; Araujo, A.; Arruda, M.D.F.; Lima, A.K.M.; Siqueira, J.D.O.; Hattori, W.T. Male and Female Breeding Strategies in a Cooperative Primate. Behav. Process. 2014, 109, 27–33. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Everett, M.G.; Johnson, J.C. Analyzing Social Networks; SAGE: London, UK, 2018. [Google Scholar]
- Puga-Gonzalez, I.; Sosa, S.; Sueur, C. Editorial: Social Networks Analyses in Primates, a Multilevel Perspective. Primates 2019, 60, 163–165. [Google Scholar] [CrossRef]
- Rose, P.E.; Croft, D.P. The Potential of Social Network Analysis as a Tool for the Management of Zoo Animals. Anim. Welf. 2015, 24, 123–138. [Google Scholar] [CrossRef]
- Croft, D.P.; Madden, J.R.; Franks, D.W.; James, R. Hypothesis Testing in Animal Social Networks. Trends Ecol. Evol. 2011, 26, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Brent, L.J.N.; Lehmann, J.; Ramos-Fernández, G. Social Network Analysis in the Study of Nonhuman Primates: A Historical Perspective. Am. J. Primatol. 2011, 73, 720–730. [Google Scholar] [CrossRef]
- Croft, D.P.; Krause, J.; Darden, S.K.; Ramnarine, I.W.; Faria, J.J.; James, R. Behavioural Trait Assortment in a Social Network: Patterns and Implications. Behav. Ecol. Sociobiol. 2009, 63, 1495–1503. [Google Scholar] [CrossRef]
- James, R.; Croft, D.P.; Krause, J. Potential Banana Skins in Animal Social Network Analysis. Behav. Ecol. Sociobiol. 2009, 63, 989–997. [Google Scholar] [CrossRef]
- Krause, J.; Croft, D.P.; James, R. Social Network Theory in the Behavioural Sciences: Potential Applications. Behav. Ecol. Sociobiol. 2007, 62, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Hanser, S.F.; McHugh, K.A. Social Network Theory: New Insights and Issues for Behavioral Ecologists. Behav. Ecol. Sociobiol. 2009, 63, 975–988. [Google Scholar] [CrossRef]
- Silk, J.; Cheney, D.; Seyfarth, R. A Practical Guide to the Study of Social Relationships. Evol. Anthropol. Issues News Rev. 2013, 22, 213–225. [Google Scholar] [CrossRef]
- Wey, T.; Blumstein, D.T.; Shen, W.; Jordán, F. Social Network Analysis of Animal Behaviour: A Promising Tool for the Study of Sociality. Anim. Behav. 2008, 75, 333–344. [Google Scholar] [CrossRef]
- Kleinhappel, T.K.; John, E.A.; Pike, T.W.; Wilkinson, A.; Burman, O.H.P. Animal Welfare: A Social Networks Perspective. Sci. Prog. 2016, 99, 68–82. [Google Scholar] [CrossRef]
- Koene, P.; Ipema, B. Social Networks and Welfare in Future Animal Management. Animals 2014, 4, 93–118. [Google Scholar] [CrossRef] [PubMed]
- McCowan, B.; Anderson, K.; Heagarty, A.; Cameron, A. Utility of Social Network Analysis for Primate Behavioral Management and Well-Being. Appl. Anim. Behav. Sci. 2008, 109, 396–405. [Google Scholar] [CrossRef]
- Sueur, C.; Pelé, M. Social Network and Decision-Making in Primates: A Report on Franco-Japanese Research Collaborations. Primates 2016, 57, 327–332. [Google Scholar] [CrossRef]
- Aureli, F.; Schino, G. Brief Touch Is Different from a Massage: Insights from Nonhuman Primates. Curr. Opin. Behav. Sci. 2022, 43, 174–180. [Google Scholar] [CrossRef]
- Di Bitetti, M.S. Evidence for an Important Social Role of Allogrooming in a Platyrrhine Primate. Anim. Behav. 1997, 54, 199–211. [Google Scholar] [CrossRef]
- Lehmann, J.; Korstjens, A.H.; Dunbar, R.I.M. Group Size, Grooming and Social Cohesion in Primates. Anim. Behav. 2007, 74, 1617–1629. [Google Scholar] [CrossRef]
- Kudo, H.; Dunbar, R.I.M. Neocortex Size and Social Network Size in Primates. Anim. Behav. 2001, 62, 711–722. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. The Social Role of Touch in Humans and Primates: Behavioural Function and Neurobiological Mechanisms. Neurosci. Biobehav. Rev. 2010, 34, 260–268. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. Functional Significance of Social Grooming in Primates. Folia Primatol. 1991, 57, 121–131. [Google Scholar] [CrossRef]
- Kanngiesser, P.; Sueur, C.; Riedl, K.; Grossmann, J.; Call, J. Grooming Network Cohesion and the Role of Individuals in a Captive Chimpanzee Group. Am. J. Primatol. 2011, 73, 758–767. [Google Scholar] [CrossRef]
- Dufour, V.; Sueur, C.; Whiten, A.; Buchanan-Smith, H.M. The Impact of Moving to a Novel Environment on Social Networks, Activity and Wellbeing in Two New World Primates. Am. J. Primatol. 2011, 73, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Sueur, C.; Jacobs, A.; Amblard, F.; Petit, O.; King, A.J. How Can Social Network Analysis Improve the Study of Primate Behavior? Am. J. Primatol. 2011, 73, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.F.; Aureli, F. Social Network Changes during Space Restriction in Zoo Chimpanzees. Primates 2019, 60, 203–211. [Google Scholar] [CrossRef]
- Sosa, S.; Sueur, C.; Puga-Gonzalez, I. Network Measures in Animal Social Network Analysis: Their Strengths, Limits, Interpretations and Uses. Methods Ecol. Evol. 2021, 12, 10–21. [Google Scholar] [CrossRef]
- Finkenwirth, C.; Burkart, J.M. Why Help? Relationship Quality, Not Strategic Grooming Predicts Infant-Care in Group-Living Marmosets. Physiol. Behav. 2018, 193, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, M.B. Consistency in Social Network Position over Changing Environments in a Seasonally Breeding Primate. Behav. Ecol. Sociobiol. 2018, 72, 11. [Google Scholar] [CrossRef]
- Cowl, V.B.; Jensen, K.; Lea, J.M.D.; Walker, S.L.; Shultz, S. Sulawesi Crested Macaque (Macaca nigra) Grooming Networks Are Robust to Perturbation While Individual Associations Are More Labile. Int. J. Primatol. 2020, 41, 105–128. [Google Scholar] [CrossRef]
- Koyama, N.F.; Ronkainen, K.; Aureli, F. Durability and Flexibility of Chimpanzee Grooming Patterns during a Period of Dominance Instability. Am. J. Primatol. 2017, 79, e22713. [Google Scholar] [CrossRef]
- Xia, D.-P.; Kyes, R.C.; Wang, X.; Sun, B.-H.; Sun, L.; Li, J.-H. Grooming Networks Reveal Intra- and Intersexual Social Relationships in Macaca Thibetana. Primates 2019, 60, 223–232. [Google Scholar] [CrossRef]
- Brent, L.J.N.; MacLarnon, A.; Platt, M.L.; Semple, S. Seasonal Changes in the Structure of Rhesus Macaque Social Networks. Behav. Ecol. Sociobiol. 2013, 67, 349–359. [Google Scholar] [CrossRef]
- Mann, J.; Stanton, M.A.; Patterson, E.M.; Bienenstock, E.J.; Singh, L.O. Social Networks Reveal Cultural Behaviour in Tool-Using Dolphins. Nat. Commun. 2012, 3, 980. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Duque, E.; Di Fiore, A.; Huck, M. The Behaviour, Evology, and Social Evolution of New World Monkeys. In The Evolution of Primate Societies; Mitani, J.C., Call, J., Kappeler, P.M., Palombit, R.A., Silk, J.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2012; pp. 43–64. [Google Scholar]
- Goldizen, A.W. Social Relationships in a Cooperatively Polyandrous Group of Tamarins (Saguinus fuscicollis). Behav. Ecol. Sociobiol. 1989, 24, 79–89. [Google Scholar] [CrossRef]
- Heymann, E.W. Social Behavior of Wild Moustached Tamarins, Saguinus mystax, at the Estación Biológica Quebrada Blanco, Peruvian Amazonia. Am. J. Primatol. 1996, 38, 101–113. [Google Scholar] [CrossRef]
- Löttker, P.; Huck, M.; Zinner, D.P.; Heymann, E.W. Grooming Relationships between Breeding Females and Adult Group Members in Cooperatively Breeding Moustached Tamarins (Saguinus mystax). Am. J. Primatol. 2007, 69, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Lazaro-Perea, C.; Arruda, M.d.F.; Snowdon, C.T. Grooming as a Reward? Social Function of Grooming between Females in Cooperatively Breeding Marmosets. Anim. Behav. 2004, 67, 627–636. [Google Scholar] [CrossRef]
- Porter, L.; Garber, P. Social Behavior of Callimicos: Mating Strategies and Infant Care. In The Smallest Anthopoids; Springer: New York, NY, USA, 2009; pp. 87–101. [Google Scholar] [CrossRef]
- Yamamoto, M.E.; Arruda, M.d.F.; Alencar, A.I.; de Sousa, M.B.C.; Araújo, A. Mating Systems and Female–Female Competition in the Common Marmoset, Callithrix Jacchus. In The Smallest Anthropoids: The Marmoset/Callimico Radiation; Ford, S.M., Porter, L.M., Davis, L.C., Eds.; Developments in Primatology: Progress and Prospects; Springer: Boston, MA, USA, 2009; pp. 119–133. [Google Scholar] [CrossRef]
- Lunn, S.F.; McNeilly, A.S. Failure of Lactation to Have a Consistent Effect on Interbirth Interval in the Common Marmoset, Callithrix Jacchus Jacchus. Folia Primatol. 1982, 37, 99–105. [Google Scholar] [CrossRef]
- Ziegler, T.E.; Savage, A.; Scheffler, G.; Snowdon, C.T. The Endocrinology of Puberty and Reproductive Functioning in Female Cotton-Top Tamarins (Saguinus oedipus) under Varying Social Conditions1. Biol. Reprod. 1987, 37, 618–627. [Google Scholar] [CrossRef]
- Erb, W.M.; Porter, L.M. Mother’s Little Helpers: What We Know (and Don’t Know) about Cooperative Infant Care in Callitrichines. Evol. Anthropol. Issues News Rev. 2017, 26, 25–37. [Google Scholar] [CrossRef]
- Erb, W.M.; Porter, L.M. Variable Infant Care Contributions in Cooperatively Breeding Groups of Wild Saddleback Tamarins. Am. J. Primatol. 2020, 82, e23190. [Google Scholar] [CrossRef]
- Finkenwirth, C.; van Schaik, C.; Ziegler, T.E.; Burkart, J.M. Strongly Bonded Family Members in Common Marmosets Show Synchronized Fluctuations in Oxytocin. Physiol. Behav. 2015, 151, 246–251. [Google Scholar] [CrossRef]
- Finkenwirth, C.; Burkart, J.M. Long-Term-Stability of Relationship Structure in Family Groups of Common Marmosets, and Its Link to Proactive Prosociality. Physiol. Behav. 2017, 173, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ginther, A.J.; Snowdon, C.T. Expectant Parents Groom Adult Sons According to Previous Alloparenting in a Biparental Cooperatively Breeding Primate. Anim. Behav. 2009, 78, 287–297. [Google Scholar] [CrossRef]
- Díaz-Muñoz, S.L. Complex Cooperative Breeders: Using Infant Care Costs to Explain Variability in Callitrichine Social and Reproductive Behavior. Am. J. Primatol. 2016, 78, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Snowdon, C.T. Social Development during the First Twenty Weeks in the Cotton-Top Tamarin (Saguinus oedipus). Anim. Behav. 1984, 32, 432–444. [Google Scholar] [CrossRef]
- Price, E.; Mcgrew, W. Departures from Monogamy in Colonies of Captive Cotton-Top Tamarins. Folia Primatol. 1991, 57, 16–27. [Google Scholar] [CrossRef]
- Savage, A.; Giraldo, L.H.; Soto, L.H.; Snowdon, C.T. Demography, Group Composition, and Dispersal in Wild Cotton-Top Tamarin (Saguinus oedipus) Groups. Am. J. Primatol. 1996, 38, 85–100. [Google Scholar] [CrossRef]
- Sánchez, S.; Peláez, F.; Fidalgo, A.; Morcillo, A.; Caperos, J.M. Changes in Body Mass of Expectant Male Cotton-Top Tamarins (Saguinus oedipus). Folia Primatol. 2008, 79, 458–462. [Google Scholar] [CrossRef]
- Sánchez, S.; Peláez, F.; Fidalgo, A.; Morcillo, A.; Caperos, J.M. Body weight increase in expectant males and helpers of cotton-top tamarin (Saguinus oedipus): A sympton of the Couvade syndrome? Psicothema 2008, 20, 825–829. [Google Scholar]
- Ziegler, T.E.; Prudom, S.L.; Schultz-Darken, N.J.; Kurian, A.V.; Snowdon, C.T. Pregnancy Weight Gain: Marmoset and Tamarin Dads Show It Too. Biol. Lett. 2006, 2, 181–183. [Google Scholar] [CrossRef]
- Price, E.C. Competition To Carry Infants in Captive Families of Cotton-Top Tamarins (Saguinus oedipus). Behaviour 1991, 118, 66–88. [Google Scholar] [CrossRef]
- Sánchez, S.; Peláez, F.; Gil-Bürmann, C. Why do cotton-top tamarin female helpers carry infants? A preliminary study. Am. J. Primatol. 2002, 57, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Washabaugh, K.F.; Snowdon, C.T.; Ziegler, T.E. Variations in Care for Cottontop Tamarin, Saguinus oedipus, Infants as a Function of Parental Experience and Group Size. Anim. Behav. 2002, 63, 1163–1174. [Google Scholar] [CrossRef]
- Snowdon, C.T.; Cronin, K.A. Cooperative Breeders Do Cooperate. Behav. Process. 2007, 76, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Bardi, M.; Petto, A.J.; Lee-Parritz, D.E. Parental Failure in Captive Cotton-Top Tamarins (Saguinus oedipus). Am. J. Primatol. 2001, 54, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Le Vin, A.L.; Mable, B.K.; Taborsky, M.; Heg, D.; Arnold, K.E. Individual Variation in Helping in a Cooperative Breeder: Relatedness versus Behavioural Type. Anim. Behav. 2011, 82, 467–477. [Google Scholar] [CrossRef]
- Price, E.C. Infant Carrying as a Courtship Strategy of Breeding Male Cotton-Top Tamarins. Anim. Behav. 1990, 40, 784–786. [Google Scholar] [CrossRef]
- Price, E.C. The Costs of Infant Carrying in Captive Cotton-Top Tamarins. Am. J. Primatol. 1992, 26, 23–33. [Google Scholar] [CrossRef]
- Price, E. Contributions to Infant Care in Captive Cotton-Top Tamarins (Saguinus oedipus): The Influence of Age, Sex, and Reproductive Status. Int. J. Primatol. 1992, 13, 125–141. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Farine, D.R.; Whitehead, H. Constructing, Conducting and Interpreting Animal Social Network Analysis. J. Anim. Ecol. 2015, 84, 1144–1163. [Google Scholar] [CrossRef]
- Whitehead, H.; James, R. Generalized Affiliation Indices Extract Affiliations from Social Network Data. Methods Ecol. Evol. 2015, 6, 836–844. [Google Scholar] [CrossRef]
- Dekker, D.; Krackhardt, D.; Snijders, T.A.B. Sensitivity of MRQAP Tests to Collinearity and Autocorrelation Conditions. Psychometrika 2007, 72, 563–581. [Google Scholar] [CrossRef] [PubMed]
- González, N.T.; Machanda, Z.; Otali, E.; Muller, M.N.; Enigk, D.K.; Wrangham, R.; Thompson, M.E. Age-Related Change in Adult Chimpanzee Social Network Integration. Evol. Med. Public Health 2021, 9, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Rathke, E.-M.; Fischer, J. Social Aging in Male and Female Barbary Macaques. Am. J. Primatol. 2021, 83, e23272. [Google Scholar] [CrossRef] [PubMed]

| Name | Age Category | Sex |
|---|---|---|
| SA * | Adult | Female |
| GA * | Adult | Male |
| GE | Adult | Male |
| PA | Adult | Female |
| PHI | Adult | Male |
| GI | Adult | Female |
| JO | Adult | Male |
| NI | Adult | Female |
| MI | Subadult | Male |
| SU | Subadult | Female |
| RA | Juvenile | Female |
| CHE | Juvenile | Male |
| DA | Juvenile | Male |
| Name | Age Category | Sex |
|---|---|---|
| LEO * | Adult | Female |
| GAR * | Adult | Male |
| MAR | Subadult | Male |
| MER | Subadult | Male |
| NER | Juvenile | Female |
| JA | Juvenile | Female |
| LEON | Juvenile | Male |
| TAR | Juvenile | Male |
| Name | Eigenvector Before | Eigenvector After |
|---|---|---|
| SA | 0.378 | 0.382 |
| GA | 0.282 | 0.226 |
| GE | 0.344 | 0.345 |
| PA | 0.290 | 0.274 |
| PHI | 0.274 | 0.344 |
| GI | 0.191 | 0.150 |
| JO | 0.329 | 0.419 |
| NI | 0.303 | 0.300 |
| MI | 0.138 | 0.297 |
| SU | 0.382 | 0.226 |
| RA | 0.303 | 0.149 |
| CHE | 0.050 | 0.181 |
| DA | 0.058 | 0.094 |
| Mean | 0.256 | 0.261 |
| Eigenvector Before | Eigenvector After | |
|---|---|---|
| LEO | 0.435 | 0.525 |
| GAR | 0.295 | 0.206 |
| MAR | 0.391 | 0.226 |
| MER | 0.391 | 0.419 |
| NER | 0.284 | 0.382 |
| JA | 0.384 | 0.339 |
| LEON | 0.284 | 0.307 |
| TAR | 0.329 | 0.317 |
| Mean | 0.349125 | 0.340125 |
| Variable | Standardized Coefficient | Standard Error | p |
|---|---|---|---|
| Grooming before the birth | 0.314 | 0.092 | 0.003 |
| Age | 0.289 | 6.259 | <0.001 |
| Sex | 0.087 | 5.58 | 0.159 |
| Variable | Standardized Coefficient | Standard Error | p |
|---|---|---|---|
| Grooming before the birth | 0.232 | 0.062 | 0.07 |
| Age | 0.002 | 10.428 | 0.506 |
| Sex | −0.188 | 9.645 | 0.109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, S.; Sánchez, S.; Fidalgo, A. Social Network Changes in Cotton-Top Tamarins (Saguinus oedipus) after the Birth of New Infants. Animals 2023, 13, 1758. https://doi.org/10.3390/ani13111758
Díaz S, Sánchez S, Fidalgo A. Social Network Changes in Cotton-Top Tamarins (Saguinus oedipus) after the Birth of New Infants. Animals. 2023; 13(11):1758. https://doi.org/10.3390/ani13111758
Chicago/Turabian StyleDíaz, Sergio, Susana Sánchez, and Ana Fidalgo. 2023. "Social Network Changes in Cotton-Top Tamarins (Saguinus oedipus) after the Birth of New Infants" Animals 13, no. 11: 1758. https://doi.org/10.3390/ani13111758
APA StyleDíaz, S., Sánchez, S., & Fidalgo, A. (2023). Social Network Changes in Cotton-Top Tamarins (Saguinus oedipus) after the Birth of New Infants. Animals, 13(11), 1758. https://doi.org/10.3390/ani13111758






