The Influence of Photoperiod, Intake of Polyunsaturated Fatty Acids, and Food Availability on Seasonal Acclimatization in Red Deer (Cervus elaphus)
Abstract
Simple Summary
Abstract
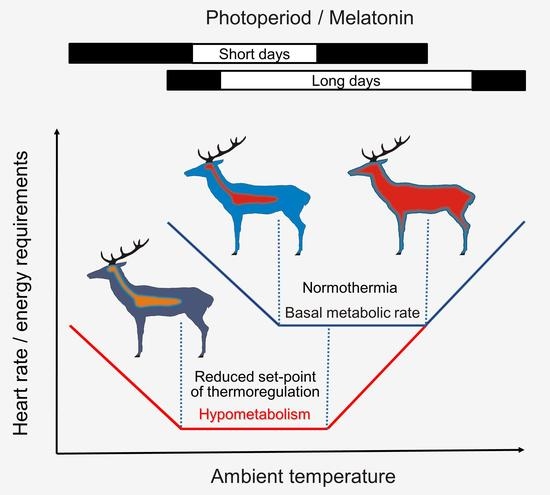
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Feeding
2.2. Biologging
2.3. Administration of Melatonin
2.4. Anaesthesia
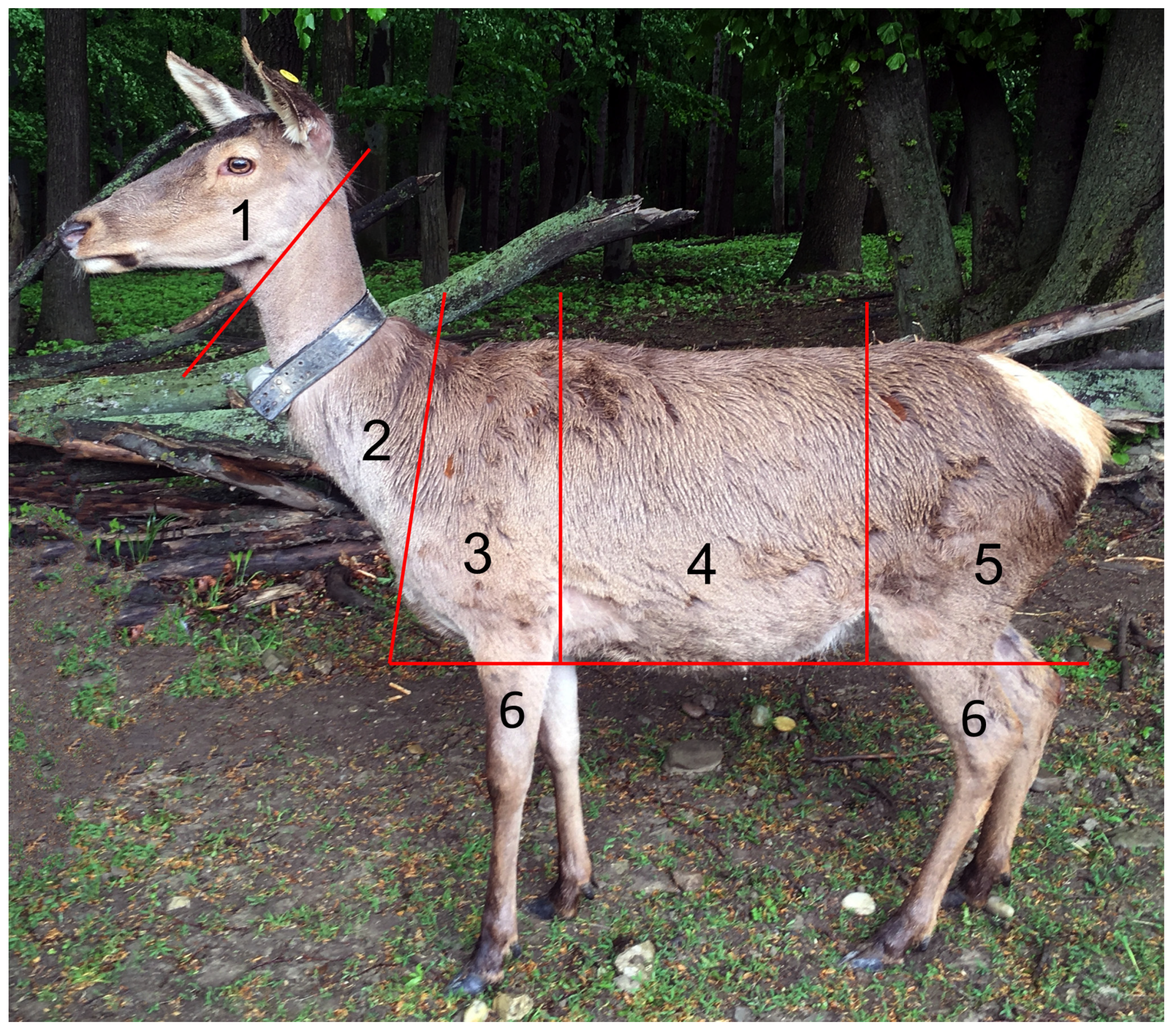
2.5. Measuring Coat Change
2.6. Data Analyses
3. Results
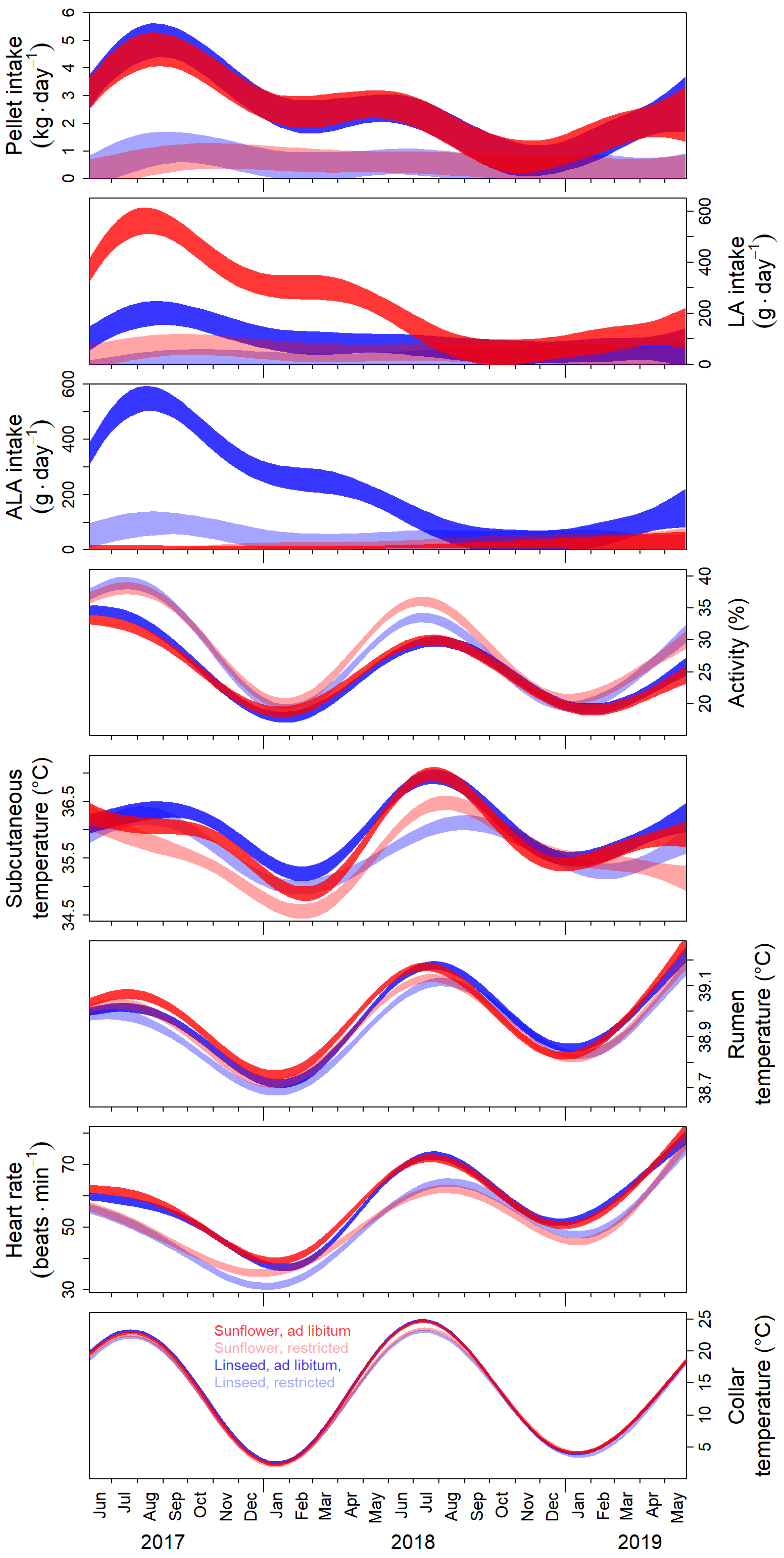
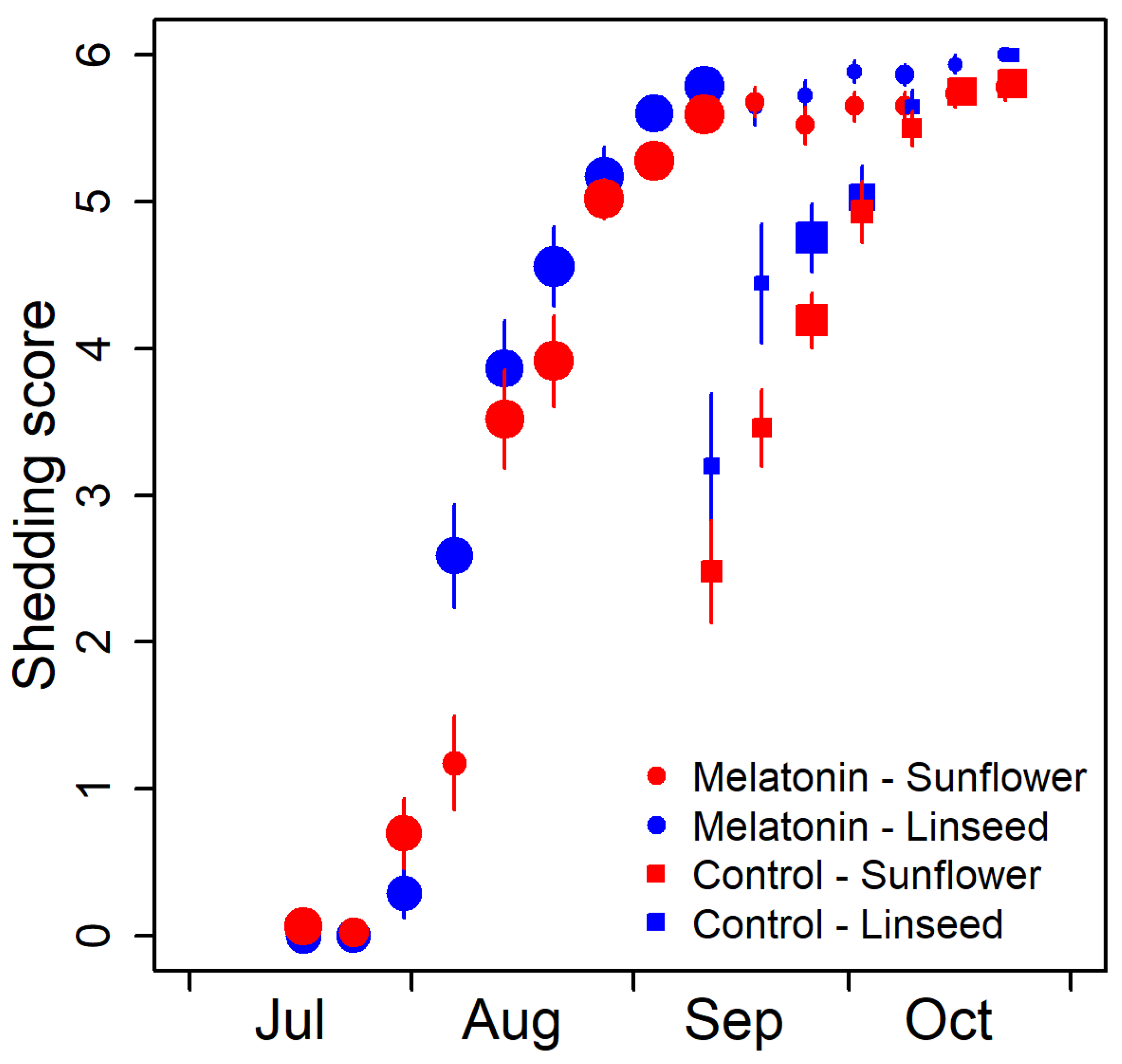
3.1. Seasonal Changes of Behavioural and Physiological Parameters
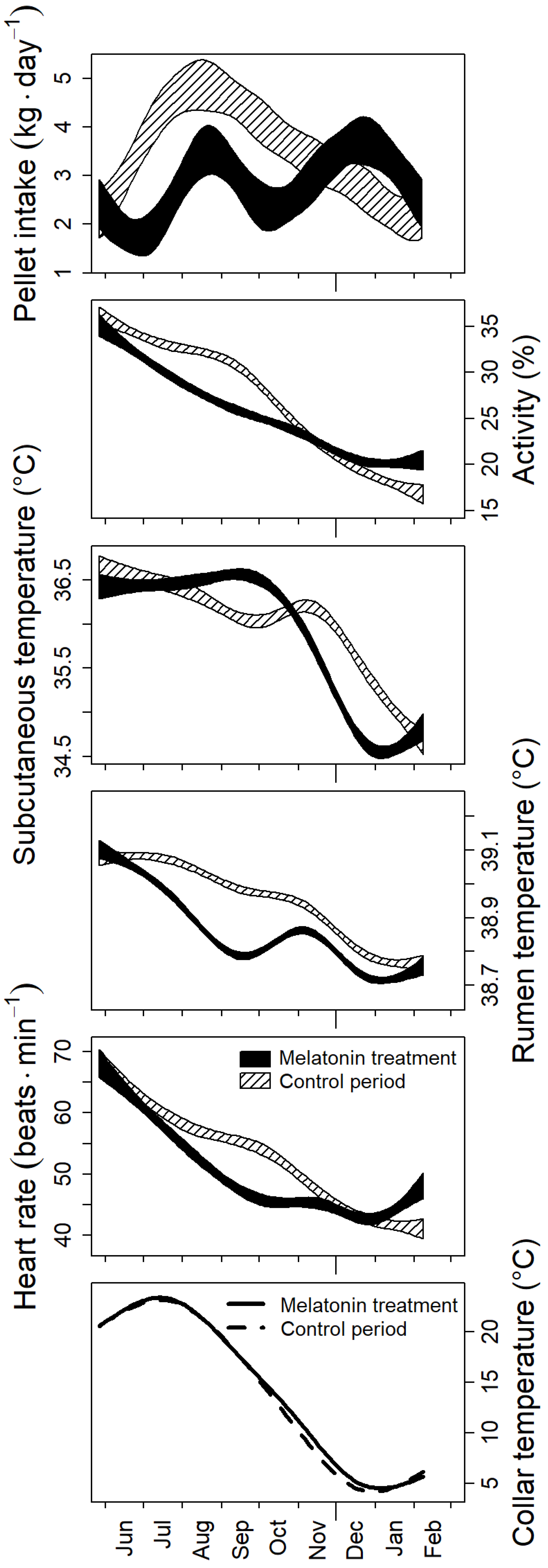
3.2. Photoperiodic Control of Seasonal Changes
3.3. Factors Influencing Coat Change
4. Discussion
4.1. Effects of Food Supplementation with Polyunsaturated Fatty Acids
4.2. Effects of Treatment with Melatonin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stien, A.; Ims, R.A.; Albon, S.D.; Fuglei, E.; Irvine, R.J.; Ropstad, E.; Halvorsen, O.; Langvatn, R.; Loe, L.E.; Veiberg, V.; et al. Congruent responses to weather variability in high arctic herbivores. Biol. Lett. 2012, 8, 1002–1005. [Google Scholar] [CrossRef]
- Tyler, N.J.C. Climate, snow, ice, crashes, and declines in populations of reindeer and caribou (Rangifer tarandus L.). Ecol. Monogr. 2010, 80, 197–219. [Google Scholar] [CrossRef]
- Geiser, F.; Ruf, T. Hibernation versus Daily Torpor in Mammals and Birds: Physiological Variables and Classification of Torpor Patterns. Physiol. Zool. 1995, 68, 935–966. Available online: https://www.jstor.org/stable/30163788 (accessed on 1 October 2022). [CrossRef]
- Arnold, W.; Giroud, S.; Valencak, T.G.; Ruf, T. Ecophysiology of Omega Fatty Acids: A Lid for Every Jar. Physiology 2015, 30, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W. Review: Seasonal differences in the physiology of wild northern ruminants. Animal 2020, 14, s124–s132. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, L.; Gerken, M.; Hambly, C.; Speakman, J.R.; Riek, A. Thyroid hormones correlate with field metabolic rate in ponies, Equus ferus caballus. J. Exp. Biol. 2016, 219, 2559–2566. [Google Scholar] [CrossRef]
- Brinkmann, L.; Gerken, M.; Hambly, C.; Speakman, J.R.; Riek, A. Saving energy during hard times: Energetic adaptations of Shetland pony mares. J. Exp. Biol. 2014, 217, 4320–4327. [Google Scholar] [CrossRef]
- Brosh, A. Heart rate measurements as an index of energy expenditure and energy balance in ruminants: A review. J. Anim. Sci. 2007, 85, 1213–1227. [Google Scholar] [CrossRef]
- Butler, P.J.; Green, J.A.; Boyd, I.L.; Speakman, J.R. Measuring metabolic rate in the field: The pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 2004, 18, 168–183. [Google Scholar] [CrossRef]
- Vesterdorf, K.; Beatty, D.T.; Barnes, A.; Maloney, S.K. Rumen temperature is a reliable proxy of core body temperature in sheep (Ovis aries). Anim. Prod. Sci. 2022, 62, 1671–1682. [Google Scholar] [CrossRef]
- Rose-Dye, T.K.; Burciaga-Robles, L.O.; Krehbiel, C.R.; Step, D.L.; Fulton, R.W.; Confer, A.W.; Richards, C.J. Rumen temperature change monitored with remote rumen temperature boluses after challenges with bovine viral diarrhea virus and Mannheimia haemolytica. J. Anim. Sci. 2011, 89, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Beatty, D.T.; Barnes, A.; Taylor, E.; Maloney, S.K. Do changes in feed intake or ambient temperature cause changes in cattle rumen temperature relative to core temperature? J. Therm. Biol. 2008, 33, 12–19. [Google Scholar] [CrossRef]
- Turbill, C.; Ruf, T.; Mang, T.; Arnold, W. Regulation of heart rate and rumen temperature in red deer: Effects of season and food intake. J. Exp. Biol. 2011, 214, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; Ruf, T.; Kuntz, R. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) II. Energy expenditure. J. Exp. Biol. 2006, 209, 4566–4573. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; Ruf, T.; Reimoser, S.; Tataruch, F.; Onderscheka, K.; Schober, F. Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). Am. J. Physiol. Reg. Int. Comp. Physiol. 2004, 286, R174–R181. [Google Scholar] [CrossRef]
- Arnold, W.; Beiglböck, C.; Burmester, M.; Guschlbauer, M.; Lengauer, A.; Schröder, B.; Wilkens, M.; Breves, G. Contrary seasonal changes of rates of nutrient uptake, organ mass, and voluntary food intake in red deer (Cervus elaphus). Am. J. Physiol. Reg. Int. Comp. Physiol. 2015, 309, R277–R285. [Google Scholar] [CrossRef]
- Loudon, A.S.I. Photoperiod and the regulation of annual and circannual cycles of food intake. Proc. Nutr. Soc. 1994, 53, 495–507. [Google Scholar] [CrossRef]
- Milne, J.A.; Loudon, A.S.I.; Sibbald, A.M.; Curlewis, J.D.; McNeilly, A.S. Effects of melatonin and a dopamine agonist and antagonist on seasonal changes in voluntary intake, reproductive activity and plasma concentrations of prolactin and tri-iodothyronine in red deer hinds. J. Endocrinol. 1990, 125, 241–249. [Google Scholar] [CrossRef]
- Heydon, M.J.; Sibbald, A.M.; Milne, J.A.; Brinklow, B.R.; Loudon, A.S.I. The interaction of food availability and endogenous physiological cycles on the grazing ecology of red deer hinds (Cervus elaphus). Funct. Ecol. 1993, 7, 216–222. [Google Scholar] [CrossRef]
- Heydon, M.J.; Milne, J.A.; Brinklow, B.R.; Loudon, A.S.I. Manipulating melatonin in red deer (Cervus elaphus): Differences in the response to food restriction and lactation on the timing of the breeding season and prolactin-dependent pelage changes. J. Exp. Zool. 1995, 273, 12–20. [Google Scholar] [CrossRef]
- Ocloo, A.; Shabalina, I.G.; Nedergaard, J.; Brand, M.D. Cold-induced alterations of phospholipid fatty acyl composition in brown adipose tissue mitochondria are independent of uncoupling protein-1. Am. J. Physiol. Reg. Int. Comp. Physiol. 2007, 293, R1086–R1093. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; Ruf, T.; Frey-Roos, F.; Bruns, U. Diet-Independent Remodeling of Cellular Membranes Precedes Seasonally Changing Body Temperature in a Hibernator. PLoS ONE 2011, 6, e18641. [Google Scholar] [CrossRef] [PubMed]
- Nagahuedi, S.; Popesku, J.T.; Trudeau, V.L.; Weber, J.-M. Mimicking the natural doping of migrant sandpipers in sedentary quails: Effects of dietary n-3 fatty acids on muscle membranes and PPAR expression. J. Exp. Biol. 2009, 212, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Maillet, D.; Weber, J.M. Relationship between n-3 PUFA content and energy metabolism in the flight muscles of a migrating shorebird: Evidence for natural doping. J. Exp. Biol. 2007, 210, 413–420. [Google Scholar] [CrossRef]
- Maillet, D.; Weber, J.-M. Performance-enhancing role of dietary fatty acids in a long-distance migrant shorebird: The semipalmated sandpiper. J. Exp. Biol. 2006, 209, 2686–2695. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Richardson, M. Photo-neuroendocrine control of seasonal cycles in body weight, pelage growth and reproduction: Lessons from the HPD sheep model. Comp. Biochem. Physiol. C 1998, 119, 283–294. [Google Scholar] [CrossRef]
- Turbill, C.; Ruf, T.; Rothmann, A.; Arnold, W. Social dominance is associated with individual differences in heart rate and energetic response to food restriction in female red deer. Physiol. Biochem. Zool. 2013, 86, 528–537. [Google Scholar] [CrossRef]
- Gasch, K.; Hykollari, A.; Habe, M.; Painer-Gigler, J.; Stalder, G.; Arnold, W. Photoperiod and intake of essential fatty acids influence the acyl composition of phospholipids and the activities of key metabolic enzymes: An experimental study with red deer (Cervus elaphus). Biology, 2023; in prep. [Google Scholar]
- García, A.; Landete-Castillejos, T.; Zarazaga, L.; Garde, J.; Gallego, L. Seasonal changes in melatonin concentrations in female Iberian red deer (Cervus elaphus hispanicus). J. Pineal Res. 2003, 34, 161–166. [Google Scholar] [CrossRef]
- Einwaller, J.; Painer, J.; Raekallio, M.; Gasch, K.; Restitutti, F.; Auer, U.; Stalder, G.L. Cardiovascular effects of intravenous vatinoxan (MK-467) in medetomidine–tiletamine–zolazepam anaesthetised red deer (Cervus elaphus). Vet. Anaesth. Analg. 2020, 47, 518–527. [Google Scholar] [CrossRef]
- Arnold, W.; Ruf, T.; Loe, L.E.; Irvine, R.J.; Ropstad, E.; Veiberg, V.; Albon, S.D. Circadian rhythmicity persists through the Polar night and midnight sun in Svalbard reindeer. Sci. Rep. 2018, 8, 14466. [Google Scholar] [CrossRef] [PubMed]
- Signer, C.; Ruf, T.; Arnold, W. Hypometabolism and basking: The strategies of Alpine ibex to endure harsh over-wintering conditions. Funct. Ecol. 2011, 25, 537–547. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 October 2022).
- Wood, S.N. Thin-plate regression splines. J. R. Stat. Soc. B 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Christensen, R.H.B. Ordinal—Regression Models for Ordinal Data. R Package Version 2022.11-16. 2022. Available online: https://CRAN.R-project.org/package=ordinal (accessed on 20 November 2022).
- Hulbert, A.J.; Abbott, S.K. Nutritional ecology of essential fatty acids: An evolutionary perspective. Aust. J. Zool. 2012, 59, 369–379. [Google Scholar] [CrossRef]
- Cherel, Y.; Charrassin, J.B.; Challet, E. Energy and protein requirements for molt in the king penguin Aptenodytes patagonicus. Am. J. Physiol. Reg. Int. Comp. Physiol. 1994, 266, R1182–R1188. [Google Scholar] [CrossRef]
- Cyr, N.E.; Wikelski, M.; Romero, L.M. Increased Energy Expenditure but Decreased Stress Responsiveness during Molt. Physiol. Biochem. Zool. 2008, 81, 452–462. [Google Scholar] [CrossRef]
- Dietz, M.W.; Daan, S.; Masman, D. Energy requirements for molt in the kestrel Falco tinnunculus. Physiol. Zool. 1992, 65, 1217–1235. Available online: https://www.jstor.org/stable/30158276 (accessed on 1 October 2022). [CrossRef]
- Saino, N.; Romano, M.; Caprioli, M.; Lardelli, R.; Micheloni, P.; Scandolara, C.; Rubolini, D.; Fasola, M. Molt, feather growth rate and body condition of male and female Barn Swallows. J. Ornithol. 2013, 154, 537–547. [Google Scholar] [CrossRef]
- Hackländer, K.; Arnold, W. Male-caused failure of female reproduction and its adaptive value in alpine marmots (Marmota marmota). Behav. Ecol. 1999, 10, 592–597. [Google Scholar] [CrossRef]
- Ruf, T.; Geiser, F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 2015, 90, 891–926. [Google Scholar] [CrossRef]
- Trondrud, L.M.; Pigeon, G.; Albon, S.; Arnold, W.; Evans, A.L.; Irvine, R.J.; Król, E.; Ropstad, E.; Stien, A.; Veiberg, V.; et al. Determinants of heart rate in Svalbard reindeer reveal mechanisms of seasonal energy management. Philos. Trans. R. Soc. B 2021, 376, 20200215. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.M.; Grøndahl, C.; Evans, A.L.; Desforges, J.-P.; Blake, J.; Hansen, L.H.; Beumer, L.T.; Mosbacher, J.B.; Stelvig, M.; Greunz, E.M.; et al. On the interplay between hypothermia and reproduction in a high arctic ungulate. Sci. Rep. 2020, 10, 1514. [Google Scholar] [CrossRef]
- Græsli, A.R.; Thiel, A.; Fuchs, B.; Singh, N.J.; Stenbacka, F.; Ericsson, G.; Neumann, W.; Arnemo, J.M.; Evans, A.L. Seasonal Hypometabolism in Female Moose. Front. Ecol. Evol. 2020, 8, 107. [Google Scholar] [CrossRef]
- Riek, A.; Stölzl, A.; Marquina Bernedo, R.; Ruf, T.; Arnold, W.; Hambly, C.; Speakman, J.R.; Gerken, M. Energy expenditure and body temperature variations in llamas living in the High Andes of Peru. Sci. Rep. 2019, 9, 4037. [Google Scholar] [CrossRef] [PubMed]
- Riek, A.; Brinkmann, L.; Gauly, M.; Perica, J.; Ruf, T.; Arnold, W.; Hambly, C.; Speakman, J.R.; Gerken, M. Seasonal changes in energy expenditure, body temperature and activity patterns in llamas (Lama glama). Sci. Rep. 2017, 7, 7600. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, L.; Riek, A.; Gerken, M. Long-term adaptation capacity of ponies: Effect of season and feed restriction on blood and physiological parameters. Animal 2017, 12, 88–97. [Google Scholar] [CrossRef]
- Brinkmann, L.; Gerken, M.; Riek, A. Adaptation strategies to seasonal changes in environmental conditions of a domesticated horse breed, the Shetland pony (Equus ferus caballus). J. Exp. Biol. 2012, 215, 1061–1068. [Google Scholar] [CrossRef]
- Ruf, T.; Vetter, S.G.; Painer, J.; Stalder, G.; Bieber, C. Atypical for northern ungulates, energy metabolism is lowest during summer in female wild boars (Sus scrofa). Sci. Rep. 2021, 11, 18310. [Google Scholar] [CrossRef]
- Anufriev, A.; Solomonov, N.; Yadrikhinskii, V.; Isaev, A. Seasonal changes in the body temperature of medium-sized and large mammals in cold climate. Dokl. Biol. Sci. 2007, 415, 317–319. [Google Scholar] [CrossRef]
- Speakman, J.R.; Chi, Q.; Ołdakowski, Ł.; Fu, H.; Fletcher, Q.E.; Hambly, C.; Togo, J.; Liu, X.; Piertney, S.B.; Wang, X.; et al. Surviving winter on the Qinghai-Tibetan Plateau: Pikas suppress energy demands and exploit yak feces to survive winter. Proc. Natl. Acad. Sci. USA 2021, 118, e2100707118. [Google Scholar] [CrossRef]
- McClune, D.W.; Kostka, B.; Delahay, R.J.; Montgomery, W.I.; Marks, N.J.; Scantlebury, D.M. Winter Is Coming: Seasonal Variation in Resting Metabolic Rate of the European Badger (Meles meles). PLoS ONE 2015, 10, e0135920. [Google Scholar] [CrossRef] [PubMed]
- Fuglesteg, B.N.; Øyvind, E.H.; Folkow, L.P.; Fuglei, E. Seasonal variations in basal metabolic rate, lower critical temperature and responses to temporary starvation in the arctic fox (Alopex lagopus) from Svalbard. Polar Biol. 2006, 29, 308–319. [Google Scholar] [CrossRef]
- Brown, K.J.; Downs, C.T. Seasonal patterns in body temperature of free-living rock hyrax (Procavia capensis). Comp. Biochem. Physiol. A 2006, 143, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Broggi, J.; Nilsson, J.-Å. Individual response in body mass and basal metabolism to the risks of predation and starvation. J. Exp. Biol. 2023, 226, jeb244744. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.M.; Lima, S.L. Nocturnal hypothermia impairs flight ability in birds: A cost of being cool. Proc. R. Soc. Lond. B 2013, 280, 20131846. [Google Scholar] [CrossRef]
- McKechnie, A.E.; Lovegrove, B.G. Avian facultative hypothermic responses: A review. Condor 2002, 104, 705–724. [Google Scholar] [CrossRef]
- Wascher, C.A.F.; Kotrschal, K.; Arnold, W. Free-living greylag geese adjust their heart rates and body core temperatures to season and reproductive context. Sci. Rep. 2018, 8, 2142. [Google Scholar] [CrossRef]
- Nilssen, K.J.; Sundsfjord, J.A.; Blix, A.S. Regulation of metabolic rate in Svalbard and Norwegian reindeer. Am. J. Physiol. 1984, 247, R837–R841. [Google Scholar] [CrossRef]
- Mautz, W.W.; Kanter, J.; Pekins, P.J. Seasonal metabolic rhythms of captive female white-tailed deer: A reexamination. J. Wildl. Manag. 1992, 56, 656–661. [Google Scholar] [CrossRef]
- Mesteig, K.; Tyler, N.J.; Blix, A.S. Seasonal changes in heart rate and food intake in reindeer (Rangifer tarandus tarandus). Acta Physiol. Scand. 2000, 170, 145–151. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Johnston, J.D.; Andersson, H.; Wagner, G.; Hazlerigg, D.G. Photorefractoriness in mammals: Dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology 2005, 146, 3782–3790. [Google Scholar] [CrossRef] [PubMed]
| Type of Pellet | Crude Fat (%) | Crude Protein (%) | NDF (%) + | ADF (%) * |
|---|---|---|---|---|
| Immersed in 10% w/w linseed oil | 23.6 | 12.8 | 30.9 | 19.6 |
| Immersed in 10% w/w sunflower seed oil | 21.8 | 11.9 | 30.8 | 19.2 |
| Immersed in 5% w/w linseed oil | 7.9 | 12.9 | 31.0 | 20.6 |
| Immersed in 5% w/w sunflower seed oil | 7.8 | 16.3 | 31.4 | 19.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasch, K.; Habe, M.; Krauss, J.S.; Painer-Gigler, J.; Stalder, G.; Arnold, W. The Influence of Photoperiod, Intake of Polyunsaturated Fatty Acids, and Food Availability on Seasonal Acclimatization in Red Deer (Cervus elaphus). Animals 2023, 13, 1600. https://doi.org/10.3390/ani13101600
Gasch K, Habe M, Krauss JS, Painer-Gigler J, Stalder G, Arnold W. The Influence of Photoperiod, Intake of Polyunsaturated Fatty Acids, and Food Availability on Seasonal Acclimatization in Red Deer (Cervus elaphus). Animals. 2023; 13(10):1600. https://doi.org/10.3390/ani13101600
Chicago/Turabian StyleGasch, Kristina, Manuela Habe, Julie Sophie Krauss, Johanna Painer-Gigler, Gabrielle Stalder, and Walter Arnold. 2023. "The Influence of Photoperiod, Intake of Polyunsaturated Fatty Acids, and Food Availability on Seasonal Acclimatization in Red Deer (Cervus elaphus)" Animals 13, no. 10: 1600. https://doi.org/10.3390/ani13101600
APA StyleGasch, K., Habe, M., Krauss, J. S., Painer-Gigler, J., Stalder, G., & Arnold, W. (2023). The Influence of Photoperiod, Intake of Polyunsaturated Fatty Acids, and Food Availability on Seasonal Acclimatization in Red Deer (Cervus elaphus). Animals, 13(10), 1600. https://doi.org/10.3390/ani13101600