Density-Dependent Seed Predation of Quercus wutaishanica by Rodents in Response to Different Seed States
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Seed Collection
2.3. Marking and Labeling of Seeds
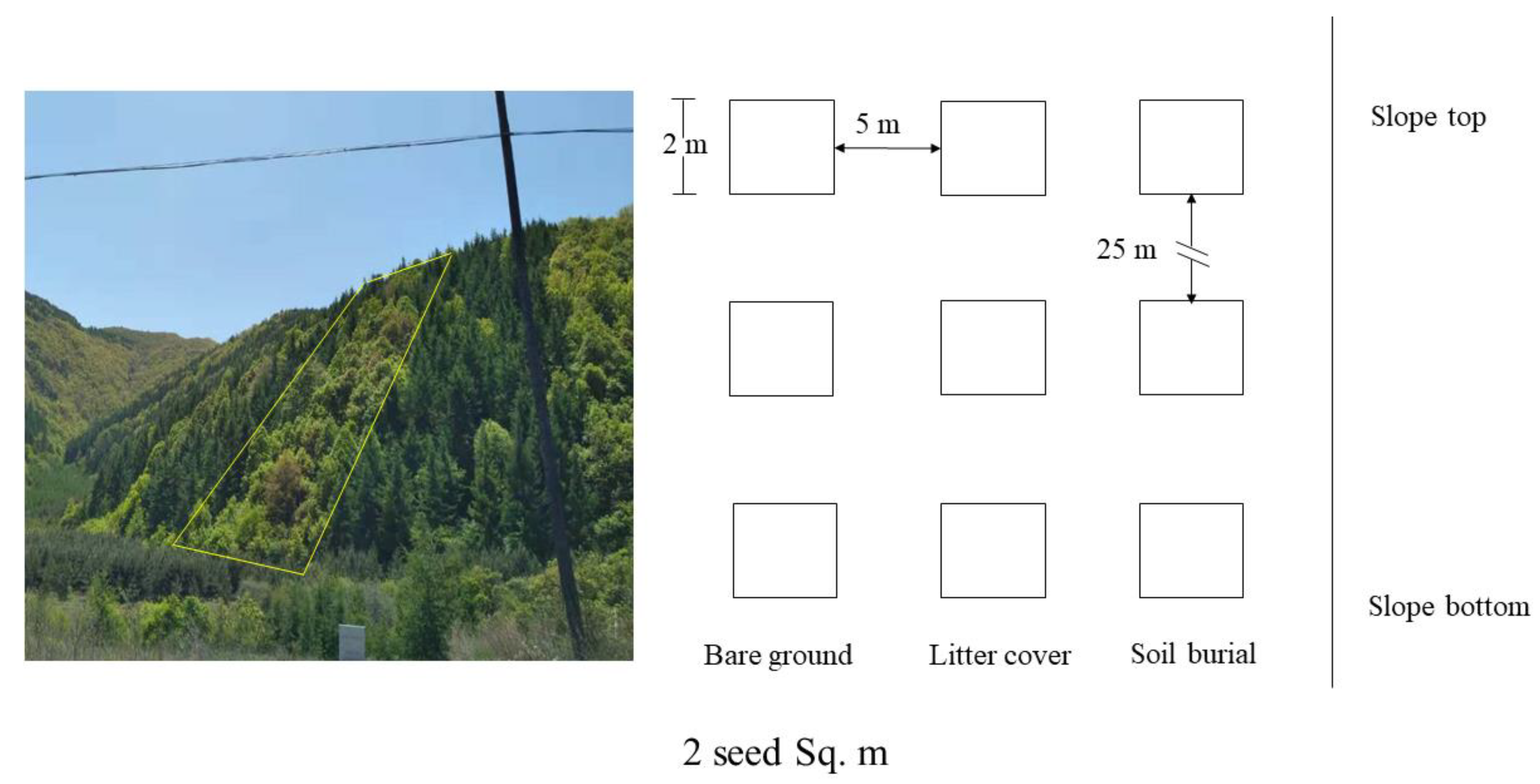
2.4. Experimental Design
2.5. Field Investigation
2.6. Data Analysis
3. Results
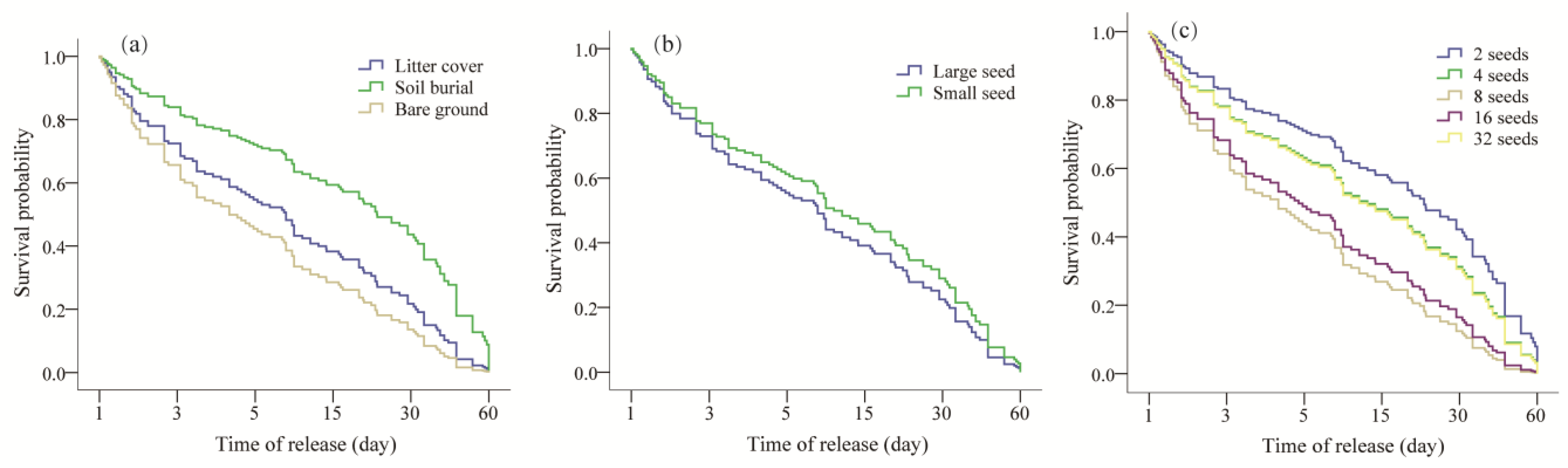
3.1. Quercus Wutaishanica Seed Dynamics
3.2. Quercus Wutaishanica Seed Fates
3.3. Dispersal Distance
4. Discussion
4.1. Microhabitat Affects the Predation and Dispersal Behavior of Rodents
4.2. The Preference of Rodents for Seeds of Different Sizes
4.3. Negatively Density-Dependent
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zwolak, R. How intraspecific variation in seed-dispersing animals matters for plants. Biol. Rev. 2018, 93, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Chen, Y.S.; Zhang, Z.B. Differences of dispersal fitness of large and small acorns of Liaodong oak (Quercus liaotungensis) before and after seed caching by small rodents in a warm temperature forest, China. For. Ecol. Manag. 2008, 225, 1243–1250. [Google Scholar] [CrossRef]
- Zhang, J.F.; Yan, X.F.; Buddhi, D.; Luo, Y.H.; Li, J.Q. Frequency-dependent predation and seedling fate: Effect of forest litter on regeneration of the Quercus wutaishanica seedling. Glob. Ecol. Conserv. 2022, 38, e02233. [Google Scholar] [CrossRef]
- Preston, S.D.; Jacobs, L.F. Conspecific pilferage but not presence affects Merriam’s kangaroo rat cache strategy. Behav. Ecol. 2001, 12, 517–523. [Google Scholar] [CrossRef]
- Haugaasen, J.M.T.; Haugaasen, T.; Peres, C.A.; Gribel, R.; Wegge, P. Seed dispersal of the Brazil nut tree (Bertholletia excelsa) by scatter-hoarding rodents in a central Amazonian forest. J. Trop. Ecol. 2010, 26, 251–262. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Schaefer, H.M. The conservation physiology of seed dispersal. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1708–1718. [Google Scholar] [CrossRef]
- Clarke, M.F.; Kramer, D.L. The placement, recovery, and loss of scatter hoards by eastern chipmunks, Tamias striatus. Behav. Ecol. 1994, 5, 353–361. [Google Scholar] [CrossRef]
- Vander Wall, S.B. How plants manipulate the scatter-hoarding behaviour of seed- dispersing animals. Philos. Trans. R. Soc. B 2010, 365, 989–997. [Google Scholar] [CrossRef]
- Gálvez, D.; Kranstauber, B.; Kays, R.W.; Janse, P.A. Scatter hoarding by the central American agouti: A test of optimal cache spacing theory. Anim. Behav. 2009, 78, 1327–1333. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. Dyn. Popul. 1971, 1, 298–312. [Google Scholar]
- Stapanian, M.A.; Smith, C.C. A model for scatter hoarding: Coevolution of fox squirrels and black walnuts. Ecology 1978, 59, 887–896. [Google Scholar] [CrossRef]
- Olendorf, R.; Rodd, F.H.; Punzalan, D.; Houde, A.E.; Hurt, C.; Reznick, D.N.; Hughes, K.A. Frequency-dependent survival in natural guppy populations. Nature 2006, 441, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Xiao, Z.S.; Guo, C. Trait-mediated seed predation, dispersal and survival among frugivore-dispersed plants in a fragmented subtropical forest, Southwest China. Integr. Zool. 2014, 9, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Celis-Diez, J.L.; Bustamante, R.O. Frequency-dependent seed size selection on Cryptocarya alba (Mol.) Looser (Lauraceae): Testing the effect of background. Biol. J. Linn. Soc. 2005, 84, 137–142. [Google Scholar] [CrossRef]
- Celis-Diez, J.L.; Bustamante, R.O.; Vasquez, R.A. Assessing frequency-dependent seed size selection: A field experiment. Biol. J. Linn. Soc. 2004, 81, 307–312. [Google Scholar] [CrossRef]
- Zhang, H.M.; Yan, C.; Chang, G.; Zhang, Z.B. Seed trait-mediated selection by rodents affects mutualistic interactions and seedling recruitment of co-occurring tree species. Oecologia 2016, 180, 475–484. [Google Scholar] [CrossRef]
- Brewer, S.W. Predation and dispersal of large and small seeds of tropical palm. Oikos 2001, 92, 245–255. [Google Scholar] [CrossRef]
- Mendes, C.P.; Ribeiro, M.C.; Galetti, M. Patch size, shape and edge distance influence seed predation on a palm species in the Atlantic forest. Ecography 2016, 39, 465–475. [Google Scholar] [CrossRef]
- Zhang, J.F.; Ge, J.R.; Yan, X.F.; Buddhi, D.; Luo, Y.H.; Li, J.Q. Frequency-dependent seedling predation by rodents: Growth and survival of Quercus wutaishanica in two habitats. J. Plant Ecol. 2022, 16, rtac086. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Jansen, P.A.; Zhang, Z.B. Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. For. Ecol. Manag. 2006, 223, 18–23. [Google Scholar] [CrossRef]
- Wang, B.; Wang, G.; Chen, J. Scatter-hoarding rodents use different foraging strategies for seeds from different plant species. Plant Ecol. 2012, 213, 1329–1336. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Henry, H.M. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2019, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, J. Seed size, more than nutrient or tannin content, affects seed caching behavior of a common genus of Old-World rodents. Ecology 2009, 90, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.F.; Wang, Z.Y.; Liu, C.Q.; Liu, G.Q. Seed trait and rodent species determine seed dispersal and predation: Evidences from semi-natural enclosures. Iforest-Biogeosci. For. 2015, 8, 207–213. [Google Scholar] [CrossRef]
- Crego, R.D.; Jimenez, J.E.; Rozzi, R. Macro-and micro-habitat selection of small rodents and their predation risk perception under a novel invasive predator at the southern end of the Americas. Mammal Res. 2018, 63, 267–275. [Google Scholar] [CrossRef]
- Sato, Y.; Ito, K.; Kudoh, H. Optimal foraging by herbivores maintains polymorphism in defence in a natural plant population. Funct. Ecol. 2017, 31, 2233–2243. [Google Scholar] [CrossRef]
- Steele, M.A.; Contreras, T.A.; Hadj-Chikh, L.Z.; Agosta, S.J.; Smallwood, P.D.; Tomlinson, C.N. Do scatter hoarders trade off increased predation risks for lower rates of cache pilferage? Behav. Ecol. 2014, 25, 206–215. [Google Scholar] [CrossRef]
- Wang, M.H.; Yi, X.F. The effects of seed detectability and seed traits on hoarding preference of two rodent species. Integr. Zool. 2001, 17, 944–952. [Google Scholar] [CrossRef]
- Steele, M.A.; Rompré, G.; Stratford, J.A.; Zhang, H.M. Scatter hoarding rodents favor higher predation risks for cache sites: The potential for predators to influence the seed dispersal process. Integr. Zool. 2015, 10, 257–266. [Google Scholar] [CrossRef]
- Vander Wall, S.B. The evolutionary ecology of nut dispersal. Bot. Rev. 2001, 67, 74–117. [Google Scholar] [CrossRef]
- Yi, X.F.; Yang, Y.Q.; Curtis, R.; Bartlow, A.W.; Agosta, S.J.; Steele, M.A. Alternative strategies of seed predator escape by early-germinating oaks in Asia and North America. Ecol. Evol. 2012, 2, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Raison, H.E.; Weale, M.E. The influence of density on frequency-dependent selection by wild birds feeding on artificial prey. Philos. Trans. R. Soc. B 1998, 265, 1031–1035. [Google Scholar] [CrossRef]
- Myers, J.A.; Vellend, M.; Gardescu, S.; Marks, P. Seed dispersal by white-tailed deer: Implications for long-distance dispersal, invasion, and migration of plants in eastern North America. Oecologia 2004, 139, 35–44. [Google Scholar] [PubMed]
- Huang, Z.Y.; Wang, Y.; Zhang, H.M.; Wu, F.Q.; Zhang, Z.B. Behavioural responses of sympatric rodents to complete pilferage. Anim. Behav. 2011, 81, 831–836. [Google Scholar] [CrossRef]
- Ratikainen, I.I.; Gill, J.A.; Gunnarsson, T.G.; Sutherland, W.J.; Kokko, H.K. When density dependence is not instantaneous: Theoretical developments and management implications. Ecol. Lett. 2010, 11, 184–198. [Google Scholar] [CrossRef]
- Farhoudi, F.; Allahyari Tabadkani, S.M.; Gholizadeh, M. Prey preference of Aphidoletes aphidimyza on Acyrthosiphon pisum: Effect of prey color and size. J. Insect Behav. 2014, 27, 776–785. [Google Scholar] [CrossRef]
- Sato, Y.; Kawagoe, T.; Sawada, Y.; Hirai, M.; Kudoh, H. Frequency-dependent herbivory by a leaf beetle, Phaedon brassicae, on hairy and glabrous plants of Arabidopsis halleri subsp. gemmifera. Evol. Ecol. 2014, 28, 545–559. [Google Scholar] [CrossRef]
- Sundaram, M.; Lichti, N.I.; Steele, M.A.; Dalgleish, H.J.; Swihart, R.K. Frequency-dependent hoarding by Sciurus carolinensis occurs with seeds of similar perceived value. J. Mammal. 2017, 98, 124–134. [Google Scholar]
- Moore, J.E.; McEuen, A.B.; Swihart, R.K.; Contreras, T.A.; Steele, M.A. Determinants of seed removal distance by scatter-hoarding rodents in deciduous forests. Ecology 2007, 88, 2529–2540. [Google Scholar] [CrossRef]
- Male, L.H.; Smulders, T.V. Hyperdispersed cache distributions reduce pilferage: A field study. Anim. Behav. 2007, 73, 717–726. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Corlett, R.T. Factors influencing repeated seed movements by scatter-hoarding rodents in an alpine forest. Sci. Rep. 2014, 4, 4786. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.S.; Zhang, Z.B.; Krebs, C.J. Long-term seed survival and dispersal dynamics in a rodent-dispersed tree: Testing the predator satiation hypothesis and the predator dispersal hypothesis. J. Ecol. 2013, 101, 1256–1264. [Google Scholar] [CrossRef]


| Covariates | Standardized Coefficients | p | R2 | |
|---|---|---|---|---|
| Fixed effects | Seed sizes | −0.433 | 0.048 | 0.3225 |
| Densities | −0.224 | 0.035 | ||
| States | −0.025 | 0.001 | ||
| Seed sizes × Densities | −0.459 | 0.053 | ||
| Densities × States | −0.763 | 0.121 | ||
| Seed sizes × Densities × States | 0.532 | 0.068 | ||
| Random effects | Variance components | R2 | ||
| Plots | 0.0058 | 0.2258 | ||
| Observation time | 0.0037 | 0.1589 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Cheng, J.; Yan, X.; Yang, H.; Shen, Y.; Ge, J.; Zhang, M.; Zhang, J.; Xu, Z. Density-Dependent Seed Predation of Quercus wutaishanica by Rodents in Response to Different Seed States. Animals 2023, 13, 1732. https://doi.org/10.3390/ani13111732
Luo Y, Cheng J, Yan X, Yang H, Shen Y, Ge J, Zhang M, Zhang J, Xu Z. Density-Dependent Seed Predation of Quercus wutaishanica by Rodents in Response to Different Seed States. Animals. 2023; 13(11):1732. https://doi.org/10.3390/ani13111732
Chicago/Turabian StyleLuo, Yonghong, Jiming Cheng, Xingfu Yan, Hui Yang, Yan Shen, Jingru Ge, Min Zhang, Jinfeng Zhang, and Zhuwen Xu. 2023. "Density-Dependent Seed Predation of Quercus wutaishanica by Rodents in Response to Different Seed States" Animals 13, no. 11: 1732. https://doi.org/10.3390/ani13111732
APA StyleLuo, Y., Cheng, J., Yan, X., Yang, H., Shen, Y., Ge, J., Zhang, M., Zhang, J., & Xu, Z. (2023). Density-Dependent Seed Predation of Quercus wutaishanica by Rodents in Response to Different Seed States. Animals, 13(11), 1732. https://doi.org/10.3390/ani13111732






