Electromyographic and Kinematic Comparison of the Leading and Trailing Fore- and Hindlimbs of Horses during Canter
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses and Horse Preparation
2.2. Equipment Set Up
2.3. Data Collection Protocol
2.4. Data Processing and Analysis
2.4.1. Kinematic Data Processing and Analysis
2.4.2. sEMG Data Processing and Analysis
2.5. Statistical Analysis
3. Results
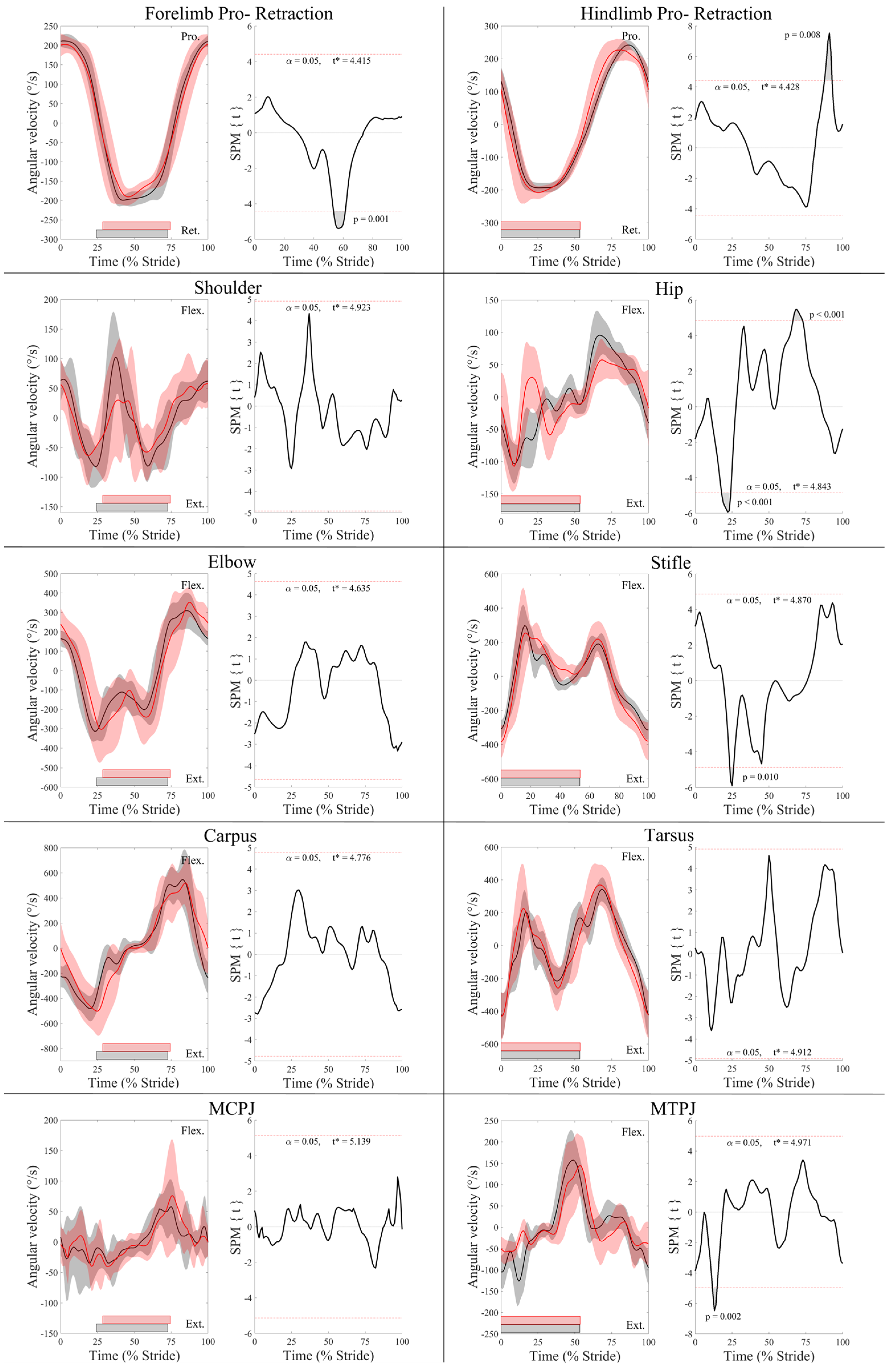
3.1. Kinematic Differences between Leading and Trailing Fore- and Hindlimbs
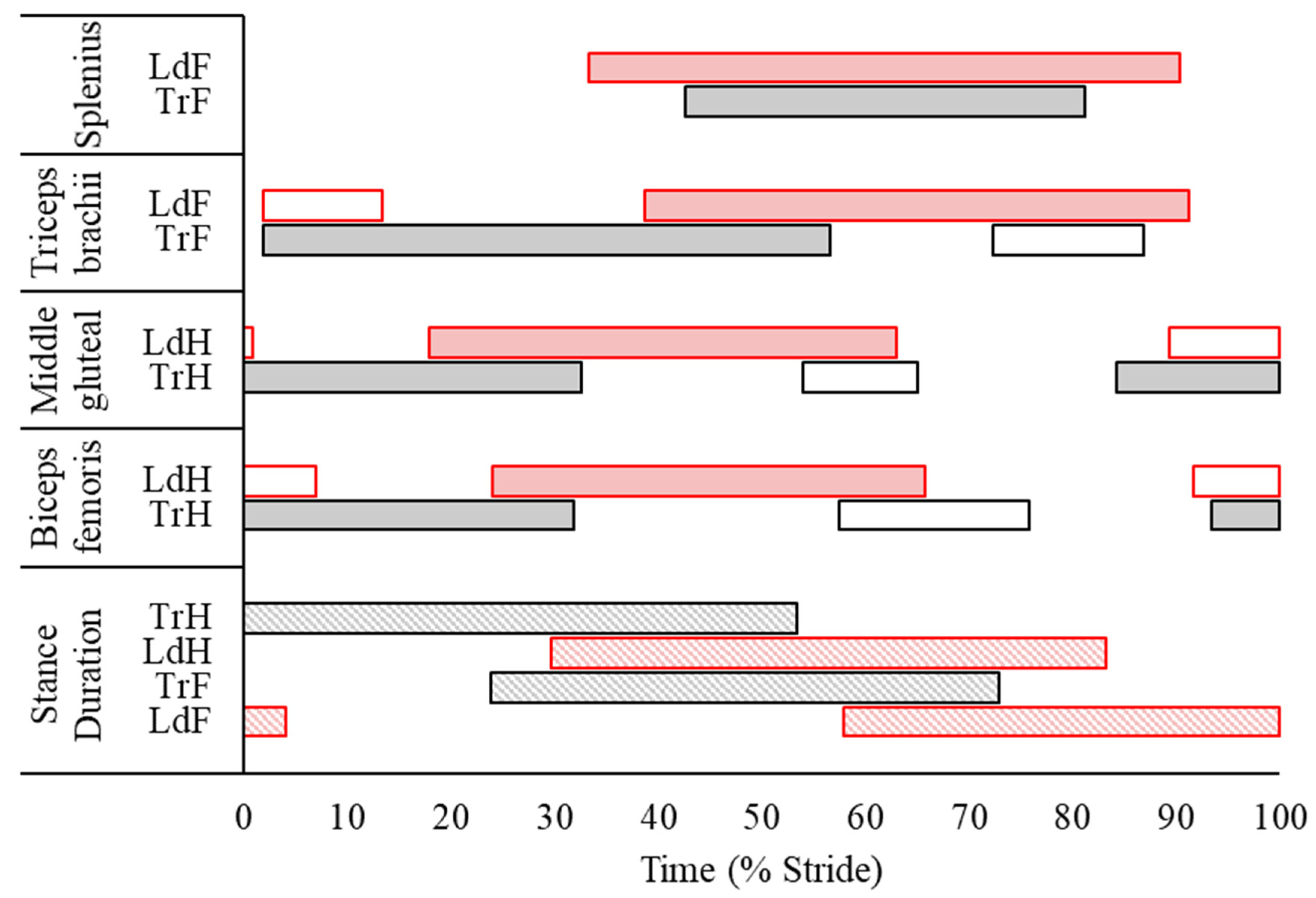
3.2. Surface Electromyography Data
4. Discussion
4.1. Electromyographic and Kinematic Differences between Leading and Trailing Forelimbs
4.2. Electromyographic and Kinematic Differences between Leading and Trailing Hindlimbs
4.3. Practical Applications for Equestrian Training
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hildebrand, M. Analysis of asymmetrical gaits. J. Mammal. 1977, 58, 131–156. [Google Scholar] [CrossRef]
- Leach, D. Recommended terminology for researchers in locomotion and biomechanics of quadrupedal animals. Cells Tissues Organs 1993, 146, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.; Ormrod, K.; Clayton, H. Standardised terminology for the description and analysis of equine locomotion. Equine Vet. J. 1984, 16, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Back, W.; Schamhardt, H.; Barneveld, A. Kinematic comparison of the leading and trailing fore- and hindlimbs at the canter. Equine Vet. J. 1997, 29, 80–83. [Google Scholar] [CrossRef]
- Merkens, H.W.; Schamhardt, H.C.; Van Osch, G.; Hartman, W. Ground reaction force patterns of Dutch Warmbloods at the canter. Am. J. Vet. Res. 1993, 54, 670. [Google Scholar]
- Clayton, H. The extended canter: A comparison of some kinematic variables in horses trained for dressage and for racing. Cells Tissues Organs 1993, 146, 183–187. [Google Scholar] [CrossRef]
- Faber, M.; Johnston, C.; Schamhardt, H.; Van Weeren, P.; Roepstorff, L.; Barneveld, A. Three-dimensional kinematics of the equine spine during canter. Equine Vet. J. 2001, 33, 145–149. [Google Scholar] [CrossRef]
- Burns, T.E.; Clayton, H.M. Comparison of the temporal kinematics of the canter pirouette and collected canter. Equine Vet. J. 1997, 29, 58–61. [Google Scholar] [CrossRef]
- Clayton, H.M. Comparison of the collected, working, medium and extended canters. Equine Vet. J. 1994, 26, 16–19. [Google Scholar] [CrossRef]
- Deuel, N.R.; Park, J.-J. The gait patterns of Olympic dressage horses. J. Appl. Biomech. 1990, 6, 198–226. [Google Scholar] [CrossRef]
- Clayton, H.M. Time-motion analysis of show jumping competitions. J. Equine Vet. Sci. 1996, 16, 262–266. [Google Scholar] [CrossRef]
- St George, L.; Hobbs, S.J.; Sinclair, J.; Richards, J.; Roddam, H. Does equestrian knowledge and experience influence selection and training practices for showjumping horses? Comp. Exerc. Physiol. 2019, 15, 123–135. [Google Scholar] [CrossRef]
- Hellsten, E.T.; Viklund, Å.; Koenen, E.; Ricard, A.; Bruns, E.; Philipsson, J. Review of genetic parameters estimated at stallion and young horse performance tests and their correlations with later results in dressage and show-jumping competition. Livest. Sci. 2006, 103, 1–12. [Google Scholar] [CrossRef]
- Wallin, L.; Strandberg, E.; Philipsson, J. Genetic correlations between field test results of Swedish Warmblood Riding Horses as 4-year-olds and lifetime performance results in dressage and show jumping. Livest. Prod. Sci. 2003, 82, 61–71. [Google Scholar] [CrossRef]
- Niki, Y.; Ueda, Y.; Masumitsu, H. A force plate study in equine biomechanics 3. The vertical and fore-aft components of floor reaction forces and motion of equine limbs at canter. Bull. Equine Res. Inst. 1984, 1984, 8–18. [Google Scholar]
- Crevier-Denoix, N.; Falala, S.; Holden-Douilly, L.; Camus, M.; Martino, J.; Ravary-Plumioen, B.; Vergari, C.; Desquilbet, L.; Denoix, J.M.; Chateau, H. Comparative kinematic analysis of the leading and trailing forelimbs of horses cantering on a turf and a synthetic surface. Equine Vet. J. 2013, 45, 54–61. [Google Scholar] [CrossRef]
- Witte, T.; Knill, K.; Wilson, A. Determination of peak vertical ground reaction force from duty factor in the horse (Equus caballus). J. Exp. Biol. 2004, 207, 3639–3648. [Google Scholar] [CrossRef]
- McGuigan, M.P.; Wilson, A.M. The effect of gait and digital flexor muscle activation on limb compliance in the forelimb of the horse Equus caballus. J. Exp. Biol. 2003, 206, 1325–1336. [Google Scholar] [CrossRef]
- Harrison, S.M.; Whitton, R.C.; King, M.; Haussler, K.K.; Kawcak, C.E.; Stover, S.M.; Pandy, M.G. Forelimb muscle activity during equine locomotion. J. Exp. Biol. 2012, 215, 2980–2991. [Google Scholar] [CrossRef]
- Jansen, M.; Van Raaij, J.; Van den Bogert, A.; Schamhardt, H.; Hartman, W. Quantitative analysis of computer-averaged electromyographic profiles of intrinsic limb muscles in ponies at the walk. Am. J. Vet. Res. 1992, 53, 2343–2349. [Google Scholar]
- Zsoldos, R.; Kotschwar, A.; Kotschwar, A.; Groesel, M.; Licka, T.; Peham, C. Electromyography activity of the equine splenius muscle and neck kinematics during walk and trot on the treadmill. Equine Vet. J. 2010, 42, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Zsoldos, R.; Kotschwar, A.; Kotschwar, A.; Rodriguez, C.; Peham, C.; Licka, T. Activity of the equine rectus abdominis and oblique external abdominal muscles measured by surface EMG during walk and trot on the treadmill. Equine Vet. J. 2010, 42, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Valette, J.; Degueurce, C.; Denoix, J. Correlation between surface electromyography and kinematics of the hindlimb of horses at trot on a treadmill. Cells Tissues Organs 1999, 165, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Licka, T.; Frey, A.; Peham, C. Electromyographic activity of the longissimus dorsi muscles in horses when walking on a treadmill. Vet. J. 2009, 180, 71–76. [Google Scholar] [CrossRef]
- Licka, T.F.; Peham, C.; Frey, A. Electromyographic activity of the longissimus dorsi muscles in horses during trotting on a treadmill. Am. J. Vet. Res. 2004, 65, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Hodson-Tole, E. Effects of treadmill inclination and speed on forelimb muscle activity and kinematics in the horse. Equine Comp. Exerc. Physiol. 2006, 3, 61–72. [Google Scholar] [CrossRef]
- Kienapfel, K.; Preuschoft, H.; Wulf, A.; Wagner, H. The biomechanical construction of the horse’s body and activity patterns of three important muscles of the trunk in the walk, trot and canter. J. Anim. Physiol. Anim. Nutr. 2018, 102, e818–e827. [Google Scholar] [CrossRef]
- St George, L.; Roy, S.; Richards, J.; Sinclair, J.; Hobbs, S.J. Surface EMG signal normalisation and filtering improves sensitivity of equine gait analysis. Comp. Exerc. Physiol. 2019, 15, 173–185. [Google Scholar] [CrossRef]
- Gellman, K.S.; Bertram, J. The equine nuchal ligament 2: Passive dynamic energy exchange in locomotion. Vet. Comp. Orthop. Traumatol. 2002, 15, 07–14. [Google Scholar]
- Dunbar, D.C.; Macpherson, J.M.; Simmons, R.W.; Zarcades, A. Stabilization and mobility of the head, neck and trunk in horses during overground locomotion: Comparisons with humans and other primates. J. Exp. Biol. 2008, 211, 3889–3907. [Google Scholar] [CrossRef]
- St George, L.; Clayton, H.M.; Sinclair, J.; Richards, J.; Roy, S.H.; Hobbs, S.J. Muscle Function and Kinematics during Submaximal Equine Jumping: What Can Objective Outcomes Tell Us about Athletic Performance Indicators? Animals 2021, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J. The use of surface electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.J.; Richards, J.; Clayton, H.M. The effect of centre of mass location on sagittal plane moments around the centre of mass in trotting horses. J. Biomech. 2014, 47, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.; St George, L.; Clayton, H.; Hobbs, S.J. A simple method for equine kinematic gait event detection. Equine Vet. J. 2017, 49, 688–691. [Google Scholar] [CrossRef]
- Back, W.; Schamhardt, H.; Savelberg, H.; Van den Bogert, A.; Bruin, G.; Hartman, W.; Barneveld, A. How the horse moves: 1. Significance of graphical representations of equine forelimb kinematics. Equine Vet. J. 1995, 27, 31–38. [Google Scholar] [CrossRef]
- Back, W.; Schamhardt, H.; Savelberg, H.; Van Den Bogert, A.; Bruin, G.; Hartman, W.; Barneveld, A. How the horse moves: 2. Significance of graphical representations of equine hind limb kinematics. Equine Vet. J. 1995, 27, 39–45. [Google Scholar] [CrossRef]
- St George, L.; Hobbs, S.J.; Richards, J.; Sinclair, J.; Holt, D.; Roy, S. The effect of cut-off frequency when high-pass filtering equine sEMG signals during locomotion. J. Electromyogr. Kinesiol. 2018, 43, 28–40. [Google Scholar] [CrossRef]
- Witte, T.; Hirst, C.; Wilson, A. Effect of speed on stride parameters in racehorses at gallop in field conditions. J. Exp. Biol. 2006, 209, 4389–4397. [Google Scholar] [CrossRef]
- Tokuriki, M.; Aoki, O. Neck muscles activity in horses during locomotion with and without a rider. In Equine Exercise Physiology 3; Persson, S., Lindholm, A., Jeffcott, L., Eds.; ICEEP Publications: Davis, CA, USA, 1991; pp. 146–150. [Google Scholar]
- Buchner, H.; Savelberg, H.; Schamhardt, H.; Barneveld, A. Limb movement adaptations in horses with experimentally induced fore-or hindlimb lameness. Equine Vet. J. 1996, 28, 63–70. [Google Scholar] [CrossRef]
- Robert, C.; Valette, J.; Denoix, J. The effects of treadmill inclination and speed on the activity of two hindlimb muscles in the trotting horse. Equine Vet. J. 2000, 32, 312–317. [Google Scholar] [CrossRef]
- Robert, C.; Valette, J.P.; Pourcelot, P.; Audigie, F.; Denoix, J.M. Effects of trotting speed on muscle activity and kinematics in saddlehorses. Equine Vet. J. 2002, 34, 295–301. [Google Scholar] [CrossRef]
- Tokuriki, M.; Aoki, O. Electromyographic activity of the hindlimb muscles during the walk, trot and canter. Equine Vet. J. 1995, 27, 152–155. [Google Scholar] [CrossRef]
- Dutto, D.J.; Hoyt, D.F.; Clayton, H.M.; Cogger, E.A.; Wickler, S.J. Joint work and power for both the forelimb and hindlimb during trotting in the horse. J. Exp. Biol. 2006, 209, 3990–3999. [Google Scholar] [CrossRef] [PubMed]
- Denoix, J.M. Biomechanics and Physical Training of the Horse; Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Biewener, A.A. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1998, 120, 73–87. [Google Scholar] [CrossRef]
- Wells, A.; Blache, D. Horses do not exhibit motor bias when their balance is challenged. Animal 2008, 2, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Byström, A.; Clayton, H.; Hernlund, E.; Rhodin, M.; Egenvall, A. Equestrian and biomechanical perspectives on laterality in the horse. Comp. Exerc. Physiol. 2020, 16, 35–45. [Google Scholar] [CrossRef]
- Eisersiö, M.; Roepstorff, L.; Rhodin, M.; Egenvall, A. A snapshot of the training schedule for 8 professional riders riding dressage. Comp. Exerc. Physiol. 2015, 11, 35–45. [Google Scholar] [CrossRef]
- Krueger, K.; Schwarz, S.; Marr, I.; Farmer, K. Laterality in horse training: Psychological and physical balance and coordination and strength rather than straightness. Animals 2022, 12, 1042. [Google Scholar] [CrossRef]
- Robert, C.; Valette, J.; Denoix, J.M. The effects of treadmill inclination and speed on the activity of three trunk muscles in the trotting horse. Equine Vet. J. 2001, 33, 466–472. [Google Scholar] [CrossRef]
- Crook, T.; Wilson, A.; Hodson-Tole, E. The effect of treadmill speed and gradient on equine hindlimb muscle activity. Equine Vet. J. 2010, 42, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Thaut, M.H.; McIntosh, G.C.; Rice, R.R. Components of EMG symmetry and variability in parkinsonian and healthy elderly gait. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control 1996, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.; Elzinga, H.; Grimmius, W.; Halbertsma, J. Speed dependence of averaged EMG profiles in walking. Gait. Posture 2002, 16, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, M.; Aoki, O.; Niki, Y.; Kurakawa, Y.; Hataya, M.; Kita, T. Electromyographic activity of cubital joint muscles in horses during locomotion. Am. J. Vet. Res. 1989, 50, 950–957. [Google Scholar] [PubMed]


| Variable | Canter Lead/Limb | p Value | |
|---|---|---|---|
| Left Lead TrH or TrF | Right Lead LdH or LdF | ||
| Stride velocity (m/s) | 4.41 ± 0.42 | 4.42 ± 0.40 | 0.729 |
| Stride duration (s) | 0.59 ± 0.02 | 0.59 ± 0.02 | 0.641 |
| Forelimb stance duration (s) | 0.29 ± 0.02 | 0.27 ± 0.02 | 0.018 * |
| Hindlimb stance duration (s) | 0.32 ± 0.03 | 0.32 ± 0.04 | 0.786 |
| Muscle | Variable | Limb | p Value | |
|---|---|---|---|---|
| TrH | LdH | |||
| Biceps femoris | ARV (%) | 62.7 ± 20.3 | 80.2 ± 14.9 | 0.179 |
| Activity duration (% stride) | 46.9 ± 13.7 | 47.5 ± 14.9 | 0.822 | |
| Peak amplitude (% stride) | 16.5 ± 5.9 | 16.7 ± 7.2 | 0.541 | |
| Activity offset (% stride) | 31.9 ± 7.8 | 36.2 ± 6.5 | 0.018 * | |
| Activity onset (% stride) | 93.5 ± 6.5 | 94.3 ± 5.7 | 0.287 | |
| Middle gluteal | ARV (%) | 77.6 ± 14.1 | 66.3 ± 19.4 | 0.046 * |
| Activity duration (% stride) | 54.8 ± 13.8 | 48.6 ± 14.5 | 0.024 * | |
| Peak amplitude (% stride) | 13.6 ± 5.2 | 16.7 ± 8.2 | 0.163 | |
| Activity offset (% stride) | 32.6 ± 7.5 | 33.5 ± 7.5 | 0.340 | |
| Activity onset (% stride) | 84.2 ± 7.3 | 88.2 ± 9.6 | 0.069 | |
| TrF | LdF | |||
| Triceps brachii | ARV (%) | 72.5 ± 18.2 | 68.5 ± 19.7 | 0.179 |
| Activity duration (% stride) | 66.4 ± 14.4 | 56.5 ± 18.0 | 0.234 | |
| Peak amplitude (% stride) | 40.4 ± 13.9 | 35.9 ± 13.7 | 0.531 | |
| Activity onset (% stride) | 101.8 ± 9.4 § | 109.1 ± 9.7 § | 0.061 | |
| Activity offset (% stride) | 56.6 ± 6.5 | 56.6 ± 9.9 | 0.368 | |
| Splenius | ARV (%) | 75.8 ± 17.0 | 64.8 ± 17.7 | 0.322 |
| Activity duration (% stride) | 51.1 ± 10.3 | 57.2 ± 12.5 | 0.192 | |
| Peak amplitude (% stride) | 59.0 ± 11.4 | 32.3 ± 15.2 | 0.000 * | |
| Activity onset (% stride) | 42.7 ± 25.9 | 103.7 ± 14.0 § | 0.002 * | |
| Activity offset (% stride) | 81.2 ± 7.0 | 60.7 ± 10.0 | 0.029 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
St. George, L.B.; Clayton, H.M.; Sinclair, J.K.; Richards, J.; Roy, S.H.; Hobbs, S.J. Electromyographic and Kinematic Comparison of the Leading and Trailing Fore- and Hindlimbs of Horses during Canter. Animals 2023, 13, 1755. https://doi.org/10.3390/ani13111755
St. George LB, Clayton HM, Sinclair JK, Richards J, Roy SH, Hobbs SJ. Electromyographic and Kinematic Comparison of the Leading and Trailing Fore- and Hindlimbs of Horses during Canter. Animals. 2023; 13(11):1755. https://doi.org/10.3390/ani13111755
Chicago/Turabian StyleSt. George, Lindsay B., Hilary M. Clayton, Jonathan K. Sinclair, Jim Richards, Serge H. Roy, and Sarah Jane Hobbs. 2023. "Electromyographic and Kinematic Comparison of the Leading and Trailing Fore- and Hindlimbs of Horses during Canter" Animals 13, no. 11: 1755. https://doi.org/10.3390/ani13111755
APA StyleSt. George, L. B., Clayton, H. M., Sinclair, J. K., Richards, J., Roy, S. H., & Hobbs, S. J. (2023). Electromyographic and Kinematic Comparison of the Leading and Trailing Fore- and Hindlimbs of Horses during Canter. Animals, 13(11), 1755. https://doi.org/10.3390/ani13111755