The Recovery Time between Early Mild Stress and Final Acute Stress Affects Survival Rate, Immunity, Health, and Physiology of Oscar (Astronotus ocellatus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and methods
2.1. Animal, Fish Ethic and Experimental Conditions
2.2. Diet Formulation and Experimental Design
2.3. Sample Collection and Growth Performance
2.4. Chemical Analysis of Diets and Fish Body Composition
2.4.1. Blood Collection and Sample Preparation
2.4.2. Hematology Profile
2.4.3. Blood Biochemistry, Antioxidant Enzymes Activities, Serum Enzymes and Cortisol
2.5. Non-Specific Immune Parameters
2.6. Acute Confinement Stress (AC Stress)
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Body Composition
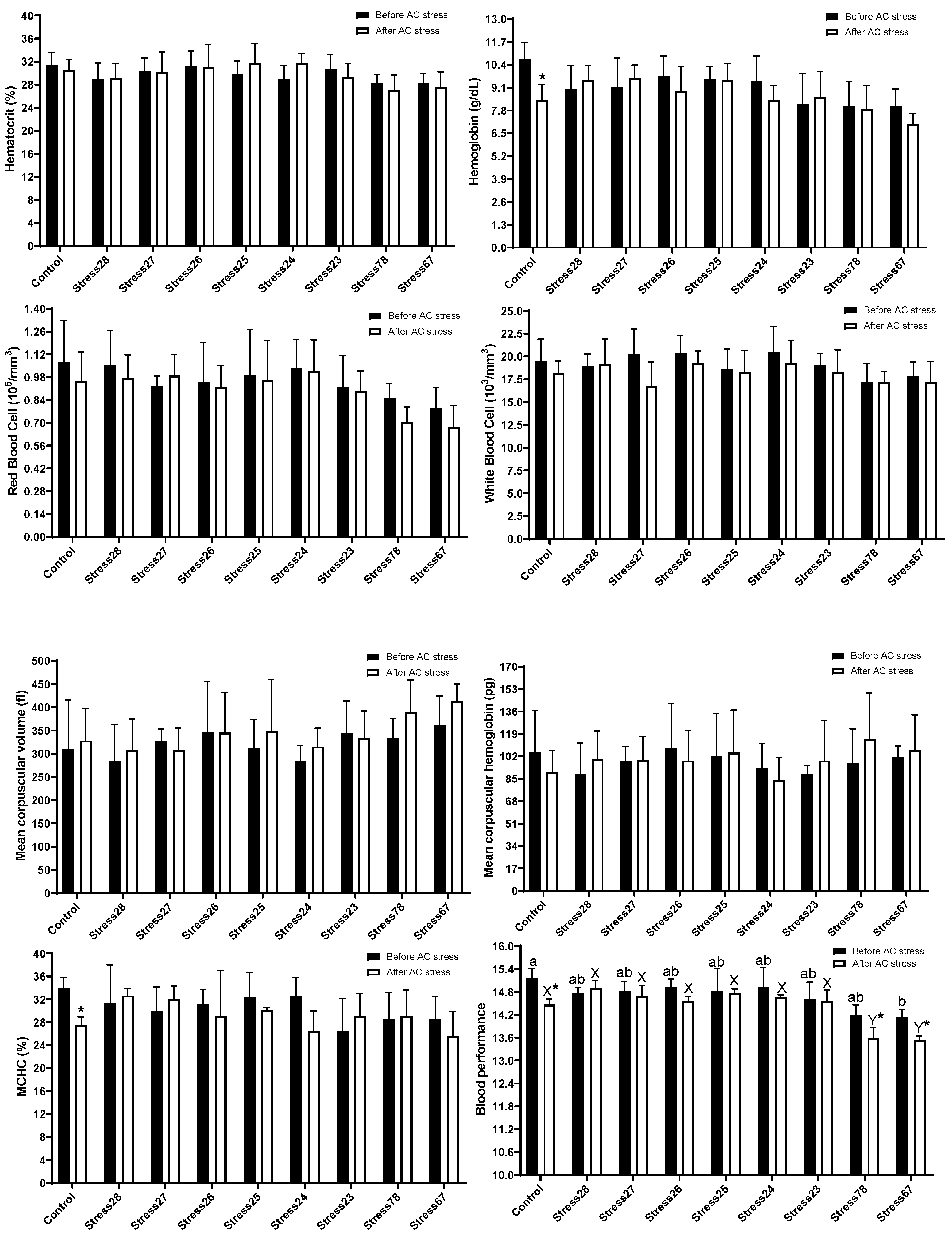
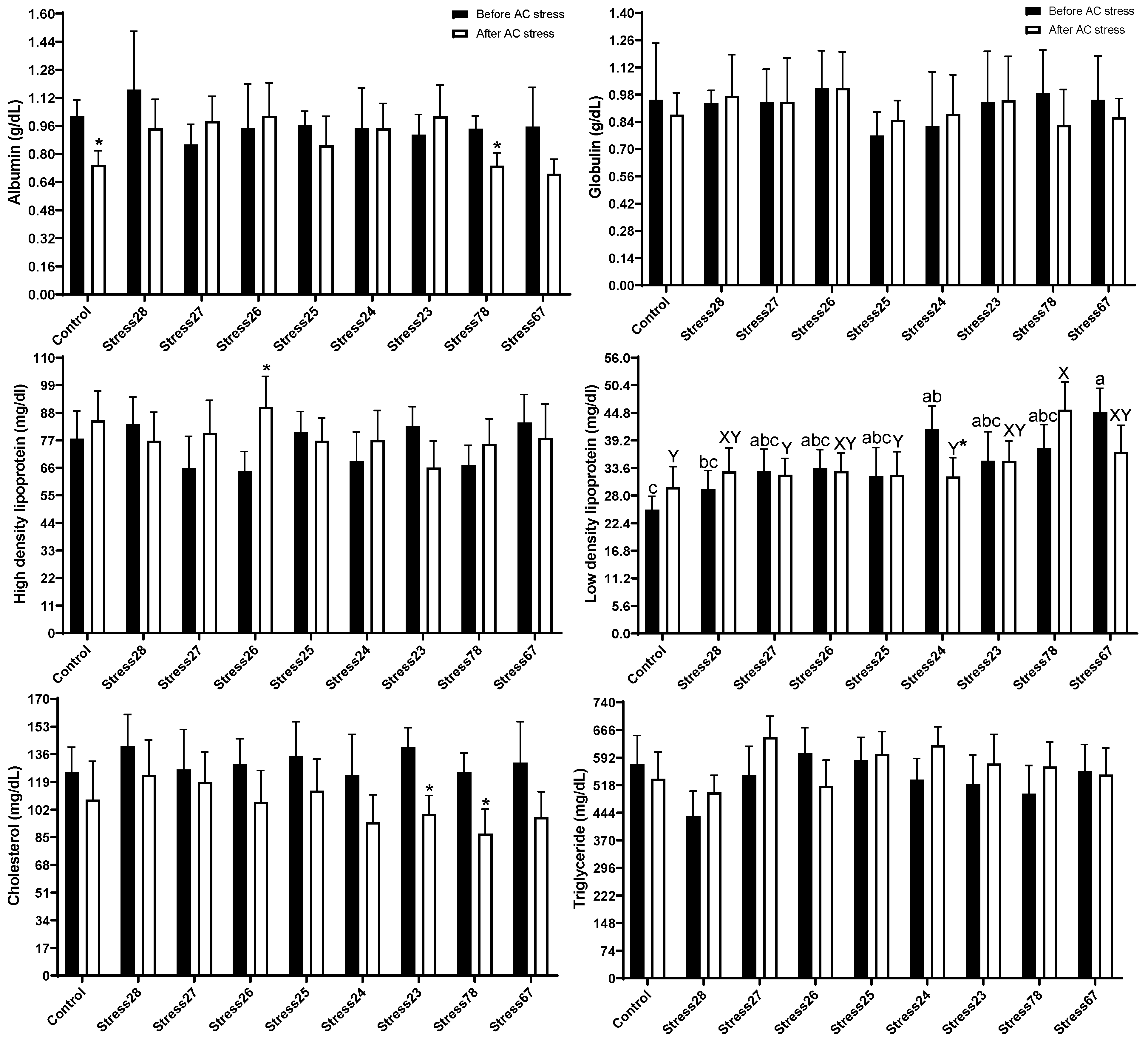
3.2. Haematology and Blood Biochemistry
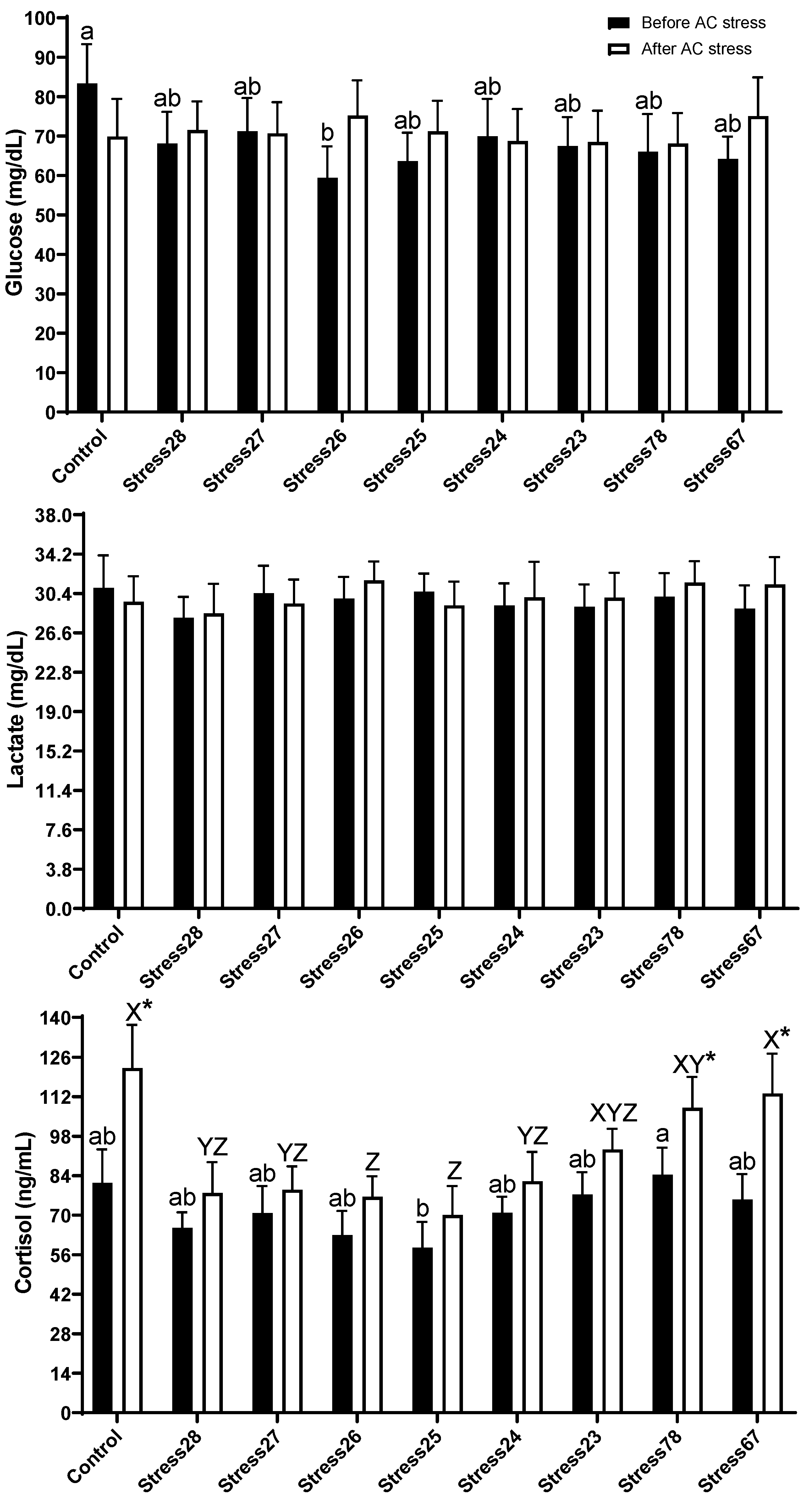
3.3. Immune and Stress Response
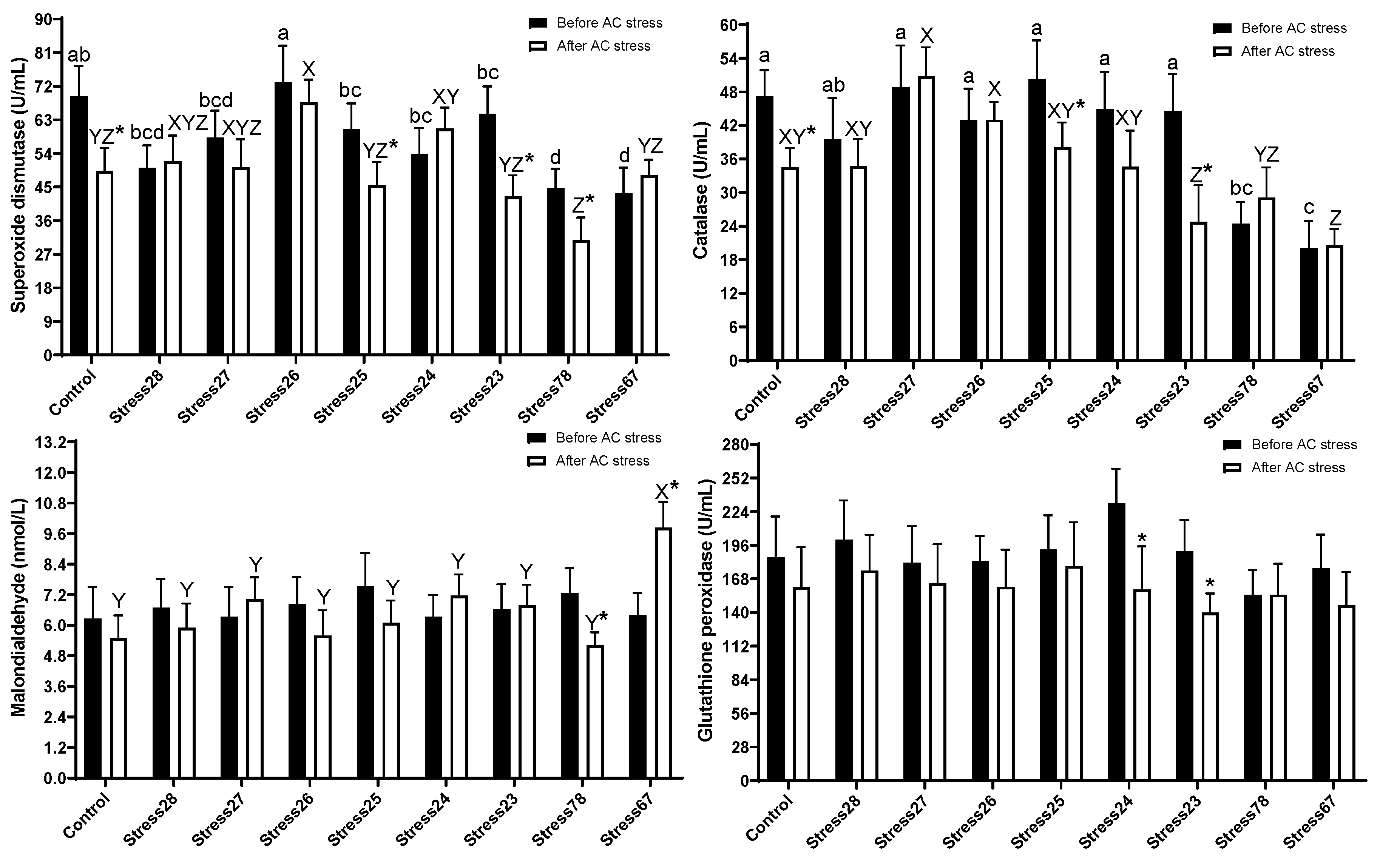
3.4. Antioxidant Enzymes Activities
3.5. Liver Enzymes
4. Discussion
4.1. Growth Performance and Body Composition
4.2. Haematology and Blood Biochemistry
4.3. Immune and Stress Response
4.4. Antioxidant Enzymes Activities
4.5. Liver Enzymes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosi Mobaraki, M.R.; Abedian Kenari, A.; Bahrami Gorji, S.; Esmaeili, M. Effect of dietary fish and vegetable oil on the growth performance, body composition, fatty acids profile, reproductive performance and larval resistance in pearl gourami (Trichogaster leeri). Aquac. Nutr. 2020, 26, 894–907. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Mancera, J.M.; Prunet, P.; Magnoni, L.J. Welfare and Stressors in Fish: Challenges Facing Aquaculture. Front. Media SA 2020, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 35, pp. 1–34. [Google Scholar]
- Esmaeili, M.; Hosseini, H.; Zare, M.; Sobhan, R.; Akhavan Rombenso, A. Early Mild Stress along with Lipid Improves the Stress Responsiveness of Oscar (Astronotus ocellatus). Aquac. Nutr. 2022, 2022, 8991678. [Google Scholar] [CrossRef]
- Pattanaik, S.S.; Sawant, P.B.; KA, M.X.; Srivastava, P.P.; Dube, K.; Sawant, B.T.; Chadha, N. Dietary carotenoprotien extracted from shrimp shell waste augments growth, feed utilisation, physio-metabolic responses and colouration in Oscar, Astronotus ocellatus (Agassiz, 1831). Aquaculture 2021, 534, 736303. [Google Scholar] [CrossRef]
- Wood, C.M.; Kajimura, M.; Sloman, K.A.; Scott, G.R.; Walsh, P.J.; Almeida-Val, V.M.; Val, A.L. Rapid regulation of Na+ fluxes and ammonia excretion in response to acute environmental hypoxia in the Amazonian oscar, Astronotus ocellatus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R2048–R2058. [Google Scholar] [CrossRef]
- Matey, V.; Iftikar, F.I.; De Boeck, G.; Scott, G.R.; Sloman, K.A.; Almeida-Val, V.M.; Val, A.L.; Wood, C.M. Gill morphology and acute hypoxia: Responses of mitochondria-rich, pavement, and mucous cells in the Amazonian oscar (Astronotus ocellatus) and the rainbow trout (Oncorhynchus mykiss), two species with very different approaches to the osmo-respiratory compromise. Can. J. Zool. 2011, 89, 307–324. [Google Scholar]
- De Boeck, G.; Wood, C.M.; Iftikar, F.I.; Matey, V.; Scott, G.R.; Sloman, K.A.; de Nazaré Paula da Silva, M.; Almeida-Val, V.M.; Val, A.L. Interactions between hypoxia tolerance and food deprivation in Amazonian oscars, Astronotus ocellatus. J. Exp. Biol. 2013, 216, 4590–4600. [Google Scholar] [CrossRef]
- Baptista, R.B.; Souza-Castro, N.; Almeida-Val, V.M.F. Acute hypoxia up-regulates HIF-1α and VEGF mRNA levels in Amazon hypoxia-tolerant Oscar (Astronotus ocellatus). Fish Physiol. Biochem. 2016, 42, 1307–1318. [Google Scholar] [CrossRef]
- Madaro, A.; Olsen, R.E.; Kristiansen, T.S.; Ebbesson, L.O.; Nilsen, T.O.; Flik, G.; Gorissen, M. Stress in Atlantic salmon: Response to unpredictable chronic stress. J. Exp. Biol. 2015, 218, 2538–2550. [Google Scholar] [CrossRef]
- Vindas, M.A.; Madaro, A.; Fraser, T.W.; Höglund, E.; Olsen, R.E.; Øverli, Ø.; Kristiansen, T.S. Coping with a changing environment: The effects of early life stress. R. Soc. Open Sci. 2016, 3, 160382. [Google Scholar] [CrossRef]
- Auperin, B.; Geslin, M. Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen. Comp. Endocrinol. 2008, 158, 234–239. [Google Scholar] [CrossRef]
- Zare, M.; Esmaeili, N.; Hosseini, H.; Akhavan, S.; Rombenso, A. How do optimum dietary protein and early mild stress events prepare fish for a stressful future? Stress responsiveness of oscar (Astronotus ocellatus). Aquac. Nutr. 2023, in press. [Google Scholar]
- Zare, M.; Esmaeili, M.; Hosseini, H.; Choupani, S.m.H.; Akhavan, S.; Rombenso, A. Fish meal replacement and early mild stress improve stress responsiveness of oscar (Astronotus ocellatus) in future stressful events. Animals 2023, 13, 1314. [Google Scholar] [CrossRef]
- Ahmadi-Noorbakhsh, S.; Mirabzadeh Ardakani, E.; Sadighi, J.; Aldavood, S.J.; Farajli Abbasi, M.; Farzad-Mohajeri, S.; Ghasemi, A.; Sharif-Paghaleh, E.; Hatami, Z.; Nikravanfard, N. Guideline for the care and use of laboratory animals in Iran. Lab Anim. 2021, 50, 303–305. [Google Scholar] [CrossRef]
- Hosseinpour Aghaei, R.; Abedian Kenari, A.; Yazdani Sadati, M.A.; Esmaeili, M. The effect of time-dependent protein restriction on growth factors, nonspecific immunity, body composition, fatty acids and amino acids in the Siberian sturgeon (Acipenser baerii). Aquac. Res. 2018, 49, 3033–3044. [Google Scholar] [CrossRef]
- Esmaeili, M.; Abedian Kenari, A.; Rombenso, A. Immunohematological status under acute ammonia stress of juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792) fed garlic (Allium sativum) powder-supplemented meat and bone meal-based feeds. Comp. Clin. Pathol. 2017, 26, 853–866. [Google Scholar] [CrossRef]
- Zaretabar, A.; Ouraji, H.; Kenari, A.A.; Yeganeh, S.; Esmaeili, M.; Amirkolaee, A.K. One step toward aquaculture sustainability of a carnivorous species: Fish meal replacement with barley protein concentrate plus wheat gluten meal in Caspian brown trout (Salmo trutta caspius). Aquac. Rep. 2021, 20, 100714. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC International; The Association: Gaithersburg, ML, USA, 2000; Volume 18. [Google Scholar]
- Kenari, A.A.; Mahmoudi, N.; Soltani, M.; Abediankenari, S. Dietary nucleotide supplements influence the growth, haemato-immunological parameters and stress responses in endangered Caspian brown trout (Salmo trutta caspius Kessler, 1877). Aquac. Nutr. 2013, 19, 54–63. [Google Scholar] [CrossRef]
- Řehulka, J.; Minařík, B.; Řehulková, E. Red blood cell indices of rainbow trout Oncorhynchus mykiss (Walbaum) in aquaculture. Aquac. Res. 2004, 35, 529–546. [Google Scholar] [CrossRef]
- Wintrobe, M. The volume and hemoglobin content of the red blood corpuscle: Simple method of calculation, normal findings, and value of such calculations in the anemias. Am. J. Med. Sci. 1929, 177, 513–522. [Google Scholar] [CrossRef]
- Esmaeili, M. Blood Performance: A New Formula for Fish Growth and Health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Clerton, P.; Troutaud, D.; Verlhac, V.; Gabaudan, J.; Deschaux, P. Dietary vitamin E and rainbow trout (Oncorhynchus mykiss) phagocyte functions: Effect on gut and on head kidney leucocytes. Fish Shellfish. Immunol. 2001, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amar, E.C.; Kiron, V.; Satoh, S.; Okamoto, N.; Watanabe, T. Effects of dietary β-carotene on the immune response of rainbow trout Oncorhynchus mykiss. Fish. Sci. 2000, 66, 1068–1075. [Google Scholar] [CrossRef]
- Hosseini, H.; Pooyanmehr, M.; Foroughi, A.; Esmaeili, M.; Ghiasi, F.; Lorestany, R. Remarkable positive effects of figwort (Scrophularia striata) on improving growth performance, and immunohematological parameters of fish. Fish Shellfish. Immunol. 2022, 120, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Abedian Kenari, A.; Esmaeili, M.; Rombenso, A. Effects of hydroalcoholic extract of honeybee pollen on growth performance, flesh quality, and immune and stress response of rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2020, 26, 1505–1519. [Google Scholar] [CrossRef]
- Pickering, A.; Pottinger, T.; Christie, P. Recovery of the brown trout, Salmo trutta L., from acute handling stress: A time-course study. J. Fish Biol. 1982, 20, 229–244. [Google Scholar] [CrossRef]
- Kavitha, P.; Rao, J.V. Oxidative stress and locomotor behaviour response as biomarkers for assessing recovery status of mosquito fish, Gambusia affinis after lethal effect of an organophosphate pesticide, monocrotophos. Pestic. Biochem. Physiol. 2007, 87, 182–188. [Google Scholar] [CrossRef]
- Douxfils, J.; Mandiki, S.; Marotte, G.; Wang, N.; Silvestre, F.; Milla, S.; Henrotte, E.; Vandecan, M.; Rougeot, C.; Mélard, C. Does domestication process affect stress response in juvenile Eurasian perch Perca fluviatilis? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 92–99. [Google Scholar] [CrossRef]
- Ericsson, M.; Fallahsharoudi, A.; Bergquist, J.; Kushnir, M.M.; Jensen, P. Domestication effects on behavioural and hormonal responses to acute stress in chickens. Physiol. Behav. 2014, 133, 161–169. [Google Scholar] [CrossRef]
- Esmaeili, M.; Carter, C.G.; Wilson, R.; Walker, S.P.; Miller, M.R.; Bridle, A.R.; Symonds, J.E. Protein metabolism in the liver and white muscle is associated with feed efficiency in Chinook salmon (Oncorhynchus tshawytscha) reared in seawater: Evidence from proteomic analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 42, 100994. [Google Scholar] [CrossRef]
- Barton, B.A.; Schreck, C.B.; Barton, L.D. Effects of chronic cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Dis. Aquat. Org. 1987, 2, 173–185. [Google Scholar] [CrossRef]
- Peters, G.; Schwarzer, R. Changes in hemopoietic tissue of rainbow trout under influence of stress. Dis. Aquat. Org. 1985, 1, 1–10. [Google Scholar] [CrossRef]
- Shearer, K.D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 1994, 119, 63–88. [Google Scholar] [CrossRef]
- Herrera, M.; Mancera, J.M.; Costas, B. The use of dietary additives in fish stress mitigation: Comparative endocrine and physiological responses. Front. Endocrinol. 2019, 10, 447. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Ravardshiri, M.; Bahram, S.; Javadian, S.R.; Bahrekazemi, M. Cinnamon Promotes Growth Performance, Digestive Enzyme, Blood Parameters, and Antioxidant Activity of Rainbow Trout (Oncorhynchus mykiss) in Low-Carbohydrate Diets. Turk. J. Fish. Aquat. Sci. 2021, 21, 309–322. [Google Scholar] [CrossRef]
- Montazeri, H.; Abedian Kenari, A.; Esmaeili, M. Soybean-based diets plus probiotics improve the profile of fatty acids, digestibility, intestinal microflora, growth performance, and the innate immunity of beluga (Huso huso). Aquac. Res. 2021, 52, 152–166. [Google Scholar] [CrossRef]
- Hosseini, H.; Esmaeili, M.; Zare, M.; Rombenso, A. Egg enrichment with n-3 fatty acids in farmed hens in sub-optimum temperature: A cold-temperament additive mix alleviates adverse effects of stress on performance and health. J. Anim. Physiol. Anim. Nutr. 2021, 106, 1333–1334. [Google Scholar] [CrossRef]
- Saleh, N.E.; Mourad, M.M.; El-Banna, S.G.; Abdel-Tawwab, M. Soybean protein concentrate as a fishmeal replacer in weaning diets for common sole (Solea solea) post-larvae: Effects on the growth, biochemical and oxidative stress biomarkers, and histopathological investigations. Aquaculture 2021, 544, 737080. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Z.; Zhou, Y.; Guo, D.; Wang, H.; Chen, X. Hepatic lipid metabolism and oxidative stress responses of grass carp (Ctenopharyngodon idella) fed diets of two different lipid levels against Aeromonas hydrophila infection. Aquaculture 2019, 509, 149–158. [Google Scholar] [CrossRef]
- Spickett, C.M.; Forman, H.J. Lipid Oxidation in Health and Disease; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bae, Y.-S.; Shin, E.-C.; Bae, Y.-S.; Van Eden, W. Stress and immunity. Front. Media SA 2019, 10, 245. [Google Scholar]
- Holzer, P.; Farzi, A.; Hassan, A.M.; Zenz, G.; Jačan, A.; Reichmann, F. Visceral inflammation and immune activation stress the brain. Front. Immunol. 2017, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Pottinger, T. Changes in blood cortisol, glucose and lactate in carp retained in anglers’ keepnets. J. Fish Biol. 1998, 53, 728–742. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Li, Z.-H.; Zlabek, V.; Velisek, J.; Grabic, R.; Machova, J.; Randak, T. Modulation of antioxidant defence system in brain of rainbow trout (Oncorhynchus mykiss) after chronic carbamazepine treatment. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 137–141. [Google Scholar] [CrossRef]
- Hegazi, M.M.; Attia, Z.I.; Ashour, O.A. Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat. Toxicol. 2010, 99, 118–125. [Google Scholar] [CrossRef]
- Tejpal, C.; Pal, A.; Sahu, N.; Kumar, J.A.; Muthappa, N.; Vidya, S.; Rajan, M. Dietary supplementation of L-tryptophan mitigates crowding stress and augments the growth in Cirrhinus mrigala fingerlings. Aquaculture 2009, 293, 272–277. [Google Scholar] [CrossRef]
- Liu, F.; Shi, H.-z.; Guo, Q.-s.; Yu, Y.-b.; Wang, A.-m.; Lv, F.; Shen, W.-b. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish. Immunol. 2016, 51, 125–135. [Google Scholar] [CrossRef]
- Dawood, M.A.; Gewaily, M.S.; Monier, M.N.; Younis, E.M.; Van Doan, H.; Sewilam, H. The regulatory roles of yucca extract on the growth rate, hepato-renal function, histopathological alterations, and immune-related genes in common carp exposed with acute ammonia stress. Aquaculture 2021, 534, 736287. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Liu, Q.; Ye, H.; Zou, C.; Xu, M.; Zhang, Y.; Ye, C. Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus coioides) under cold stress. Aquaculture 2019, 498, 545–555. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Gupta, S.K.; Yousefi, M.; Kulikov, E.V.; Drukovsky, S.G.; Petrov, A.K.; Mirghaed, A.T.; Hoseinifar, S.H.; Van Doan, H. Mitigation of transportation stress in common carp, Cyprinus carpio, by dietary administration of turmeric. Aquaculture 2022, 564, 737380. [Google Scholar] [CrossRef]


| Control | Stress28 | Stress27 | Stress26 | Stress25 | Stress24 | Stress23 | Stress78 | Stress67 | |
|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress |
| Week 2 | Without stress | stress | stress | stress | stress | stress | stress | Without stress | Without stress |
| Week 3 | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | stress | Without stress | Without stress |
| Week 4 | Without stress | Without stress | Without stress | Without stress | Without stress | stress | Without stress | Without stress | Without stress |
| Week 5 | Without stress | Without stress | Without stress | Without stress | stress | Without stress | Without stress | Without stress | Without stress |
| Week 6 | Without stress | Without stress | Without stress | stress | Without stress | Without stress | Without stress | Without stress | stress |
| Week 7 | Without stress | Without stress | stress | Without stress | Without stress | Without stress | Without stress | stress | stress |
| Week 8 | Without stress | stress | Without stress | Without stress | Without stress | Without stress | Without stress | stress | Without stress |
| Week 9 | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress |
| Calculation of weight of all fishes, growth, sampling of blood and other factors | |||||||||
| Week 10 | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress | Without stress |
| Final stress (AC stress) and sampling for serum and hematology parameters. | |||||||||
| Control | Stress28 | Stress27 | Stress26 | Stress25 | Stress24 | Stress23 | Stress78 | Stress67 | |
|---|---|---|---|---|---|---|---|---|---|
| Initial weight (g) | 5.57 ± 0.98 | 5.46 ± 0.68 | 6.27 ± 0.65 | 5.40 ± 1.05 | 5.60 ± 1.35 | 6.17 ± 0.67 | 5.40 ± 0.66 | 5.47 ± 0.67 | 5.80 ± 0.95 |
| Final weight (g) | 47.03 ± 7.16 | 46.96 ± 8.11 | 43.44 ± 8.95 | 43.53 ± 6.70 | 41.60 ± 7.37 | 44.86 ± 7.32 | 39.40 ± 4.62 | 32.23 ± 6.67 | 35.87 ± 6.21 |
| Weight gain (g) | 41.42 ± 6.26 | 41.50 ± 8.48 | 37.19 ± 8.61 | 38.13 ± 7.33 | 36.00 ± 8.16 | 38.70 ± 7.99 | 34.00 ± 4.30 | 26.78 ± 7.34 | 30.05 ± 7.16 |
| SGR (%/day) | 3.39 ± 0.11 | 3.41 ± 0.43 | 3.06 ± 0.26 | 3.32 ± 0.46 | 3.20 ± 0.60 | 3.14 ± 0.44 | 3.15 ± 0.16 | 2.80 ± 0.47 | 2.89 ± 0.54 |
| FCR | 1.89 ± 0.28 | 1.98 ± 0.31 | 2.09 ± 0.17 | 2.16 ± 0.19 | 2.25 ± 0.31 | 2.15 ± 0.37 | 2.30 ± 0.29 | 2.04 ± 0.34 | 2.09 ± 0.25 |
| DFI (%/day) | 4.72 ± 0.62 | 4.96 ± 0.66 | 4.93 ± 0.73 | 5.32 ± 0.54 | 5.36 ± 0.54 | 5.18 ± 1.22 | 5.52 ± 0.67 | 4.55 ± 0.95 | 4.76 ± 0.90 |
| HSI (%) | 2.33 + 0.31 | 2.66 + 0.31 | 2.45 + 0.28 | 2.42 + 0.22 | 2.43 ± 0.23 | 2.46 ± 0.19 | 2.42 ± 0.27 | 2.61 ± 0.34 | 2.46 ± 0.45 |
| VSI (%) | 5.87 ± 0.91 | 6.00 ± 0.62 | 6.23 ± 0.40 | 6.13 ± 0.38 | 6.10 ± 0.79 | 5.90 ± 0.80 | 6.10 ± 0.46 | 6.33 ± 0.45 | 5.90 ± 0.70 |
| Condition factor | 2.32 ± 0.46 | 2.59 ± 0.83 | 2.30 ± 0.59 | 2.31 ± 0.62 | 2.14 ± 0.72 | 2.37 ± 0.58 | 1.97 ± 0.35 | 1.85 ± 0.65 | 1.83 ± 0.15 |
| Survival rate (%) # | 94.44 ± 6.36 | 94.44 ± 6.36 | 91.67 ± 4.17 | 93.06 ± 4.81 | 93.06 ± 6.36 | 91.67 ± 4.17 | 94.44 ± 6.36 | 91.67 ± 4.17 | 93.06 ± 6.36 |
| Survival rate after AC (%) | 60.00 ± 10.00 b | 80.00 ± 0.00 a | 83.33 ± 5.77 a | 76.67 ± 5.77 ab | 80.00 ± 10.00 a | 73.33 ± 15.28 ab | 73.33 ± 5.77 ab | 63.33 ± 11.55 b | 70.00 ± 5.77 ab |
| Control | Stress28 | Stress27 | Stress26 | Stress25 | Stress24 | Stress23 | Stress78 | Stress67 | |
|---|---|---|---|---|---|---|---|---|---|
| Protein | 178.5 ± 15.7 | 176.9 ± 10.8 | 183.1 ± 10.1 | 180.2 ± 8.7 | 176.4 ± 9.5 | 179.3 ± 8.2 | 177.6 ± 4.4 | 171.8 ± 8.9 | 179.8 ± 8.7 |
| Fat | 59.1 ± 5.6 | 64.4 ± 8.7 | 60.7 ± 4.1 | 55.8 ± 4.9 | 60.5 ± 5.3 | 59.4 ± 4.3 | 62.3 ± 5.8 | 60.2 ± 4.2 | 60.6 ± 3.4 |
| Ash | 31.6 ± 2.5 | 28.6 ± 3.8 | 29.6 ± 2.5 | 29.3 ± 4.1 | 30.6 ± 4.0 | 31.6 ± 3.5 | 34.3 ± 2.1 | 31.0 ± 2.8 | 32.3 ± 3.8 |
| Moisture | 683.0 ± 23.2 | 674.1 ± 24.2 | 686.1 ± 29.7 | 679.3 ± 22.9 | 692.1 ± 38.6 | 673.3 ± 39.2 | 701.5 ± 21.8 | 699.9 ± 13.1 | 704.4 ± 17.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zare, M.; Heidari, E.; Hosseini Choupani, S.M.; Akhavan, S.R.; Rombenso, A.; Esmaeili, N. The Recovery Time between Early Mild Stress and Final Acute Stress Affects Survival Rate, Immunity, Health, and Physiology of Oscar (Astronotus ocellatus). Animals 2023, 13, 1606. https://doi.org/10.3390/ani13101606
Zare M, Heidari E, Hosseini Choupani SM, Akhavan SR, Rombenso A, Esmaeili N. The Recovery Time between Early Mild Stress and Final Acute Stress Affects Survival Rate, Immunity, Health, and Physiology of Oscar (Astronotus ocellatus). Animals. 2023; 13(10):1606. https://doi.org/10.3390/ani13101606
Chicago/Turabian StyleZare, Mahyar, Elaheh Heidari, Seyedeh Mahsa Hosseini Choupani, Sobhan R. Akhavan, Artur Rombenso, and Noah Esmaeili. 2023. "The Recovery Time between Early Mild Stress and Final Acute Stress Affects Survival Rate, Immunity, Health, and Physiology of Oscar (Astronotus ocellatus)" Animals 13, no. 10: 1606. https://doi.org/10.3390/ani13101606
APA StyleZare, M., Heidari, E., Hosseini Choupani, S. M., Akhavan, S. R., Rombenso, A., & Esmaeili, N. (2023). The Recovery Time between Early Mild Stress and Final Acute Stress Affects Survival Rate, Immunity, Health, and Physiology of Oscar (Astronotus ocellatus). Animals, 13(10), 1606. https://doi.org/10.3390/ani13101606






