High and Hyper: Fentanyl Induces Psychomotor Side-Effects in Healthy Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Socialization, Enrichment and Training
2.3. Study Design
2.4. Drugs and Chemicals
2.5. Anesthesia and Central Venous Catheter Placement
2.6. Experimental Procedure
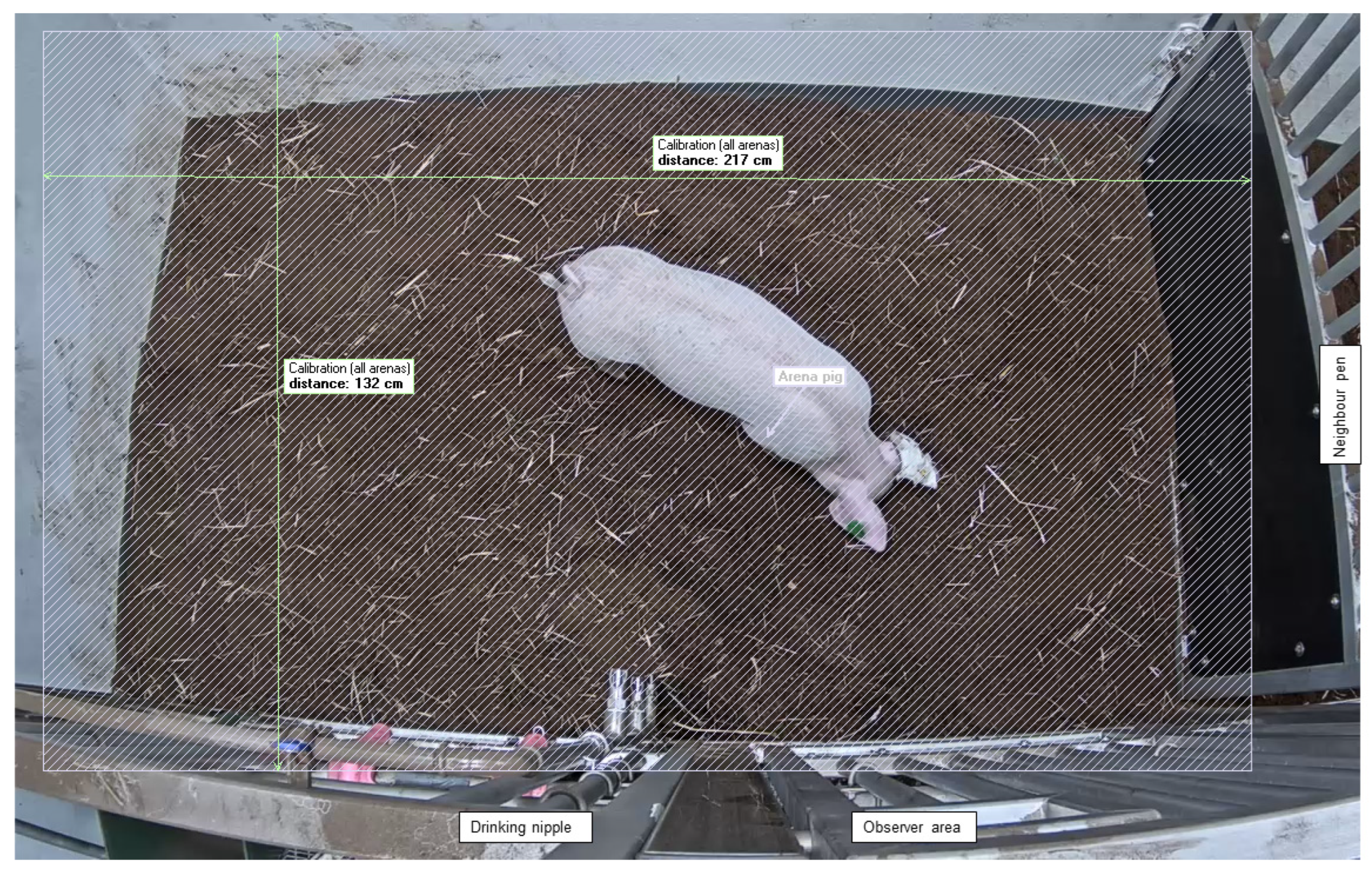
2.7. Data Collection and Processing
2.8. Data Analysis
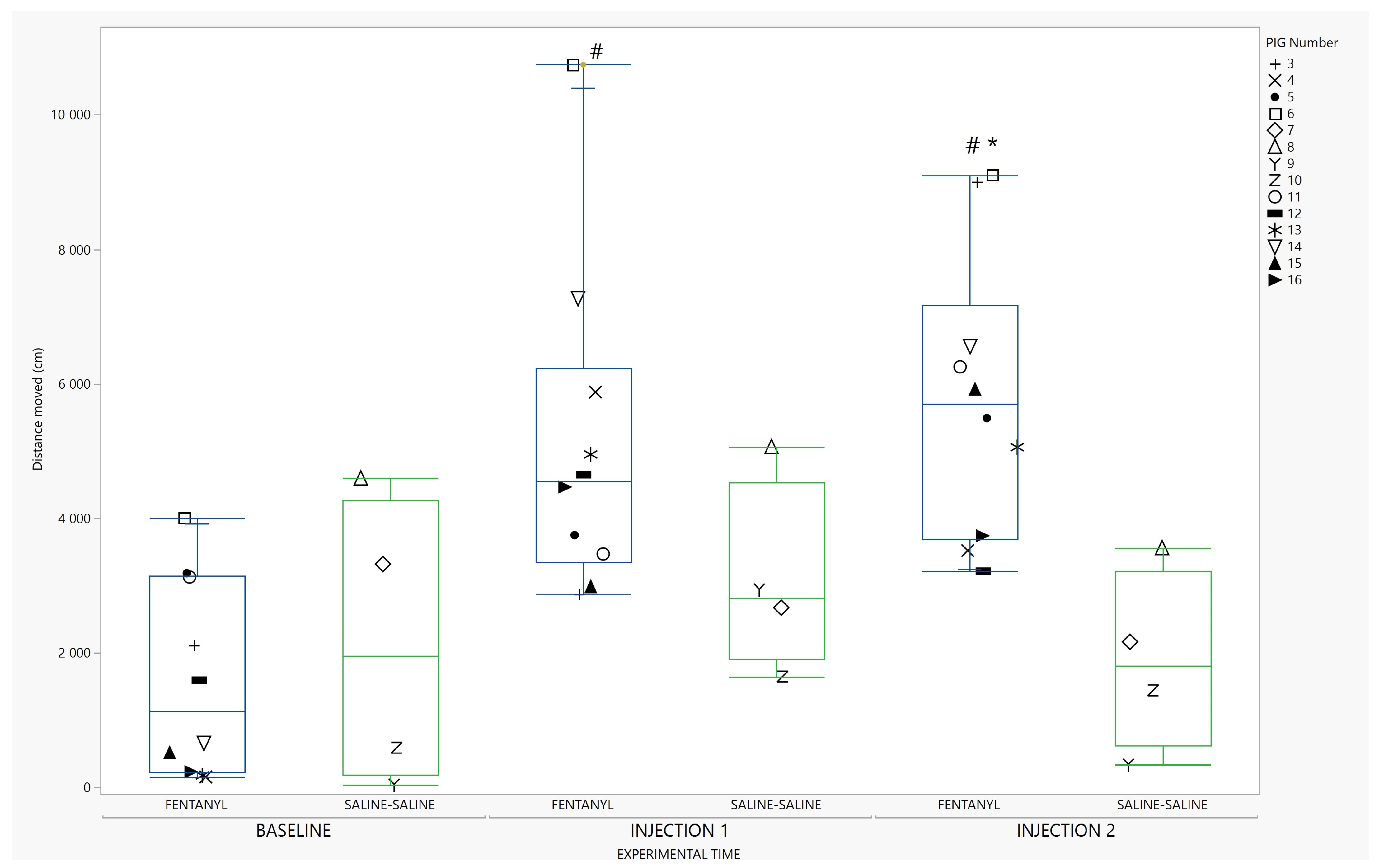
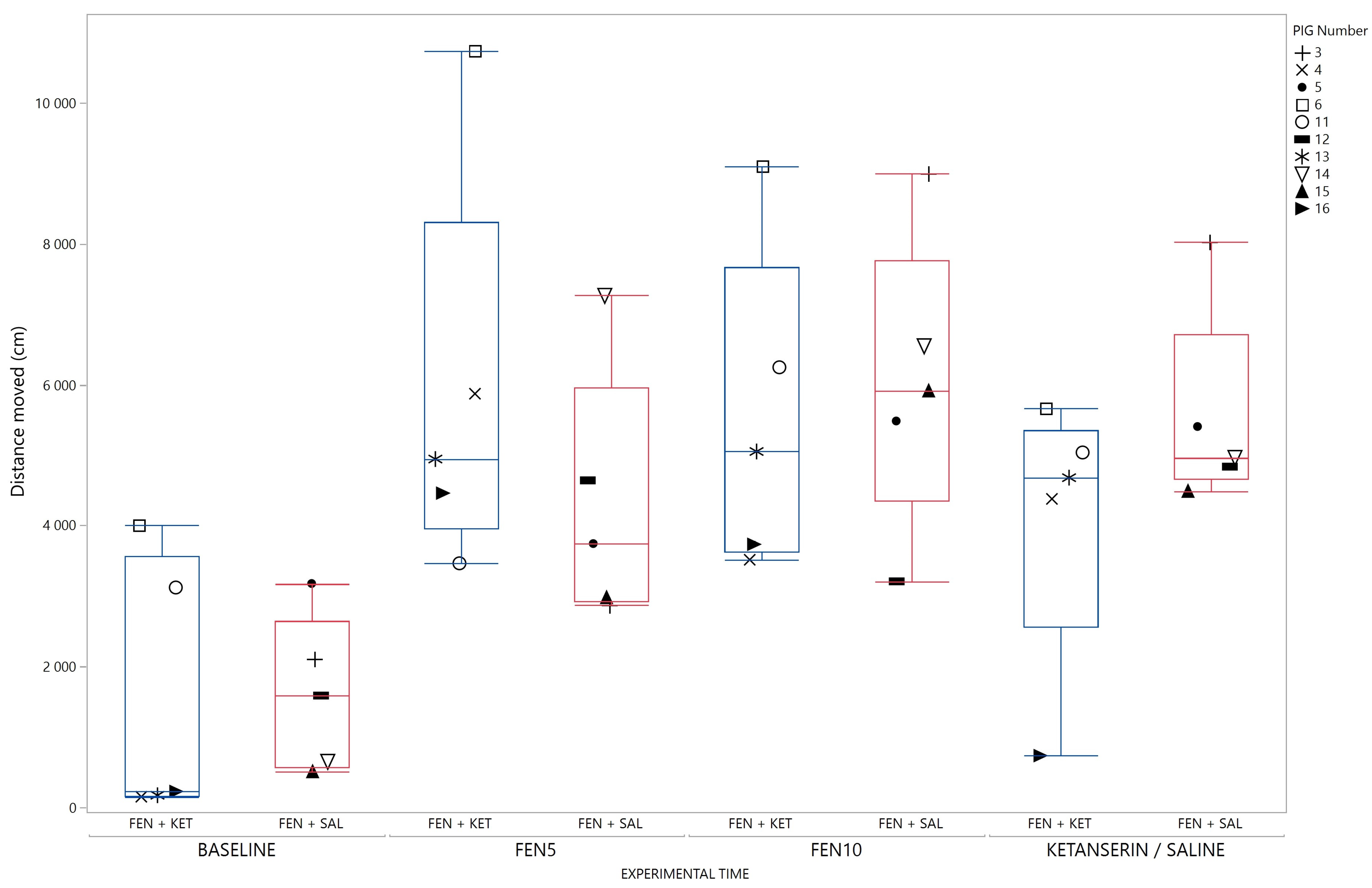
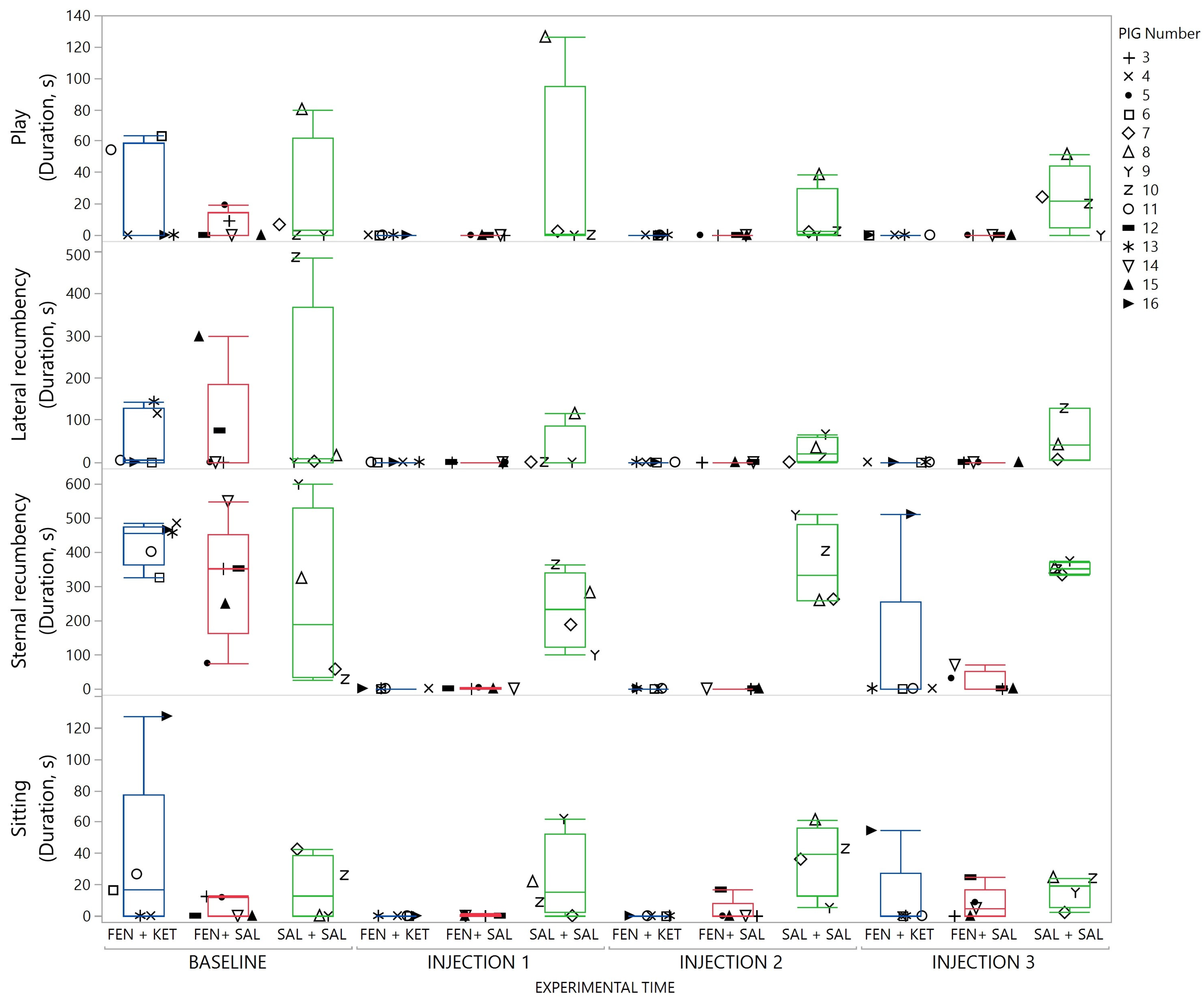
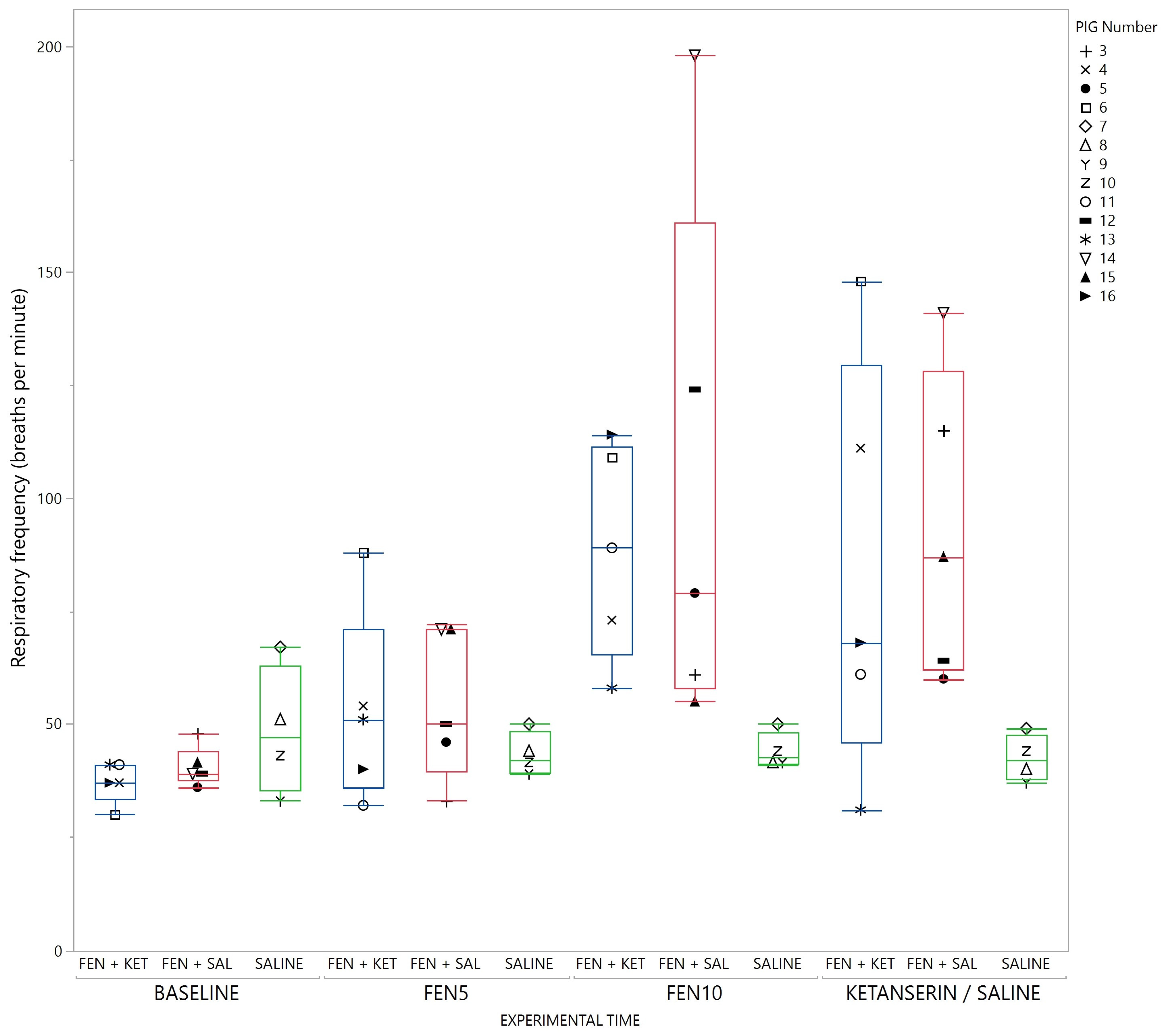
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walters, E.M.; Wells, K.D.; Bryda, E.C.; Schommer, S.; Prather, R.S. Swine models, genomic tools and services to enhance our understanding of human health and diseases. Lab. Anim. 2017, 46, 167–172. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, K.; Dicks, N.; Glanzner, W.G.; Agellon, L.B.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 2015, 6, 293. [Google Scholar] [CrossRef] [PubMed]
- 2010/63/EU, D. Directive on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 25 September 2020).
- Bradbury, A.G.; Eddleston, M.; Clutton, R.E. Pain management in pigs undergoing experimental surgery; a literature review (2012–4). Br. J. Anaesth. 2016, 116, 37–45. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Austin, J. Pain and Laboratory Animals: Publication Practices for Better Data Reproducibility and Better Animal Welfare. PLoS ONE 2016, 11, e0155001. [Google Scholar] [CrossRef]
- Fugazzola, M.C.; Wever, K.E.; van de Lest, C.; de Grauw, J.; Salvatori, D. Reporting of anaesthesia and pain management in preclinical large animal models of articular cartilage repair—A long way to go. Osteoarthr. Cartil. Open 2022, 4, 100261. [Google Scholar] [CrossRef]
- Hay, M.; Vulin, A.; Génin, S.; Sales, P.; Prunier, A. Assessment of pain induced by castration in piglets: Behavioral and physiological responses over the subsequent 5 days. Appl. Anim. Behav. Sci. 2003, 82, 201–218. [Google Scholar] [CrossRef]
- Hansson, M.; Lundeheim, N.; Nyman, G.; Johansson, G. Effect of local anaesthesia and/or analgesia on pain responses induced by piglet castration. Acta Vet. Scand. 2011, 53, 34. [Google Scholar] [CrossRef] [PubMed]
- 2010/37/EU. COMMISSION REGULATION (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origina. Available online: https://health.ec.europa.eu/system/files/2016-11/reg_2010_37_en_0.pdf (accessed on 18 January 2023).
- Bailey, J. Does the Stress of Laboratory Life and Experimentation on Animals Adversely Affect Research Data? A Critical Review. Altern. Lab. Anim. 2018, 46, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, L.M.; Augustsson, H.; Jensen-Waern, M.; Nyman, G. The Effect of Transdermal Delivery of Fentanyl on Activity in Growing Pigs. Acta Vet. Scand. 2005, 46, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, L.M.; Nyman, G.; Augustsson, H.; Jacobson, M.; Jensen-Waern, M. Effects of epidural morphine and transdermal fentanyl analgesia on physiology and behaviour after abdominal surgery in pigs. Lab. Anim. 2006, 40, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Harvey-Clark, C.J.; Gilespie, K.; Riggs, K.W. Transdermal fentanyl compared with parenteral buprenorphine in post-surgical pain in swine: A case study. Lab. Anim. 2000, 34, 386–398. [Google Scholar] [CrossRef]
- Goutchtat, R.; Chetboun, M.; Wiart, J.-F.; Gaulier, J.-M.; Pattou, F.; Allorge, D.; Hubert, T. Long-Term Analgesia following a Single Application of Fentanyl Transdermal Solution in Pigs. Eur. Surg. Res. 2021, 62, 115–120. [Google Scholar] [CrossRef]
- Hofmeister, E.H.; Egger, C.M. Transdermal Fentanyl Patches in Small Animals. J. Am. Anim. Hosp. Assoc. 2004, 40, 468–478. [Google Scholar] [CrossRef]
- Jeal, W.; Benfield, P. Transdermal fentanyl. A review of its pharmacological properties and therapeutic efficacy in pain control. Drugs 1997, 53, 109–138. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, L.; Gigliuto, C.; de Gregori, M.; Malafoglia, V.; Raffaeli, W.; Compagnone, C.; Visai, L.; Petrini, P.; Avanzini, M.A.; Muscoli, C.; et al. Pain assessment in animal models: Do we need further studies? J. Pain Res. 2014, 7, 227–236. [Google Scholar] [CrossRef]
- Carregaro, A.B.; Luna, S.P.L.; Mataqueiro, M.I.; de Queiroz-Neto, A. Effects of buprenorphine on nociception and spontaneous locomotor activity in horses. Am. J. Vet. Res. 2007, 68, 246–250. [Google Scholar] [CrossRef]
- Harkins, J.D.; Queiroz-Neto, A.; Mundy, G.D.; West, D.; Tobin, T. Development and characterization of an equine behaviour chamber and the effects of amitraz and detomidine on spontaneous locomotor activity. J. Vet. Pharmacol. Ther. 1997, 20, 396–401. [Google Scholar] [CrossRef]
- Mama, K.R.; Pascoe, P.J.; Steffey, E.P. Evaluation of the interaction of mu and kappa opioid agonists on locomotor behavior in the horse. Can. J. Vet. Res. 1993, 57, 106–109. [Google Scholar]
- Davidson, C.D.; Pettifer, G.R.; Henry, J.D., Jr. Plasma fentanyl concentrations and analgesic effects during full or partial exposure to transdermal fentanyl patches in cats. J. Am. Vet. Med. Assoc. 2004, 224, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Pavlovsky, V.H.; Corona, D.; Hug, P.J.; Kummerlen, D.; Graage, R.; Bettschart-Wolfensberger, R. Butorphanol induces anxiety-like -behaviour and distress in piglets. Schweiz. Arch. Tierheilkd. 2021, 163, 485–491. [Google Scholar] [CrossRef]
- Tao, R.; Karnik, M.; Ma, Z.; Auerbach, S.B. Effect of fentanyl on 5-HT efflux involves both opioid and 5-HT1A receptors. Br. J. Pharmacol. 2003, 139, 1498–1504. [Google Scholar] [CrossRef]
- Kitamura, S.; Kawano, T.; Kaminaga, S.; Yamanaka, D.; Tateiwa, H.; Locatelli, F.M.; Yokoyama, M. Effects of fentanyl on serotonin syndrome-like behaviors in rats. J. Anesth. 2016, 30, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Gillman, P.K. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br. J. Anaesth. 2005, 95, 434–441. [Google Scholar] [CrossRef]
- Baldo, B.A. Opioid analgesic drugs and serotonin toxicity (syndrome): Mechanisms, animal models, and links to clinical effects. Arch. Toxicol. 2018, 92, 2457–2473. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A.; Rose, M.A. The anaesthetist, opioid analgesic drugs, and serotonin toxicity: A mechanistic and clinical review. Br. J. Anaesth. 2020, 124, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T. The role of the serotonergic system in motor control. Neurosci. Res. 2018, 129, 32–39. [Google Scholar] [CrossRef]
- Perrier, J.F.; Delgado-Lezama, R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J. Neurosci. 2005, 25, 7993–7999. [Google Scholar] [CrossRef]
- Ryden, A.; Manell, E.; Biglarnia, A.; Hedenqvist, P.; Strandberg, G.; Ley, C.; Hansson, K.; Nyman, G.; Jensen-Waern, M. Nursing and training of pigs used in renal transplantation studies. Lab. Anim. 2020, 54, 469–478. [Google Scholar] [CrossRef]
- Lervik, A.; Raszplewicz, J.; Ranheim, B.; Solbak, S.; Toverud, S.F.; Haga, H.A. Dexmedetomidine or fentanyl? Cardiovascular stability and analgesia during propofol-ketamine total intravenous anaesthesia in experimental pigs. Vet. Anaesth. Analg. 2018, 45, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Calis, K.A.; Kohler, D.R.; Corso, D.M. Transdermally administered fentanyl for pain management. Clin. Pharm. 1992, 11, 22–36. [Google Scholar] [PubMed]
- Loscher, W.; Witte, U.; Fredow, G.; Ganter, M.; Bickhardt, K. Pharmacodynamic effects of serotonin (5-HT) receptor ligands in pigs: Stimulation of 5-HT2 receptors induces malignant hyperthermia. Naunyn Schmiedebergs Arch. Pharmacol. 1990, 341, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Weinger, M.B.; Cline, E.J.; Smith, N.T.; Koob, G.F. Ketanserin pretreatment reverses alfentanil-induced muscle rigidity. Anesthesiology 1987, 67, 348–354. [Google Scholar] [CrossRef]
- Hauser, D.S.; Mevissen, M.; Weiss, R.; Portier, C.J.; Scholtysik, G.; Studer, U.E.; Danuser, H. Effects of ketanserin and DOI on spontaneous and 5-HT-evoked peristalsis of the pig ureter in vivo. Br. J. Pharmacol. 2002, 135, 1026–1032. [Google Scholar] [CrossRef]
- Clutton, R.E. Opioid analgesia in horses. Vet. Clin. N. Am. Equine Pract. 2010, 26, 493–514. [Google Scholar] [CrossRef]
- Kamata, M.; Nagahama, S.; Kakishima, K.; Sasaki, N.; Nishimura, R. Comparison of behavioral effects of morphine and fentanyl in dogs and cats. J. Vet. Med. Sci. 2012, 74, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Knych, H.K.; Steffey, E.P.; Casbeer, H.C.; Mitchell, M.M. Disposition, behavioural and physiological effects of escalating doses of intravenously administered fentanyl to young foals. Equine Vet. J. 2015, 47, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Rickli, A.; Liakoni, E.; Hoener, M.C.; Liechti, M.E. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: Link to clinical reports of serotonin syndrome. Br. J. Pharmacol. 2018, 175, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Heckman, C.J.; Enoka, R.M. Motor unit. Compr. Physiol. 2012, 2, 2629–2682. [Google Scholar] [CrossRef] [PubMed]
- Heckman, C.J.; Johnson, M.; Mottram, C.; Schuster, J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 2008, 14, 264–275. [Google Scholar] [CrossRef]
- DuPre, A.; Teitler, M. Ketanserin. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–16. [Google Scholar]
- Perrier, J.F.; Cotel, F. Serotonergic modulation of spinal motor control. Curr. Opin. Neurobiol. 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Dunbar, M.J.; Tran, M.A.; Whelan, P.J. Endogenous extracellular serotonin modulates the spinal locomotor network of the neonatal mouse. J. Physiol. 2010, 588, 139–156. [Google Scholar] [CrossRef]
- van der Starre, P.J.; Reneman, R.S. The alpha-adrenergic receptor blocking effect of ketanserin and the interaction between alpha-adrenergic and S2-serotonergic receptor blockade. J. Cardiovasc. Pharmacol. 1988, 11 (Suppl. 1), S54–S61. [Google Scholar] [PubMed]
- Janssen, P.A. Pharmacology of potent and selective S2-serotonergic antagonists. J. Cardiovasc. Pharmacol. 1985, 7 (Suppl. 7), S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Digranes, N.; Hoeberg, M.; Lervik, A.; Nordgreen, J.; Haga, H.A. Serotonin Antagonist Ketanserin Reverse Fentanyl Induced Shivering in Pigs. 2023; unpublished manuscript. [Google Scholar]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 2013, 109, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, J.K.; Blackshaw, A.W.; McGlone, J.J. Startle-Freeze Behavior in Weaned Pigs. Int. J. Comp. Psychol. 1998, 11. [Google Scholar] [CrossRef]
- Carregaro, A.B.; Neto, F.J.; Beier, S.L.; Luna, S.P. Cardiopulmonary effects of buprenorphine in horses. Am. J. Vet. Res. 2006, 67, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Posner, L.P.; Pavuk, A.A.; Rokshar, J.L.; Carter, J.E.; Levine, J.F. Effects of opioids and anesthetic drugs on body temperature in cats. Vet. Anaesth. Analg. 2010, 37, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.R.; Figueroa, C.D.; Choma, J.C.; Campagnol, D.; Bettini, C.M. Effects of methadone, alone or in combination with acepromazine or xylazine, on sedation and physiologic values in dogs. Vet. Anaesth. Analg. 2008, 35, 519–527. [Google Scholar] [CrossRef]
- Adler, M.W.; Geller, E.B.; Rosow, C.E.; Cochin, J. The opioid system and temperature regulation. Annu. Rev. Pharmacol. Toxicol. 1988, 28, 429–449. [Google Scholar] [CrossRef]
- Held, S.; Spinka, M. Animal Play and Animal Welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Pascoe, P.J.; Taylor, P.M. Effects of dopamine antagonists on alfentanil-induced locomotor activity in horses. Vet. Anaesth. Analg. 2003, 30, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto-Hardman, B.D.; Steffey, E.P.; Weiner, D.; McKemie, D.S.; Kass, P.; Knych, H.K. Pharmacokinetics and selected pharmacodynamics of morphine and its active metabolites in horses after intravenous administration of four doses. J. Vet. Pharmacol. Ther. 2019, 42, 401–410. [Google Scholar] [CrossRef]
- KuKanich, B.; Wiese, J.A. Opioids. In Lumb and Jones’ Veterinary Anesthesia and Analgesia, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Garofalo, N.A.; Teixeira Neto, F.J.; Pereira, C.D.; Pignaton, W.; Vicente, F.; Alvaides, R.K. Cardiorespiratory and neuroendocrine changes induced by methadone in conscious and in isoflurane anaesthetised dogs. Vet. J. 2012, 194, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.B.; Cotes, L.C.; Kahvegian, M.A.P.; Rizzo, M.F.C.I.; Otsuki, D.A.; Ferrigno, C.R.A.; Fantoni, D.T. Evaluation of the effects of methadone and tramadol on postoperative analgesia and serum interleukin-6 in dogs undergoing orthopaedic surgery. BMC Vet. Res. 2014, 10, 194. [Google Scholar] [CrossRef]
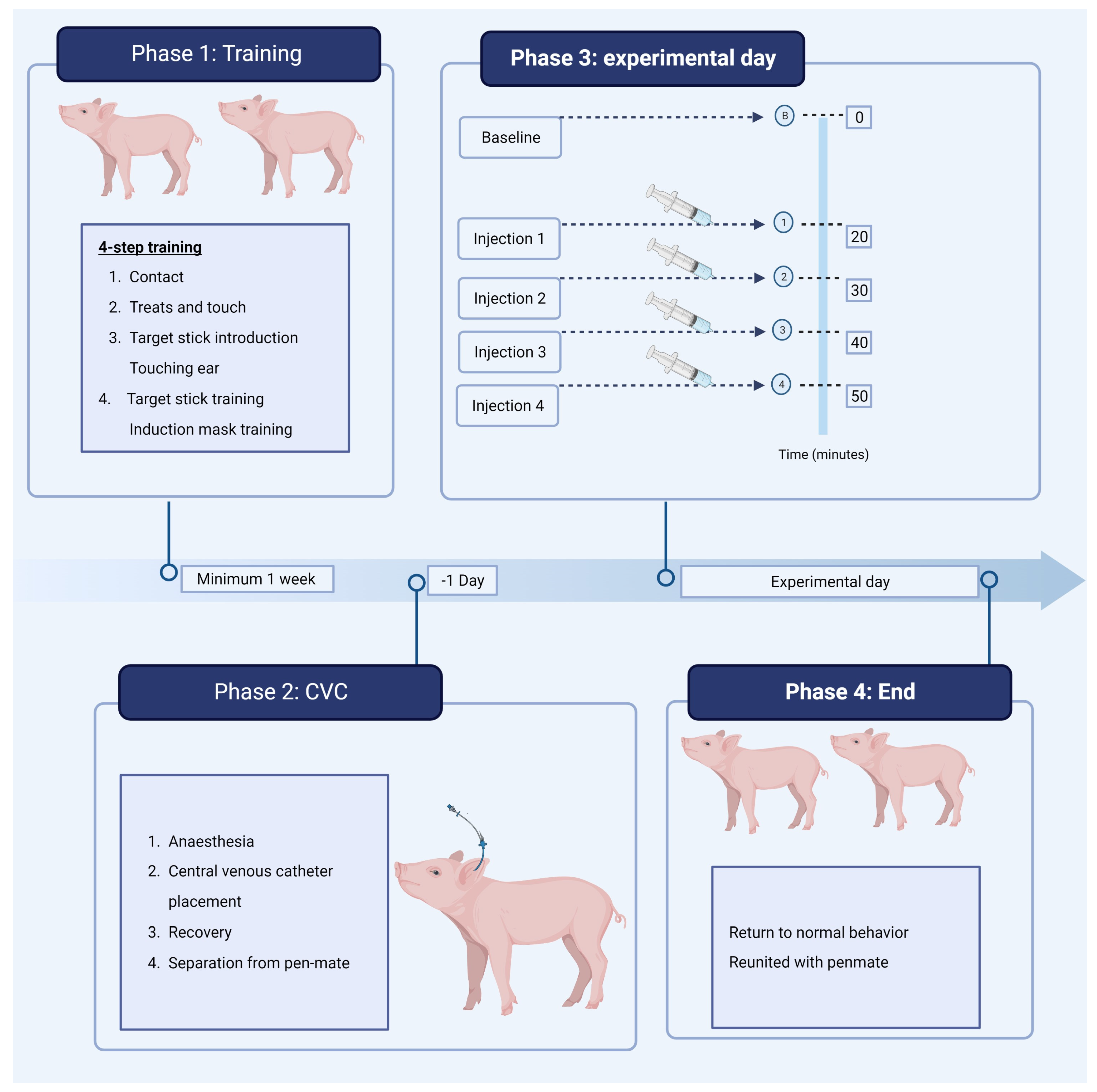




| Steps | Trainer Intervention |
|---|---|
| Step 1 | Trainer enters the pen area. Pigs are allowed to adapt to human presence. Trainer sits in the pen without interacting with the pigs. |
| Step 2 | Introduction of treats (apples, grapes, and Norwegian unleavened bread). Tossing treats in the pen, followed by providing treats by the hand of the trainer. Touching the back, ears and the head of pig while providing treats. Talking to the pigs. |
| Step 3 | Introduction to target stick by positive reinforcement rewarded by treat when touching the target. |
| Step 4 | Training pigs with target stick to stand still, touching their ears, and move within the animal experimental facility and into the transport trolly. Accustom pigs to facemask with positive reinforcement. |
| Phase Experimental Group | Baseline | Injection 1 | Injection 2 | Injection 3 | Injection 4 |
|---|---|---|---|---|---|
| Fentanyl-ketanserin (n = 5) | - | Fentanyl 5 µg/kg | Fentanyl 10 µg/kg | Ketanserin 1 mg/kg | Saline |
| Fentanyl-saline (n = 5) | - | Fentanyl 5 µg/kg | Fentanyl 10 µg/kg | Saline | Ketanserin 1 mg/kg |
| Saline-saline (n = 4) | - | Saline | Saline | Saline | Saline |
| Behavior | Description | Outcome Variables (per 10 min) |
|---|---|---|
| Normal gait-posture | Normal gait and posture. Normal balance and length of steps. | Duration (s) |
| Stiff gait-posture | Small movements of limbs and body with stiff short steps. Extended legs. “Stepping gait”. | Duration (s) |
| Ataxic gait-posture | Ataxic gait-posture, with reduced control of hindlegs. | Duration (s) |
| Lateral recumbency | Lateral recumbency | Duration (s) |
| Sternal recumbency | Sternal recumbency with head down or up | Duration (s) |
| Rooting behavior | Exploring peat or floor with snout in-ground | Duration (s) |
| Play | Playful running, jumping (vertical and horizontal bouncy movements) and rolling | Duration (s) |
| Backward locomotion | Walking backwards | Duration (s) |
| Circling | Circling around hindquarters | Duration (s) |
| Sitting | Sitting with the rear end of the body in contact with the ground. | Duration (s) |
| Water nipple | Spilling or drinking water | Frequency |
| Freeze | Fixed body posture with a stiff, extended neck, while staring right ahead | Frequency |
| Jumping on wall | Attempting to or successfully jumping on wall | Frequency |
| Undefined | Behaviors not described further such as scratching, rolling in water, defecation/urination | Duration (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Digranes, N.; Haga, H.A.; Nordgreen, J. High and Hyper: Fentanyl Induces Psychomotor Side-Effects in Healthy Pigs. Animals 2023, 13, 1671. https://doi.org/10.3390/ani13101671
Digranes N, Haga HA, Nordgreen J. High and Hyper: Fentanyl Induces Psychomotor Side-Effects in Healthy Pigs. Animals. 2023; 13(10):1671. https://doi.org/10.3390/ani13101671
Chicago/Turabian StyleDigranes, Nora, Henning Andreas Haga, and Janicke Nordgreen. 2023. "High and Hyper: Fentanyl Induces Psychomotor Side-Effects in Healthy Pigs" Animals 13, no. 10: 1671. https://doi.org/10.3390/ani13101671
APA StyleDigranes, N., Haga, H. A., & Nordgreen, J. (2023). High and Hyper: Fentanyl Induces Psychomotor Side-Effects in Healthy Pigs. Animals, 13(10), 1671. https://doi.org/10.3390/ani13101671






