The Application of 3D Anatomy for Teaching Veterinary Clinical Neurology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
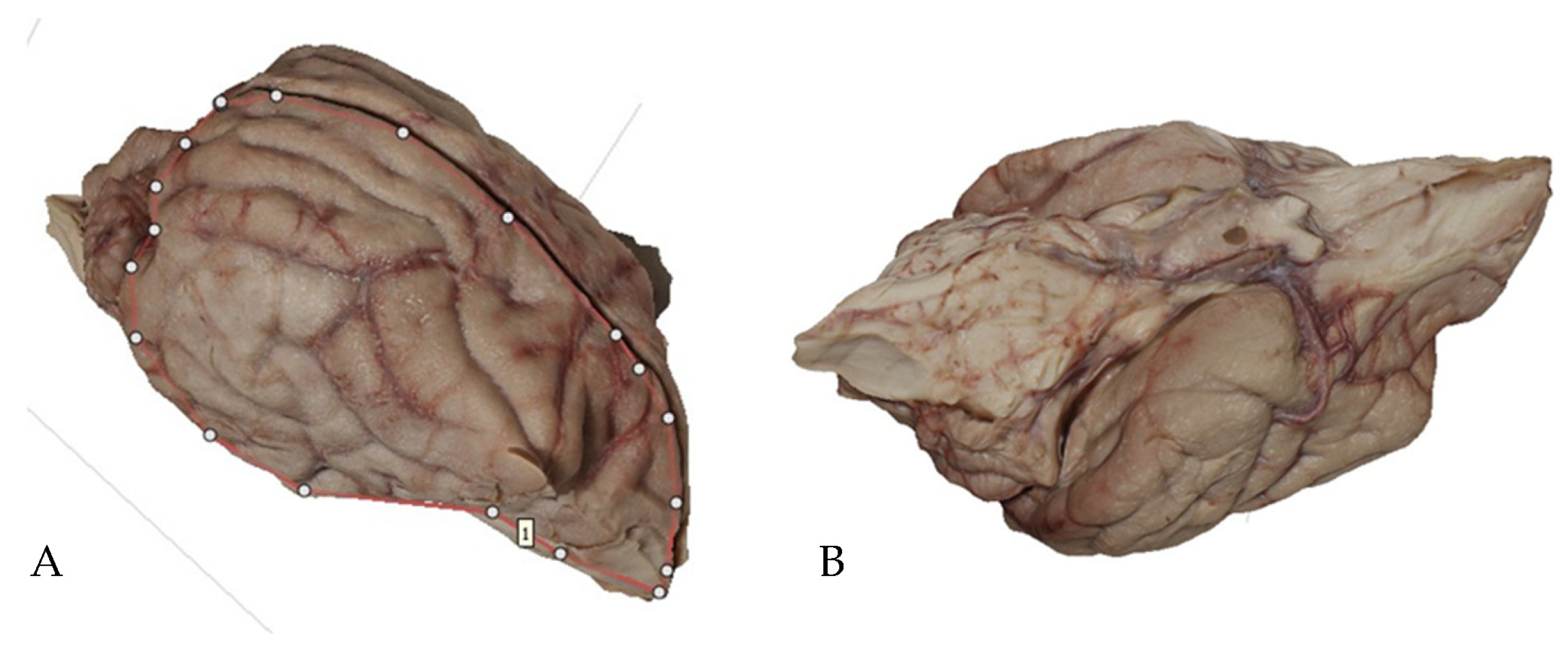
2.2. Methods
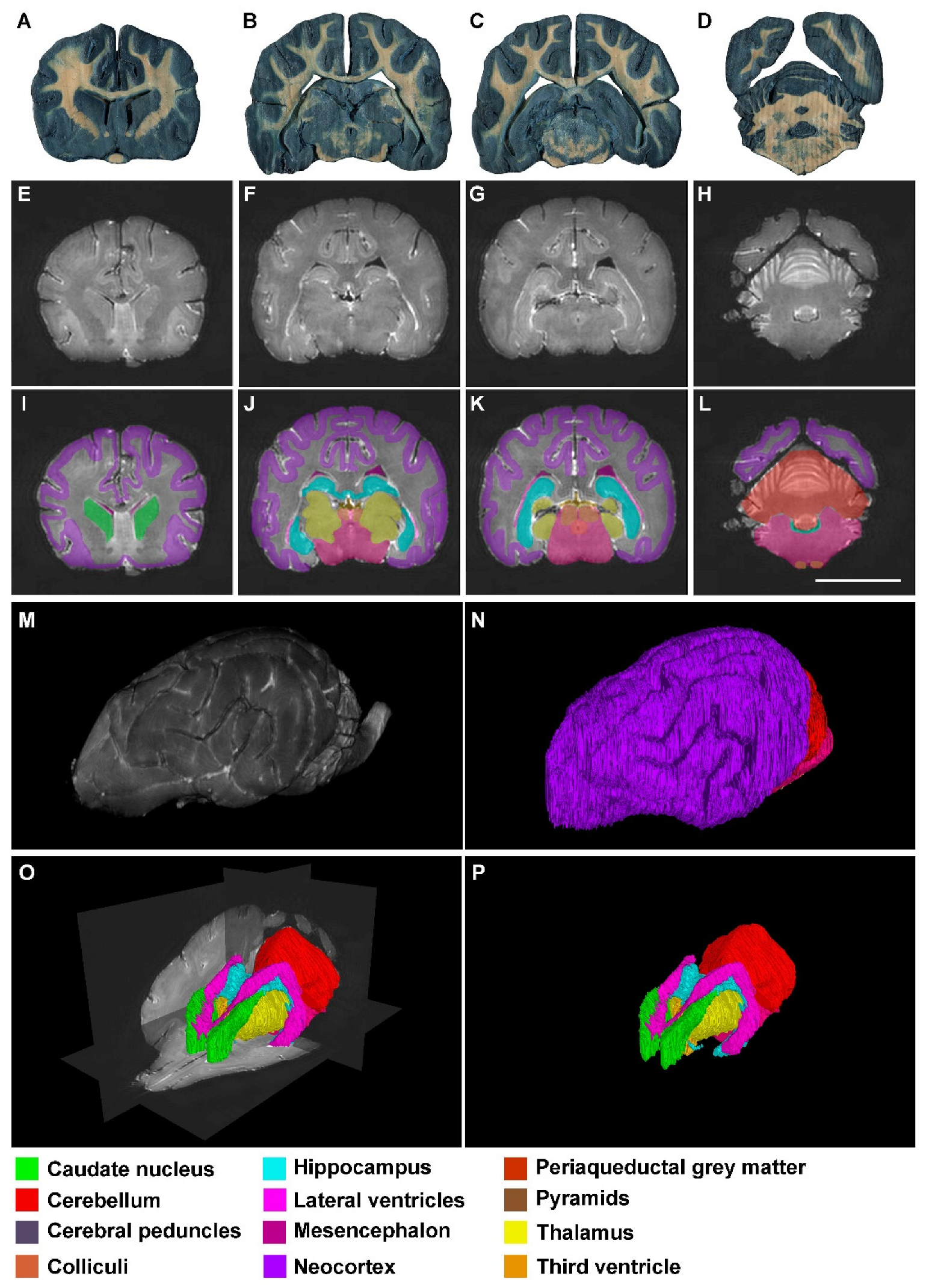
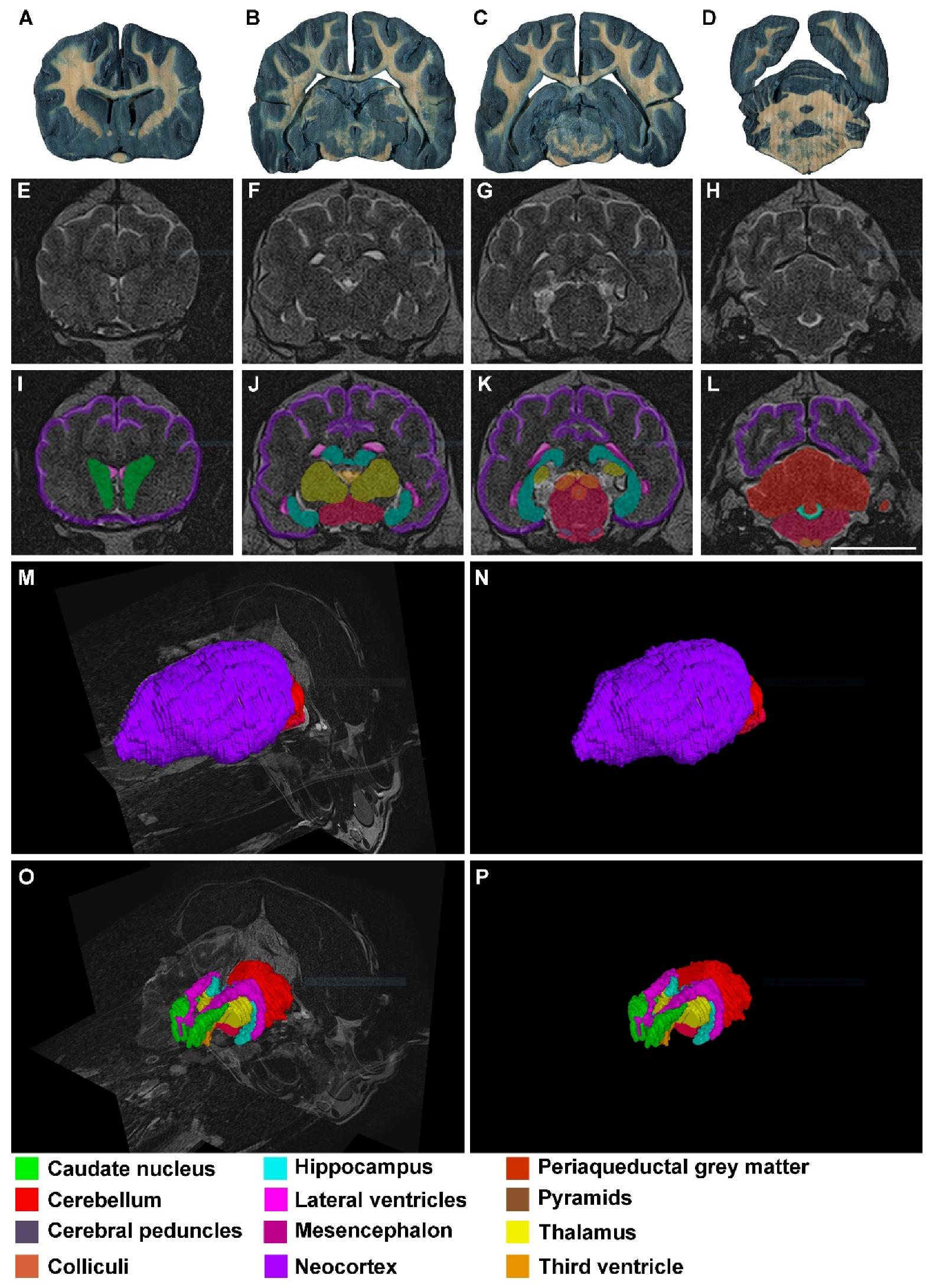
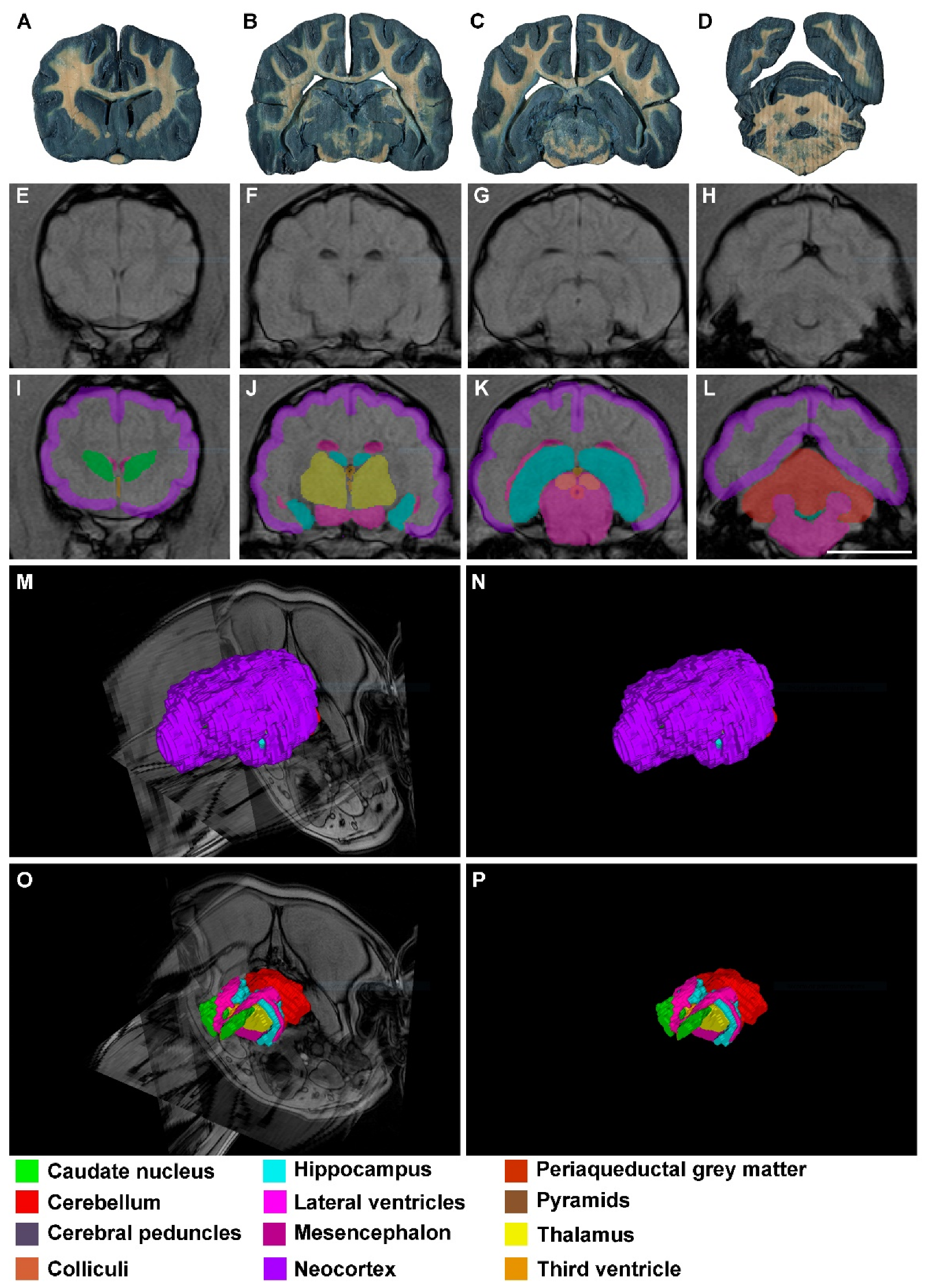
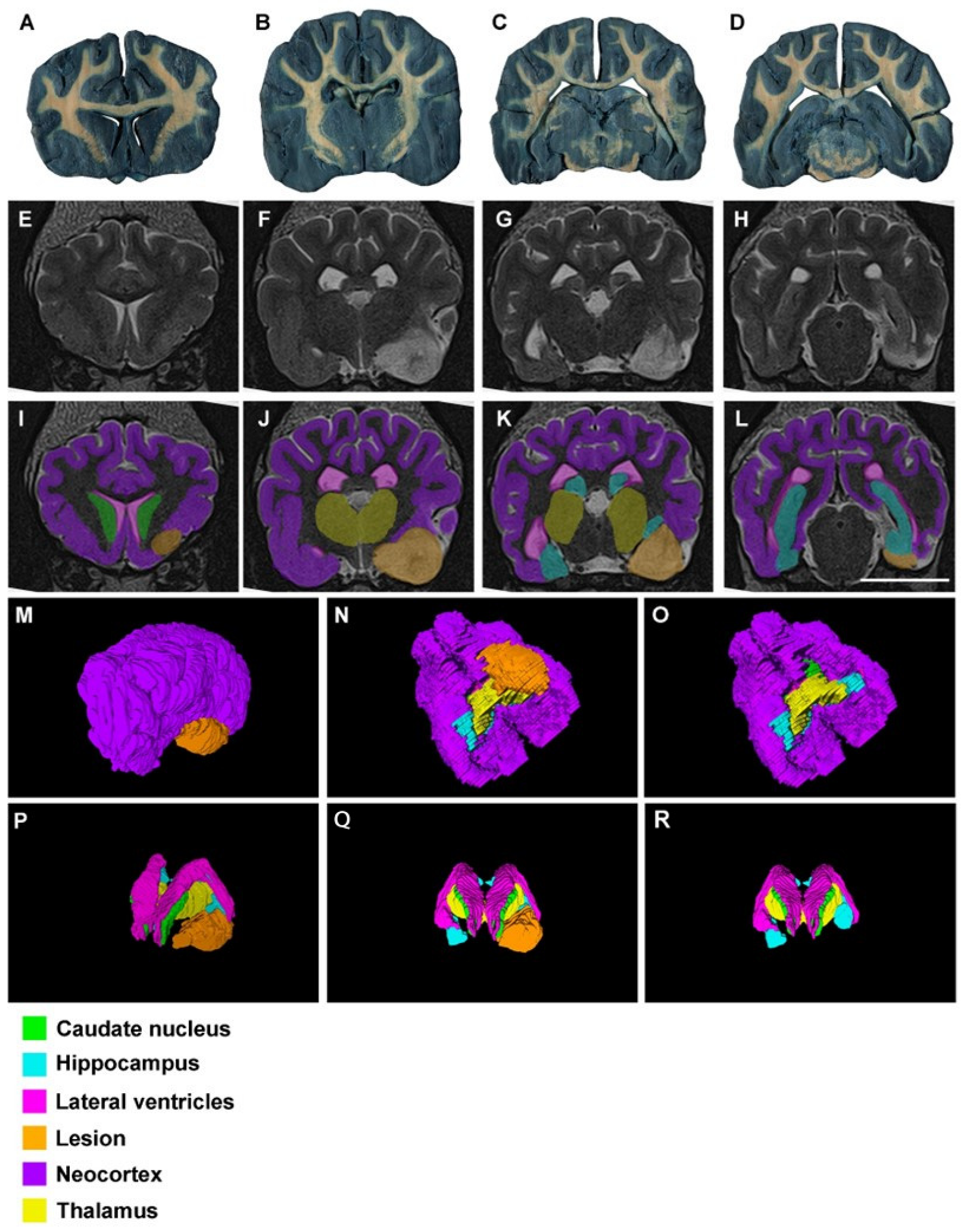
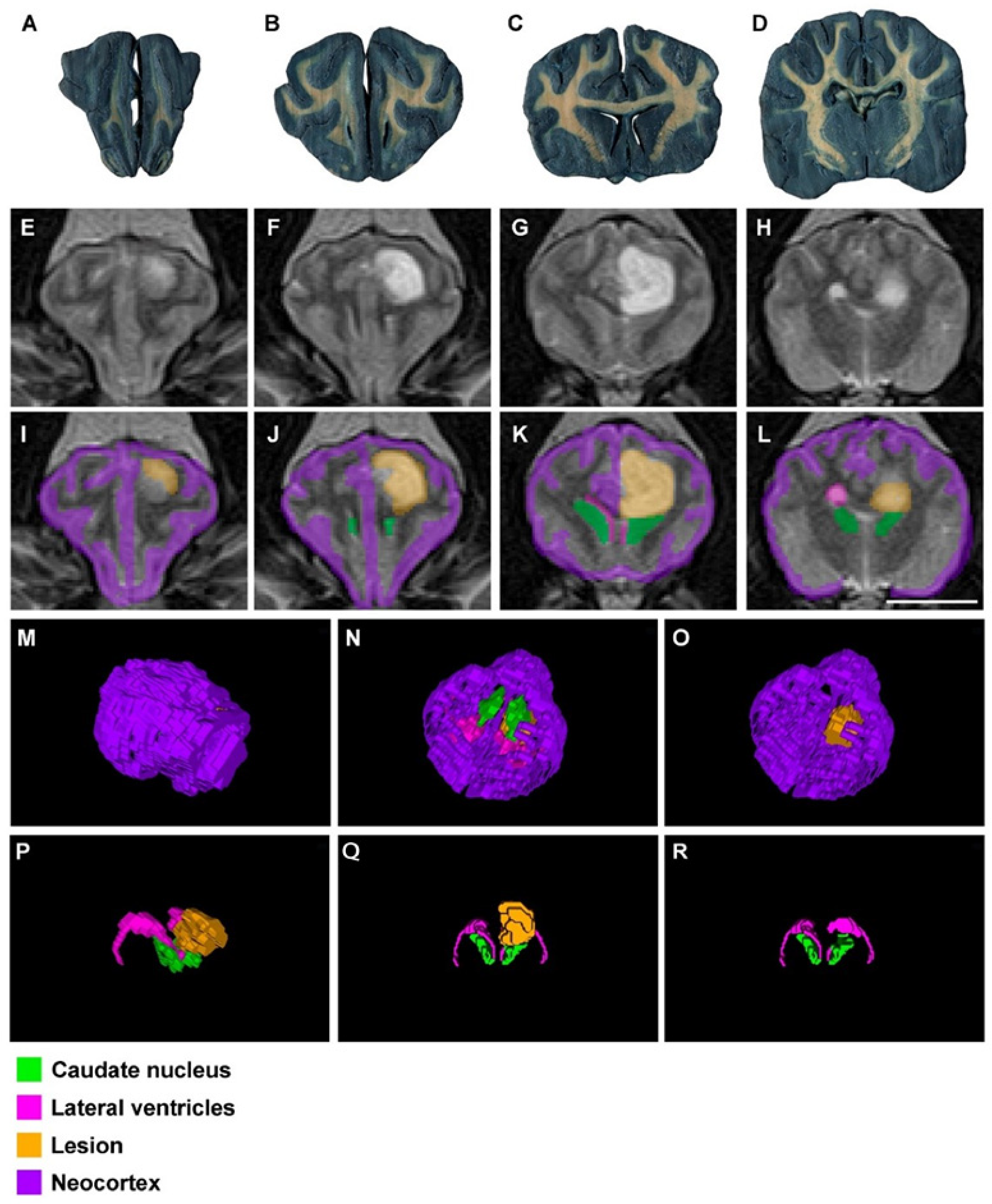
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wakuri, H.; Zhang, Y.; Liu, B.; Yang, W. What is anatomy for? Considerations on the body as a whole and whole anatomy. Okajimas Folia Anat. Jpn. 1998, 75, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld-Tacher, R.M.; Horn, T.J.; Scheviak, T.A.; Royal, K.D.; Hudson, L.C. Evaluation of 3D additively manufactured canine brain models for teaching veterinary neuroanatomy. J. Vet. Med. Educ. 2017, 44, 612–619. [Google Scholar] [CrossRef]
- Foss, K.D.; Seals, C.D.A.; Hague, D.W.; Mitek, A.E. Effectiveness of supplementary materials in teaching the veterinary neurologic Examination. J. Vet. Med. Educ. 2022, 49, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Khayruddeen, L.; Livingstone, D.; Ferguson, E. Creating a 3D learning tool for the growth and development of the craniofacial skeleton. Adv. Exp. Med. Biol. 2019, 1138, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.; Firmino-Machado, J.; Tsisar, S.; Viana, B.; Pinto-Sousa, M.; Vieira-Marques, P.; Cruz-Correia, R.; Ferreira, M.A. The role of anatomy computer-assisted learning on spatial abilities of Medical students. Anat. Sci. Educ. 2019, 12, 138–153. [Google Scholar] [CrossRef]
- Memon, I. Cadaver dissection is obsolete in medical training! A misinterpreted notion. Med. Princ. Pract. 2018, 27, 201–210. [Google Scholar] [CrossRef]
- Araujo Cuauro, J.C. Aspectos históricos de la enseñanza de la anatomía humana desde la época primitiva hasta el siglo XXI en el desarrollo de las ciencias morfológicas. Rev. Argent. Anat. Online 2018, 9, 87–97. [Google Scholar]
- Van Ginnekeng, C.J.; Vanthournout, G. Rethinking the learning and evaluation environment of a veterinary course in gross anatomy: The implementation of an assessment and development center and an E-Learning platform. J. Vet. Med. Educ. 2005, 32, 537–543. [Google Scholar] [CrossRef]
- Guevar, J. The evolution of educational technology in veterinary anatomy education. Adv. Exp. Med. Biol. 2020, 1260, 13–25. [Google Scholar] [CrossRef]
- Krucker, T.; Lang, A.; Meyer, E.P. New polyurethane-based material for vascular corrosion casting with improved physical and imaging characteristics. Microsc. Res. Tech. 2006, 69, 138–147. [Google Scholar] [CrossRef]
- Riederer, B.M. Plastination and its importance in teaching anatomy. Critical points for long-term preservation of human tissue. J. Anat. 2014, 224, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.A.; Dobson, H. Radiology as a tool for teaching veterinary anatomy. J. Vet. Med. Educ. 2003, 30, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, G.S.; Solorio, L.; Broome, A.M.; Salem, N.; Kolthammer, J.; Shah, T.; Flask, C.; Duerk, J.L. Whole animal imaging. Rev. Syst. Biol. Med. 2010, 2, 398–421. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.; Bellemare, C.; Toulouse, J.; Wells, G.A. Spatial abilities training in anatomy education: A systematic review. Anat. Sci. Educ. 2020, 13, 71–79. [Google Scholar] [CrossRef]
- Christ, R.; Guevar, J.; Poyade, M.; Rea, P.M. Proof of concept of a workflow methodology for the creation of basic canine head anatomy veterinary education tool using augmented reality. PLoS ONE 2018, 13, e0195866. [Google Scholar] [CrossRef] [PubMed]
- Hadžiomerović, N.; Hadžiomerović, A.I.; Avdić, R.; Muminović, A.; Tandir, F.; Bejdić, P.; Pandžić, A. Students’ performance in teaching neuroanatomy using traditional and technology-based methods. Anat. Histol. Embryol. 2023, 52, 115–122. [Google Scholar] [CrossRef]
- Raffan, H.; Guevar, I.; Poyade, M.; Rea, P.M. Canine neuroanatomy: Development of a 3D reconstruction and interactive ap-plication for. PLoS ONE 2017, 12, e0168911. [Google Scholar] [CrossRef]
- Falkingham, P.L. Acquisition of high-resolution three-dimensional models using free, open-source, photogrammetric software. Palaeontol. Electron. 2012, 15, 15.1.1T. [Google Scholar] [CrossRef]
- Herrero, M.J.; Pérez-Fortes, A.P.; Escavy, J.I.; Insua-Arévalo, J.M.; De La Horra, R.; López-Acevedo, F.; Trigos, L. 3D model generated from UAV photogrammetry and semi-automated rock mass characterization. Comput. Geosci. 2022, 163, 105121. [Google Scholar] [CrossRef]
- Forlizzi, V.; Miranda-Solís, F.; Pérez Cruz, J.C.; Cahuantico-Choquevilca, L.A.; Morán, G.; Baldoncini, M. Tinción de Mulligan en Neuroanatomía: Protocolización de la técnica. Rev. Argent. Anat. Online 2020, 11, 31–34. [Google Scholar]
- Bythell, J.; Pan, P.; Lee, J. Three-dimensional morphometric measurements of reef corals using underwater photogrammetry techniques. Coral Reefs 2001, 20, 193–199. [Google Scholar] [CrossRef]
- Krontiris-Litowitz, J. Using truncated lectures, conceptual exercises, and manipulatives to improve learning in the neuroanatomy classroom. Adv. Physiol. Educ. 2008, 32, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Petersson, H.; Sinkvist, D.; Wang, C.; Smedby, O. Web-based interactive 3D visualization as a tool for improved anatomy learning. Anat. Sci. Ed. 2009, 2, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Charchanti, A.V.; Troupis, T.G. Modernization of an anatomy class: From conceptualization to implementation. A case for integrated multimodal–multidisciplinary teaching. Anat. Sci. Educ. 2012, 5, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Preece, D.; Williams, S.B.; Lam, R.; Weller, R. “Let’s get physical”: Advantages of a physical model over 3D computer models and textbooks in learning imaging anatomy. Anat. Sci. Educ. 2013, 6, 216–224. [Google Scholar] [CrossRef]
- Ghosh, S.K. Cadaveric dissection as an educational tool for anatomical sciences in the 21st century. Anat. Sci. Educ. 2017, 10, 286–299. [Google Scholar] [CrossRef]
- Langlois, J.; Bellemare, C.; Toulouse, J.; Wells, G.A. Spatial abilities and anatomy knowledge assessment: A systematic review. Anat. Sci. Educ. 2017, 10, 235–241. [Google Scholar] [CrossRef]
- Joseph, M.; Singh, B. Recent advances and changing face of anatomy teaching and Learning in medical education. Natl. J. Clin. Anat. 2019, 8, 49–52. [Google Scholar] [CrossRef]
- Yuen, J. What is the role of 3D printing in undergraduate anatomy education? A scoping review of current literature and recommendations. Med. Sci. Educ. 2020, 30, 1321–1329. [Google Scholar] [CrossRef]
- Bernard, F.; Richard, P.; Kahn, A.; Fournier, H.D. Does 3D stereoscopy support anatomical education? Surg. Radiol. Anat. 2020, 42, 843–852. [Google Scholar] [CrossRef]
- Bogomolova, K.; Vorstenbosch, M.A.T.M.; El Messaoudi, I.; Holla, M.; Hovius, S.E.R.; van der Hage, J.A.; Hierck, B.P. Effect of binocular disparity on learning anatomy with stereoscopic augmented reality visualization: A double center randomized controlled trial. Anat. Sci. Educ. 2023, 16, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Chytas, D.; Salmas, M.; Demesticha, T.; Noussios, G.; Paraskevas, G.; Chrysanthou, C.; Asouhidou, I.; Katsourakis, A.; Fiska, A. A review of the use of virtual reality for teaching radiology in conjunction with anatomy. Cureus 2021, 13, e20174. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Naaz, S.; Patra, A.; Ravi, K.S.; Khanal, L. Effectiveness of 3D-printed models prepared from radiological data for anatomy education: A meta-analysis and trial sequential analysis of 22 randomized, controlled, crossover trials. J. Educ. Health Promot. 2022, 11, 353. [Google Scholar] [CrossRef]
- Manson, A.; Poyade, M.; Rea, P. A recommended workflow methodology in the creation of an educational and training application incorporating a digital reconstruction of the cerebral ventricular system and cerebrospinal fluid circulation to aid anatomical understanding. BMC Med. Imaging 2015, 15, 44. [Google Scholar] [CrossRef]
- Ocampo-Navia, M.I.; Gómez-Vega, J.C. Techniques for an anatomic approach to the study of the brain: A review. Univ. Med. 2021, 62, 22–32. [Google Scholar] [CrossRef]
- Jacquesson, T.; Simon, E.; Dauleac, C.; Margueron, L.; Robinson, P.; Mertens, P. Stereoscopic three-dimensional visualization: Interest for neuroanatomy teaching in medical school. Surg. Radiol. Anat. 2020, 42, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, H.; Al Mushaiqri, M.; Albalushi, H.; Al-Zaabi, A.A.; Roychoudhury, S.; Das, S. Teaching, learning and assessing anatomy with artificial intelligence: The road to a better future. Int. J. Environ. Res. Public Health 2022, 19, 14209. [Google Scholar] [CrossRef]
- Leandro, R.M.; Foz Filho, R.P.P.; De Silvio, M.M.; Quilici, A.P.; Sattin, M.M.; Paretsis, B.F.; Souza, V.A. Construction of the equine digestive system: A tool for teaching topographical anatomy. J. Vet. Med. Educ. 2019, 46, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Xiberta, P.; Boada, I. IVET, an interactive veterinary education tool. J. Anim. Sci. 2019, 97, 932–944. [Google Scholar] [CrossRef]
- Suñol, A.; Aige, V.; Morales, C.; López-Beltran, M.; Feliu-Pascual, A.L.; Puig, J. Use of three-dimensional printing models for veterinary medical education: Impact on learning how to identify canine vertebral fractures. J. Vet. Med. Educ. 2019, 46, 523–532. [Google Scholar] [CrossRef]
- Little, W.B.; Artemiou, E.; Fuentealba, C.; Conan, A.; Sparks, C. Veterinary students and faculty partner in developing a virtual three-dimensional (3D) interactive touch screen canine anatomy table. Med. Sci. Educ. 2019, 29, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wilhite, R.; Wölfel, I. 3D Printing for veterinary anatomy: An overview. Anat. Histol. Embryol. 2019, 48, 609–620. [Google Scholar] [CrossRef] [PubMed]
- DeBose, K. Virtual anatomy: Expanding veterinary student learning. J. Med. Libr. Assoc. 2020, 108, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, G.; Eberspächer-Schweda, M.C.; Hatamikia, S.; Königshofer, M.; Baumgartner, D.; Kramer, A.M.; Schaffarich, P.; Agis, H.; Moscato, F.; Unger, E. 3D printed biomimetic rabbit airway simulation model for nasotracheal intubation training. Front. Vet. Sci. 2020, 7, 587524. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, T.H.; Ryu, J.Y.; Kim, D.K.; Oh, E.J.; Kim, H.M.; Shim, J.-H.; Yun, W.-S.; Huh, J.B.; Moon, S.H.; et al. Osteogenesis of 3D-printed PCL/TCP/bdECM scaffold using adipose-derived stem cells aggregates; an experimental study in the canine mandible. Int. J. Mol. Sci. 2021, 22, 5409. [Google Scholar] [CrossRef] [PubMed]
- Canright, A.; Bescoby, S.; Dickson, J. Evaluation of a 3D computer model of the equine paranasal sinuses as a tool for veterinary anatomy education. J. Vet. Med. Educ. 2023, 50, 234–242. [Google Scholar] [CrossRef]
- Plewa, B.; Skieresz-Szewczyk, K.; Jackowiak, H. Three-dimensional characteristic of fungiform papillae and its taste buds in European bison (Bison bonasus), cattle (Bos taurus), and Bison bonasus hybrid. BMC Vet. Res. 2022, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Senos, R.; Ramos Leite, C.A.; Dos Santos Tolezano, F.; Roberto-Rodrigues, M.; Pérez, W. Using videos in active learning: An experience in veterinary anatomy. Anat. Histol. Embryol. 2023, 52, 50–54. [Google Scholar] [CrossRef]
- Kapoor, K.; Singh, A. Veterinary anatomy teaching from real to virtual reality: An unprecedented shift during COVID-19 in socially distant era. Anat. Histol. Embryol. 2022, 51, 163–169. [Google Scholar] [CrossRef]
- Hammerton, C.; Yip, S.W.L.; Manobharath, N.; Myers, G.; Sturrock, A. Are 3D printed models acceptable in assessment? Clin. Teach. 2022, 19, 221–228. [Google Scholar] [CrossRef]
- Montero-Crespo, M.; Dominguez-Alvaro, M.; Rondon-Carrillo, P.; Alonso-Nanclares, L.; DeFelipe, J.; Blazquez-Llorca, L. Three-dimensional synaptic organization of the human hippocampal CA1 field. Elife 2020, 9, e57013. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blázquez-Llorca, L.; Morales de Paz, L.; Martín-Orti, R.; Santos-Álvarez, I.; Fernández-Valle, M.E.; Castejón, D.; García-Real, M.I.; Salgüero-Fernández, R.; Pérez-Lloret, P.; Moreno, N.; et al. The Application of 3D Anatomy for Teaching Veterinary Clinical Neurology. Animals 2023, 13, 1601. https://doi.org/10.3390/ani13101601
Blázquez-Llorca L, Morales de Paz L, Martín-Orti R, Santos-Álvarez I, Fernández-Valle ME, Castejón D, García-Real MI, Salgüero-Fernández R, Pérez-Lloret P, Moreno N, et al. The Application of 3D Anatomy for Teaching Veterinary Clinical Neurology. Animals. 2023; 13(10):1601. https://doi.org/10.3390/ani13101601
Chicago/Turabian StyleBlázquez-Llorca, Lidia, Lubna Morales de Paz, Rosario Martín-Orti, Inmaculada Santos-Álvarez, María E. Fernández-Valle, David Castejón, María I. García-Real, Raquel Salgüero-Fernández, Pilar Pérez-Lloret, Nerea Moreno, and et al. 2023. "The Application of 3D Anatomy for Teaching Veterinary Clinical Neurology" Animals 13, no. 10: 1601. https://doi.org/10.3390/ani13101601
APA StyleBlázquez-Llorca, L., Morales de Paz, L., Martín-Orti, R., Santos-Álvarez, I., Fernández-Valle, M. E., Castejón, D., García-Real, M. I., Salgüero-Fernández, R., Pérez-Lloret, P., Moreno, N., Jiménez, S., Herrero-Fernández, M. J., & González-Soriano, J. (2023). The Application of 3D Anatomy for Teaching Veterinary Clinical Neurology. Animals, 13(10), 1601. https://doi.org/10.3390/ani13101601








