Genome-Wide Signal Selection Analysis Revealing Genes Potentially Related to Sheep-Milk-Production Traits
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Whole-Genome-Resequencing Analysis
2.3. Population Structures and Phylogenetic Analysis
2.4. Genome-Wide Signal-Selection Scan and Gene Annotation
2.5. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes
2.6. Validation of RT-qPCR Experiment
2.7. The Relationship between Gene Expression and Milk Production
3. Results
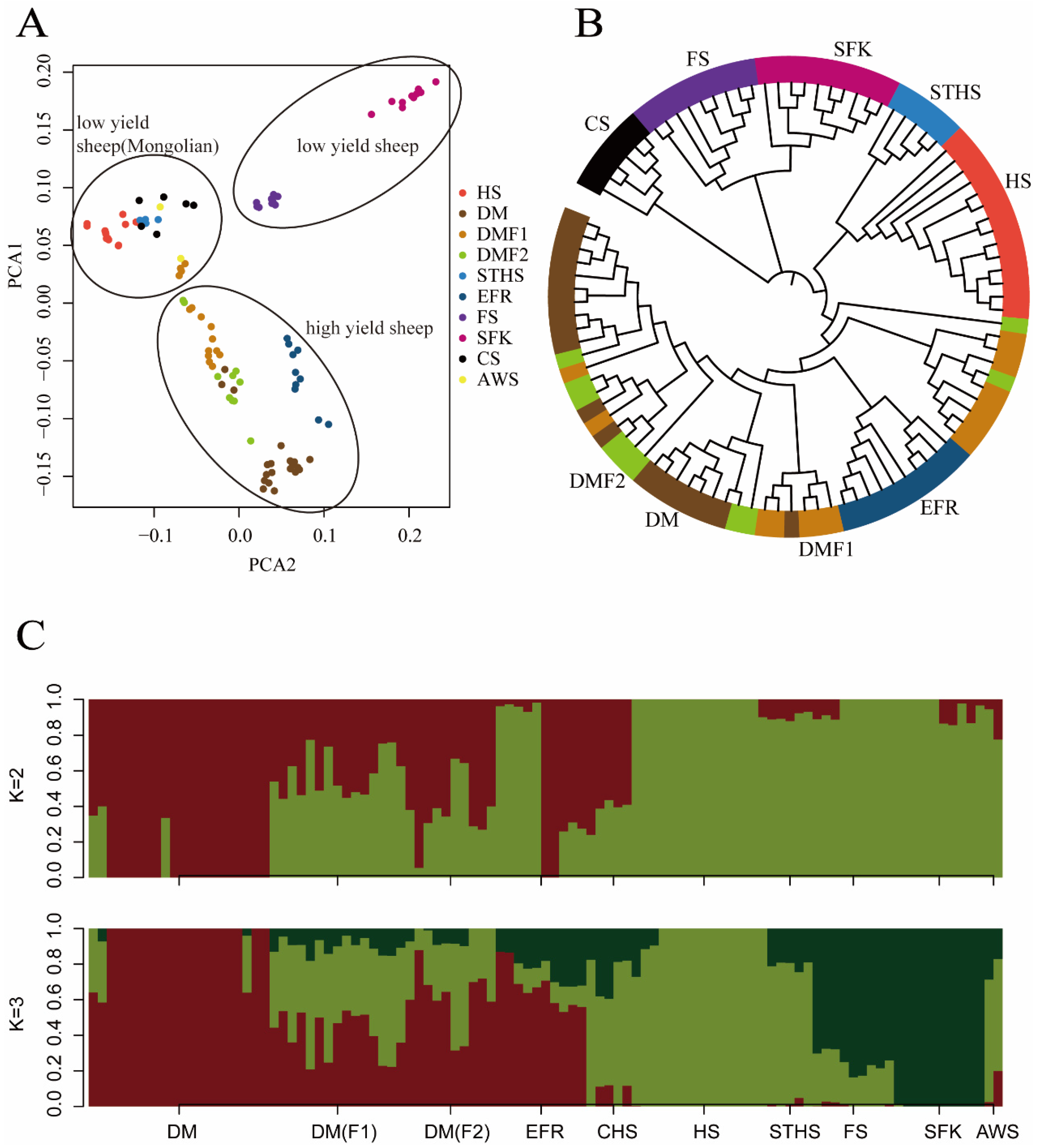
3.1. Genomic Variants and Principal Component Analysis
3.2. Neighbor-Joining-Tree Analysis
3.3. Ancestor Analysis
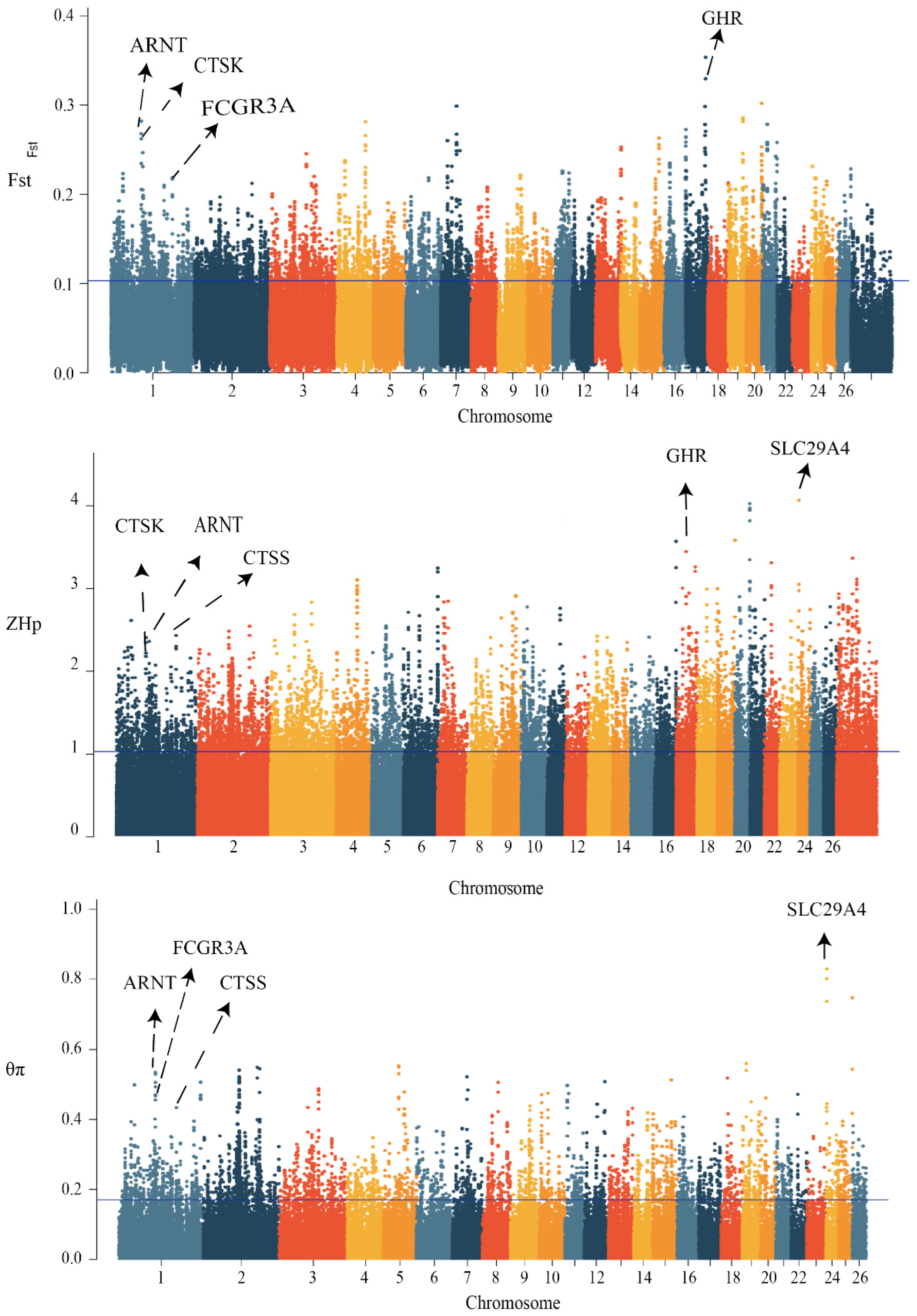
3.4. Signal-Selection Analysis and Gene Ontology
3.5. RT-qPCR Results
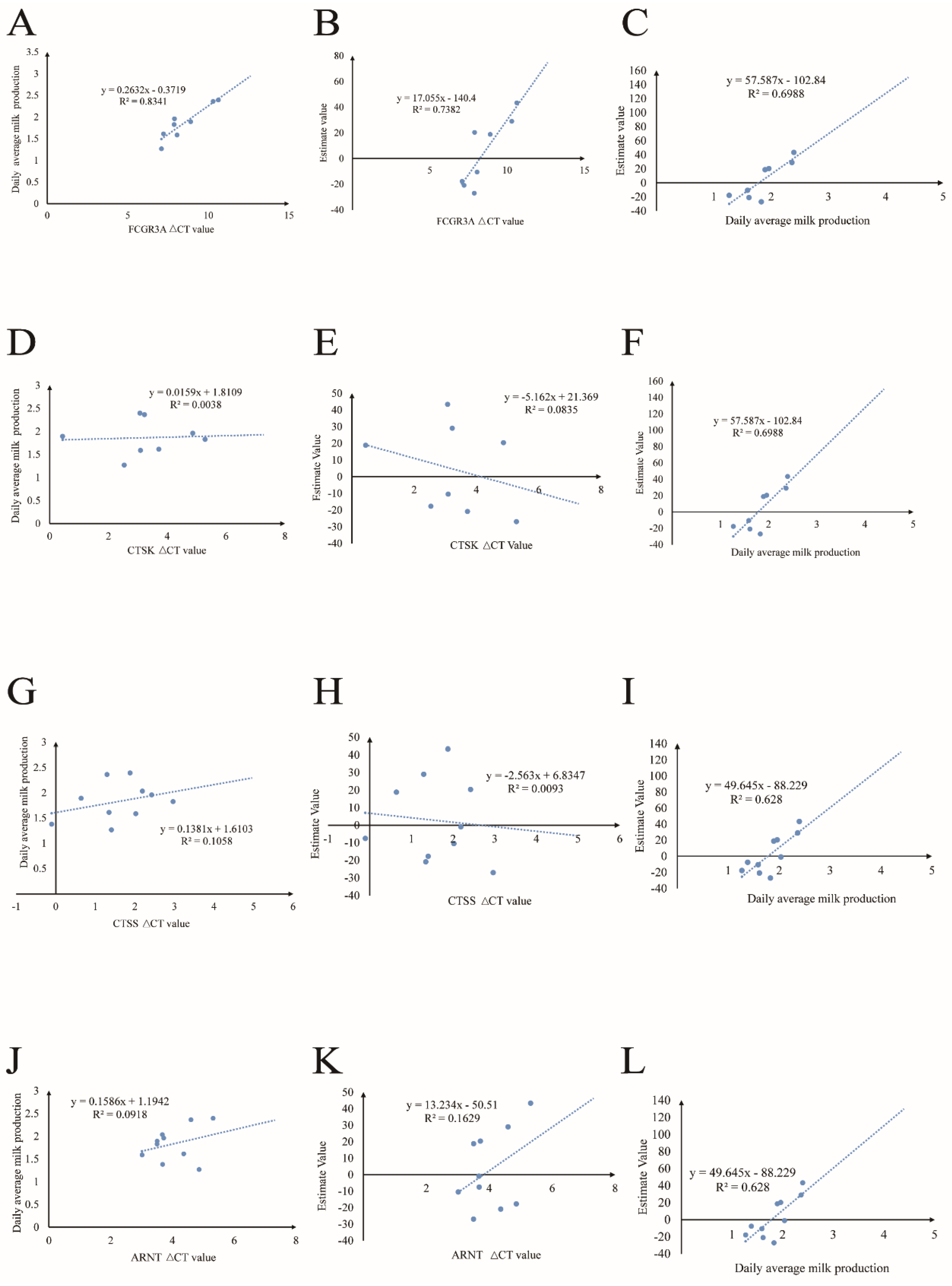
3.6. The Relationship between Gene Expression and Milk Production
4. Discussion
Candidate Genes Potentially Associated with Some Milk Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, S.F.; Mehrotra, A.; Charles, S.; Ganai, N.A. Analysis of selection signatures reveals important insights into the adaptability of high-altitude Indian sheep breed Changthangi. Gene. 2021, 799, 145809. [Google Scholar] [CrossRef] [PubMed]
- Zygoyiannis, D. Sheep production in the world and in Greece. Small Rumin. Res. 2006, 62, 143–147. [Google Scholar] [CrossRef]
- Seykora, A.J.; McDaniel, B.T. Genetics statistics and relationships of teat and udder traits, somatic cell counts, and milk production. J. Dairy Sci. 1986, 69, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Champion, F.; Dockes, A.; Lagriffoul, G.; Mottet, A.; Morin, E.; Neumeistier, D.; Perrot, C. Bergers Demain en Brebis Laitières; études sur la production ovine laitière à l’horizon 2020; Éléments de diagnostic et propositions d’actions; Collection Résultats, Institut de l’Elevage: Paris, France, 2013; pp. 7–47. [Google Scholar]
- Correddu, F.; Gaspa, G.; Cesarani, A.; Macciotta, N.P.P. Phenotypic and genetic characterization of the occurrence of noncoagulating milk in dairy sheep. J. Dairy Sci. 2022, 105, 6773–6782. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valladares, M.; Martín-Ramos, E.; Esteban-Ballesteros, M.; Balaña-Fouce, R.; Rojo-Vázquez, F.A. Effect of level of infection by gastrointestinal nematodes and anthelmintic treatment on milk yield in dairy sheep. Parasite 2021, 28, 71. [Google Scholar] [CrossRef]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef]
- Jandal, J.M. Comparative aspects of goat and sheep milk. Small Rumin. Res. 1996, 22, 177–185. [Google Scholar] [CrossRef]
- Davies, G.; Genini, S.; Bishop, S.C.; Giuffra, E. An assessment of opportunities to dissect host genetic variation in resistance to infectious diseases in livestock. Animals 2009, 3, 415–436. [Google Scholar] [CrossRef]
- Merz, A.; Stephan, R.; Johler, S. Staphylococcus aureus Isolates from Goat and Sheep Milk Seem to Be Closely Related and Differ from Isolates Detected from Bovine Milk. Front. Microbiol. 2016, 7, 319. [Google Scholar] [CrossRef]
- Sutera, A.M.; Portolano, B.; Gerlando, R.D.; Sardina, M.T.; Mastrangelo, S.; Tolone, M. Determination of milk production losses and variations of fat and protein percentages according to different levels of somatic cell count in Valle del Belice dairy sheep. Small Rumin. Res. 2018, 162, 39–42. [Google Scholar] [CrossRef]
- Banos, G.; Bramis, G.; Bush, S.J.; Clark, E.L.; McCulloch, M.E.B.; Smith, J.; Schulze, G.; Arsenos, G.; Hume, D.A.; Psifidi, A. The genomic architecture of mastitis resistance in dairy sheep. BMC Genom. 2017, 18, 624. [Google Scholar] [CrossRef] [PubMed]
- Sutera, A.M.; Moscarelli, A.; Mastrangelo, S.; Sardina, M.T.; Tolone, M. Genome-Wide Association Study Identifies New Candidate Markers for Somatic Cells Score in a Local Dairy Sheep. Front. Genet. 2021, 12, 643531. [Google Scholar] [CrossRef]
- Sutera, R.; Mastrangelo, D. Gerlando Genome-wide association studies for milk production traits in Valle del Belice sheep using repeated measures. Anim. Genet. 2019, 50, 311–314. [Google Scholar] [CrossRef]
- Di Gerlando, R.; Sutera, A.M.; Mastrangelo, S.; Tolone, M.; Portolano, B.; Sottile, G.; Bagnato, A.; Strillacci, M.G.; Sardina, M.T.; Davoli, R. Genome-wide association study between CNVs and milk production traits in Valle del Belice sheep. PLoS ONE 2019, 14, e0215204. [Google Scholar] [CrossRef] [PubMed]
- Marina, H.; Gutiérrez-Gil, B.; Esteban-Blanco, C.; Suárez-Vega, A.; Pelayo, R.; Arranz, J.J. Analysis of Whole Genome Resequencing Datasets from a Worldwide Sample of Sheep Breeds to Identify Potential Causal Mutations Influencing Milk Composition Traits. Animals 2020, 10, 1542. [Google Scholar] [CrossRef] [PubMed]
- Bai, U.; Su, X.; Zheng, Z.; Zhang, L.; Ma, Y.; Dou, Y.; Zhang, X.; Su, G.; Li, G.; Zhang, L. Comparative metabolomics analysis of Small-Tailed Han and DairyMeade ovine milk. Eur. Food Res. Technol. 2021, 247, 2869–2876. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, Z.; Zhang, L.; Dou, Y.; Bai, U.; Liu, X.; Zhang, X.; Su, X.; Zhang, L. Effects Study of Early Weaning Time on the Growth and Development of Dairy Sheep Lamb. Int. J. Food Sci. Agric. 2021, 247, 2869–2876. [Google Scholar] [CrossRef]
- Su, X.; Zheng, Z.; Zhang, L.; Bai, U.; Ma, Y.; Dou, Y.; Zhang, X.; Su, G.; Zhou, N.; Li, G.; et al. Proteomic profiling of ovine milk after grading up. J. Dairy Res. 2021, 88, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, L.; Wang, W.; Zhang, D.; Zhao, Y.; Chen, J.; Xu, D.; Zhao, L.; Li, F.; Zhang, X. Whole genome re-sequencing reveals artificial and natural selection for milk traits in East Friesian sheep. Front. Vet. Sci. 2022, 9, 1034211. [Google Scholar] [CrossRef]
- McKusick, B.; Thomas, D.; Berger, Y. Effect of weaning system on commercial milk production and lamb growth of East Friesian dairy sheep. J. Dairy Sci. 2001, 84, 1660–1668. [Google Scholar] [CrossRef]
- Talafha, A.Q.; Ababneh, M.M. Awassi sheep reproduction and milk production. Trop. Anim. Health Prod. 2011, 43, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huitong, Z.; Hickford, J.G.H.; Hao, Z.; Shen, J.; Luo, Y.; Hu, J.; Liu, X.; Li, S. Comparison of the transcriptome of the ovine mammary gland in lactating and non-lactating small-tailed han sheep. Front. Genet. 2020, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gil, B.; El-Zarei, M.F.; Alvarez, L.; Bayón, Y.; De La Fuente, L.F.; San Primitivo, F.; Arranz, J.-J. Quantitative trait loci underlying milk production traits in sheep. Anim. Gen. 2009, 40, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Yue, G. Reproductive characteristics of Chinese Hu sheep. Anim. Reprod. Sci. 1996, 44, 223–230. [Google Scholar] [CrossRef]
- Hao, Z.; Zhou, H.; Hickford, J.G.H.; Gong, H.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Zhao, M.; Luo, Y. Identification and characterization of circular RNA in lactating mammary glands from two breeds of sheep with different milk production profiles using RNA-Seq. Genomics. 2020, 112, 2186–2193. [Google Scholar] [CrossRef]
- Slen, S.; Clark, R.; Hironaka, R. A comparison of milk production and its relation to lamb growth in five breeds of sheep. Can. J. Anim. Sci. 1963, 43, 16–21. [Google Scholar] [CrossRef]
- Maijala, K. Productivity of pure Finnsheep in Finland and abroad. Livest. Prod. Sci. 1977, 4, 355–377. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Shen, M.; Xie, X.L.; Liu, G.J.; Xu, Y.X.; Lv, F.H.; Yang, H.; Yang, Y.L.; Liu, C.B.; et al. Whole-genome resequencing of wild and domestic sheep identifies genes associated with morphological and agronomic traits. Nat. Commun. 2020, 11, 2815. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.F.; Truong, S.K.; Mullet, J.E. RIG: Recalibration and interrelation of genomic sequence data with the GATK. G3 (Bethesda Md.) 2015, 5, 655–665. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, Y.; Wang, Z.; Gou, Z.; Lyu, J.; Li, W.; Yu, Y.; Shu, L.; Zhao, Y.; Ma, Y.; et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Karyadi, D.M.; Hartley, S.W.; Frone, M.N.; Sampson, J.N.; Howell, R.M.; Neglia, J.P.; Arnold, M.A.; Hicks, B.D.; Jones, K.; et al. Subsequent Neoplasm Risk Associated With Rare Variants in DNA Damage Response and Clinical Radiation Sensitivity Syndrome Genes in the Childhood Cancer Survivor Study. JCO Precis Oncol. 2020, 4, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef]
- Shriner, D. Overview of Admixture Mapping. Curr. Protoc. Hum. Genet. 2017, 94, 1.23.21–21.23.28. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP: Phylogeny Inference Package Version 3.68; Department of Genome Sciences and Department of Biology, University of Washington: Seattle, WA, USA, 2008. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- GX, E.; Basang, W.D.; Zhu, Y.B. Whole-genome analysis identifying candidate genes of altitude adaptive ecological thresholds in yak populations. J. Anim. Breed Genet. 2019, 136, 371–377. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Ferreira, A.M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, X.; Wang, L.; Zhang, L.; Li, G.; Zheng, Z. GLIS1, a potential candidate gene affect fat deposition in sheep tail. Mol. Biol. Rep. 2021, 48, 4925–4931. [Google Scholar] [CrossRef]
- Suárez-Vega, A.; Gutiérrez-Gil, B.; Klopp, C.; Robert-Granie, C.; Tosser-Klopp, G.; Arranz, J.J. Characterization and Comparative Analysis of the Milk Transcriptome in Two Dairy Sheep Breeds using RNA Sequencing. Sci. Rep. 2015, 5, 18399. [Google Scholar] [CrossRef]
- Li, C.; Cai, W.; Zhou, C.; Yin, H.; Zhang, Z.; Loor, J.J.; Sun, D.; Zhang, Q.; Liu, J.; Zhang, S. RNA-Seq reveals 10 novel promising candidate genes affecting milk protein concentration in the Chinese Holstein population. Sci. Rep. 2016, 6, 26813. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, L.; Augustino, S.M.; Duan, T.; Hall, T.J.; MacHugh, D.E.; Dou, J.; Zhang, Y.; Wang, Y.; Yu, Y. Identification of novel molecular markers of mastitis caused by Staphylococcus aureus using gene expression profiling in two consecutive generations of Chinese Holstein dairy cattle. J. Anim. Sci. Biotechnol. 2020, 11, 98. [Google Scholar] [CrossRef]
- Seroussi, E.; Blum, S.E.; Krifucks, O.; Shirak, A.; Jacoby, S.; Leitner, G. Basal levels of CD18 antigen presenting cells in cow milk associate with copy number variation of Fc gamma receptors. Genes 2020, 11, 952. [Google Scholar] [CrossRef]
- Sodhi, M.; Sharma, M.; Sharma, A.; Verma, P.; Mohanty, A.; Kataria, R.S.; Shandilya, U.K.; Kumari, P.; Mukesh, M. Expression profile of different classes of proteases in milk derived somatic cells across different lactation stages of indigenous cows (Bos indicus) and riverine buffaloes (Bubalus bubalis). Anim. Biotechnol. 2023, 34, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Seol, M.B.; Bong, J.J.; Baik, M. Involvement of Cathepsin D in Apoptosis of Mammary Epithelial Cells. Asian-Australas. J. Anim. Sci. 2006, 19, 1100–1105. [Google Scholar] [CrossRef]
- Suárez-Vega, A.; Gutiérrez-Gil, B.; Arranz, J.J. Transcriptome expression analysis of candidate milk genes affecting cheese-related traits in 2 sheep breeds. J. Dairy Sci. 2016, 99, 6381–6390. [Google Scholar] [CrossRef]
- Wysolmerski, J.J. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone 2013, 54, 230–236. [Google Scholar] [CrossRef]
- Lotinun, S.; Ishihara, Y.; Nagano, K.; Kiviranta, R.; Carpentier, V.T.; Neff, L.; Parkman, V.; Ide, N.; Hu, D.; Dann, P. Cathepsin K–deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J. Clin. Investig. 2019, 129, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Tijet, N.; Boutros, P.C.; Moffat, I.D.; Okey, A.B.; Tuomisto, J.; Pohjanvirta, R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol. Pharm. 2006, 69, 140–153. [Google Scholar] [CrossRef]
- Girolami, F.; Spalenza, V.; Carletti, M.; Sacchi, P.; Rasero, R.; Nebbia, C. Modulation of aryl hydrocarbon receptor target genes in circulating lymphocytes from dairy cows bred in a dioxin-like PCB contaminated area. Sci. Total Environ. 2013, 450, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Basham, K.J.; Leonard, C.J.; Kieffer, C.; Shelton, D.N.; McDowell, M.E.; Bhonde, V.R.; Looper, R.E.; Welm, B.E. Dioxin exposure blocks lactation through a direct effect on mammary epithelial cells mediated by the aryl hydrocarbon receptor repressor. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 143, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.L.; Lew, B.J.; Lawrence, B.P. TCDD exposure disrupts mammary epithelial cell differentiation and function. Reprod. Toxicol. 2009, 28, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Vorderstrasse, B.A.; Fenton, S.E.; Bohn, A.A.; Cundiff, J.A.; Lawrence, B.P. A novel effect of dioxin: Exposure during pregnancy severely impairs mammary gland differentiation. Toxicol. Sci. Off. J. Soc. Toxicol. 2004, 78, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Dettori, M.L.; Pazzola, M.; Paschino, P.; Amills, M.; Vacca, G.M. Association between the GHR, GHRHR, and IGF1 gene polymorphisms and milk yield and quality traits in Sarda sheep. J. Dairy Sci. 2018, 101, 9978–9986. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Park, M.-J. Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 145. [Google Scholar] [CrossRef]

| Breed | Abbreviation | Number | Milk Yield per Lactation/kg | Data Source | Classification | Reference |
|---|---|---|---|---|---|---|
| Dairy Meade sheep | DM | 11 | 350–600 | M-Natural farm | High yield | [18,19] |
| Dairy Meade (F1) sheep | DMF1 | 15 | 350 | M-Natural farm | High yield | [18,20] |
| Dairy Meade (F2) sheep | DMF2 | 10 | 500 | M-Natural farm | High yield | [18,20] |
| East Friesian sheep | EFR | 10 | 500–700 | NCBI/ PRJNA624020 | High yield | [21,22] |
| Dairy Meade sheep | DM | 9 | 500 | NCBI/ PRJNA624020 | High yield | [19] |
| Awassi sheep | AWS | 2 | 300–500 | NCBI/ MW260509 | High yield | [22] |
| Small-tailed Han sheep | STHS | 5 | 100 | M-Natural farm | Low yield | [23] |
| Churra sheep | CS | 6 | 100–200 | NCBI/ PRJNA395499 | Low yield | [24] |
| Hu sheep | HS | 14 | 100–240 | NCBI/ PRJNA624020 | Low yield | [25,26] |
| Suffolk sheep | SFK | 10 | – | NCBI/ PRJNA624020 | Low yield | [27] |
| Finland sheep | FS | 9 | – | NCBI/ PRJNA624020 | Low yield | [28] |
| Gene | Primer Sequence | Length | Synthesis Scale | Format |
|---|---|---|---|---|
| FCGR3A-F * | CTTAGGACAAATGAAGGCTCTGA | 23 | 10 nmol | TE-100 um |
| FCGR3A-R * | CTGCCTCTCCACCACGAAT | 19 | 10 nmol | TE-100 um |
| ARNT-F | CAGGCCGGGTGGTATATGTC | 20 | 10 nmol | TE-100 um |
| ARNT-R | TGGAAAGCTGCTCACGAAGT | 20 | 10 nmol | TE-100 um |
| CTSS-F | AAGCTGGTGTCTCTGAGTGC | 20 | 10 nmol | TE-100 um |
| CTSS-R | CGCCATTGCAGCCCTTATTC | 20 | 10 nmol | TE-100 um |
| CTSK-F | ATGCAAGCCTGACCTCCTTC | 20 | 10 nmol | TE-100 um |
| CTSK-R | CCAGTTTCTCCCCAGCTGT | 21 | 10 nmol | TE-100 um |
| GAPDH-F | TCGGAGTGAACGGATTTGGC | 20 | 10 nmol | TE-100 um |
| GAPDH-R | CCGTTCTCTGCCTTGACTGT | 20 | 10 nmol | TE-100 um |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhao, Y.; Liang, B.; Pu, Y.; Jiang, L.; Ma, Y. Genome-Wide Signal Selection Analysis Revealing Genes Potentially Related to Sheep-Milk-Production Traits. Animals 2023, 13, 1654. https://doi.org/10.3390/ani13101654
Li R, Zhao Y, Liang B, Pu Y, Jiang L, Ma Y. Genome-Wide Signal Selection Analysis Revealing Genes Potentially Related to Sheep-Milk-Production Traits. Animals. 2023; 13(10):1654. https://doi.org/10.3390/ani13101654
Chicago/Turabian StyleLi, Ruonan, Yuhetian Zhao, Benmeng Liang, Yabin Pu, Lin Jiang, and Yuehui Ma. 2023. "Genome-Wide Signal Selection Analysis Revealing Genes Potentially Related to Sheep-Milk-Production Traits" Animals 13, no. 10: 1654. https://doi.org/10.3390/ani13101654
APA StyleLi, R., Zhao, Y., Liang, B., Pu, Y., Jiang, L., & Ma, Y. (2023). Genome-Wide Signal Selection Analysis Revealing Genes Potentially Related to Sheep-Milk-Production Traits. Animals, 13(10), 1654. https://doi.org/10.3390/ani13101654








