Consistently Inconsistent Perceptual Illusions in Nonhuman Primates: The Importance of Individual Differences
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
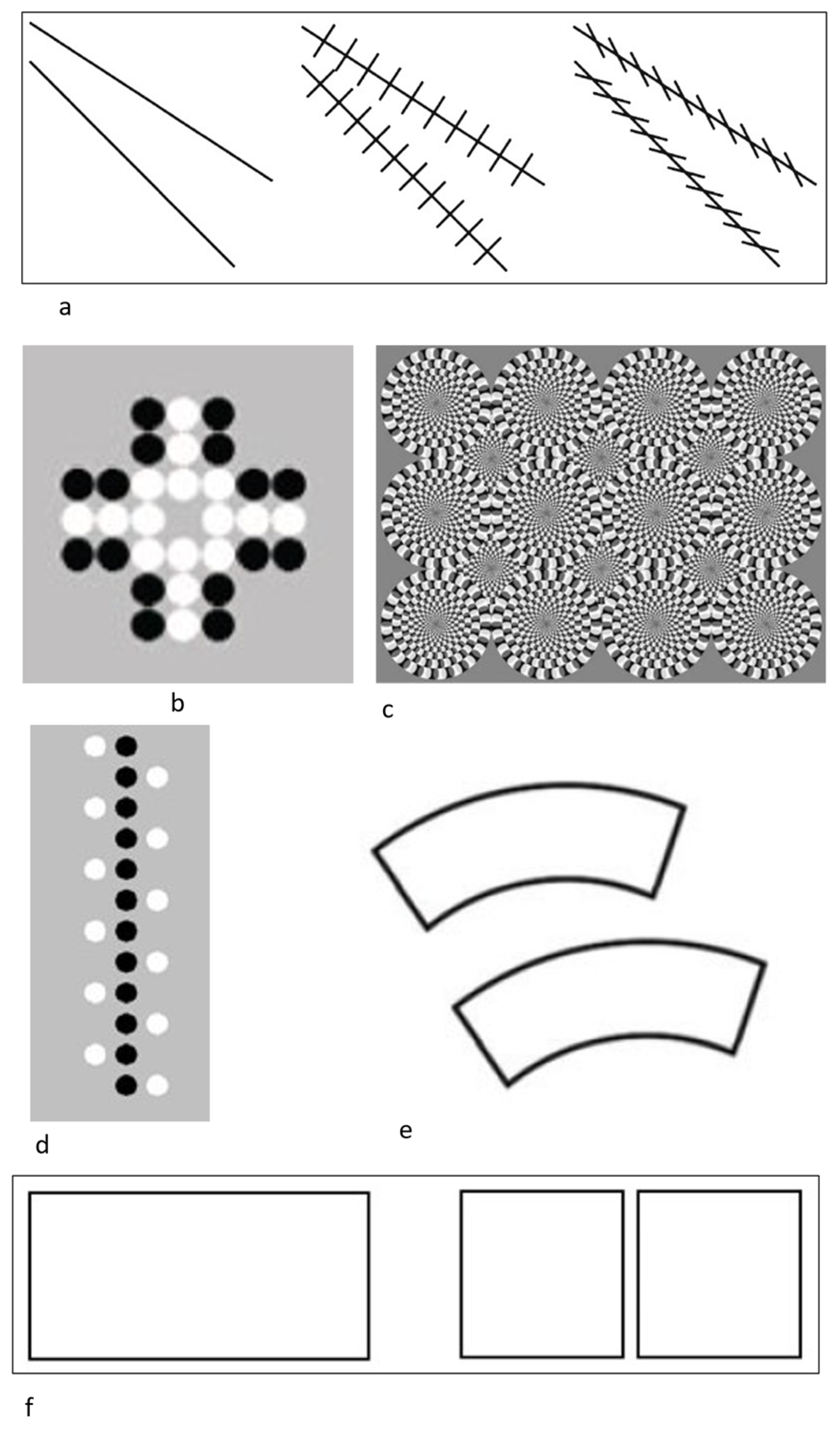
Agrillo et al. (2014a): To assess possible susceptibility to the Zöllner illusion (Figure 1a), rhesus monkeys were trained to select the narrower of two gaps at the end of two convergent lines presented on a computer screen. After training, three conditions were presented. In two control conditions, there were no crosshatches present or those crosshatches were perpendicular, and so no illusory experience could occur. In the illusory condition, the crosshatches were aligned in the manner that induced the illusion in humans (i.e., oblique crosshatching lines). The results showed that rhesus monkeys perceived the Zöllner illusion in the same direction as humans [41].
Agrillo et al. (2014b): Rhesus monkeys and capuchin monkeys were assessed for their susceptibility to the Solitaire illusion (Figure 1b, [42,43]). The task involved choosing one of two arrays based on that array having more white dots. The Solitaire configuration (clustered central dots forming a better Gestalt) typically leads to overestimation relative to the same number of dots located on the periphery of the array. Approximately half of the monkeys showed some susceptibility to this illusion in the first experiment, but there were substantial individual differences. An attempted replication within the same study with the same monkeys largely failed, providing evidence that this illusion was fleeting, at best, in these species.
Agrillo et al. (2015): This experiment assessed whether rhesus monkeys experienced illusory motion using the Rotating Snake Illusion (Figure 1c; [44]). Real motion is perceived based on a specific luminance pattern comprising concentric circles (black–dark grey–white–light grey). Monkeys were first trained to choose dynamic arrays with moving stimuli over static arrays. They then were given a choice between the Rotating Snake Illusion and a control image that was highly similar but alternated color patterns in a way that does not produce the experience of illusory movement for humans. In a second variation of the task, monkeys saw a single stimulus and had to classify it as having movement present or not. In training trials, real movement was present, and in test trials with the Rotating Snake Illusion, the question was how the monkeys would classify that stimulus. Some monkeys responded in a manner consistent with experiencing illusory motion, although the effect was subtle relative to some other illusory experiences. A second experiment required the monkeys to learn to choose the Rotating Snake stimulus over a static stimulus, and then compared performance to when two static stimuli were presented, and one was the reinforced choice. Most monkeys performed better and learned faster when asked to choose the Rotating Snake image, again suggesting some experience of illusory motion.
Parrish et al. (2015): In this study [45], monkeys and adult humans were tested on the Delboeuf illusion, in which small concentric rings lead to the overestimation of central dot size (assimilation effects) and large concentric rings lead underestimation of central dot size. Rhesus monkeys and capuchin monkeys were trained to select the larger of two black dots encircled by large and small concentric rings in Experiment 1. Adult humans and several monkeys perceived the illusion in the standard direction, but the majority of monkeys perceived a reversed illusion (selecting the dot encircled by the large ring as larger) or did not perceive the illusion in either direction. To rule out an alternate explanation (that the monkeys were responding on the basis of outer ring size vs. central dot), we trained monkeys to classify a central dot that was encircled by an outer ring of variable size as ‘small’ or ‘large’ in Experiment 2. Adult humans and the majority of monkeys perceived the illusion in the standard direction, underscoring the importance of methodological design in the study of visual illusions.
Parrish et al. (2016): In this experiment [36], we presented the Solitaire illusion (see Figure 1b) as described above via computerized testing to preschool children and task-naïve capuchin monkeys that had limited experience with computerized testing to further explore the role of experience in the emergence (or not) of this illusion. The task-naïve capuchin monkeys perceived the illusion, but there were large individual differences in susceptibility akin to our previous results [43]. Younger children performed similarly to monkeys in terms of the variance in illusion susceptibility, whereas older children were more consistent in their perception of the Solitaire illusion. Furthermore, individual susceptibility by capuchin monkeys to the Solitaire illusion did not correlate with a related numerosity illusion, the density bias [46,47].
Parrish et al. (2019): In this experiment studying linear numerosity illusions (a variant of the Solitaire array described above), rhesus monkeys and capuchin monkeys were trained to choose the array with a larger number of black dots relative to white dots. The illusory stimuli were those in which the central, contiguous array was black, whereas the white dots were peripheral to that central arrangement (Figure 1d). Humans tend to perceive the central, linear array as being more numerous than an equal number of dots on the periphery as the central dots form a better Gestalt. Approximately half of the monkeys showed the same illusory effect, although it was subtle [48]. Additionally, again, there were substantial individual differences, with one monkey showing a reverse bias to that seen in humans.
Agrillo et al. (2019): This study assessed the Jastrow illusion (Figure 1e) in capuchin monkeys and rhesus monkeys [49]. In this illusion, humans typically overestimate the size of the bottom figure relative to the identically sized top figure due to their spatial layout. Despite learning that they needed to select the larger of two stimuli in a computerized two-choice discrimination task, none of the monkeys we tested showed susceptibility to this illusion when identically sized images were arranged in the Jastrow pattern [50]. Susceptibility to the illusion may be supported by global processing mechanisms, which emerge more readily for human subjects relative to nonhuman primates.
Parrish et al. (2020): This experiment assessed the density bias in capuchin monkeys using arrays of food as the choice options [46]. This bias emerges when densely arranged stimuli are overestimated or preferred relative to an equal number of sparsely arranged items. Monkeys saw arrangements of food items in which those items were sparsely distributed or densely arranged. Most monkeys were biased to select a denser food set over the same number of food items in a sparsely arranged set, suggesting that they misperceived those items as being numerous. These results were consistent with computerized testing of the density bias with these same monkeys [47], although those results are not included in the current review as individual performances were not reported.
Parrish and Beran (2021): In this experiment, rhesus monkeys were trained to choose the larger of two rectangular stimuli on a computer screen. After becoming proficient at this, they were given trials in which either of both choice options were of low contrast to the background white color (i.e., gray rectangles) or were of high contrast (i.e., black rectangles). In other trials, the background was black, and so white rectangles were of high contrast and gray rectangles were again lower contrast. For humans, higher contrast stimuli are often overestimated in terms of their size [51], likely due to perceptual fluency of high-contrast relative to low-contrast stimuli. This result was found for some of the monkeys as well [52].
McKeon et al. (2022): Rhesus monkeys were presented with the “one is more” illusion [53] to determine whether they would show a comparable illusion to that recently reported in humans [54]. When presented with continuous objects rather than multiple discrete objects, humans experience the continuous objects as being longer compared to the discrete objects of equal length. Monkeys were trained to choose the longer of two truly different-length items on a computer screen, and then they were given probe trials in which a continuous stimulus and a discrete stimulus of equal length were presented (Figure 1f). Unlike humans, the monkeys showed no preferences in these trials, and overall they performed very highly by ignoring the discrete/continuous relation and instead focusing on the true length of stimuli to make judgments.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 50 Optical Illusions That’ll Blow Your Mind. Available online: https://parade.com/1312776/marynliles/optical-illusions/ (accessed on 16 November 2022).
- Two Years Later, We Finally Know Why People Saw “The Dress” Differently. Available online: https://slate.com/technology/2017/04/heres-why-people-saw-the-dress-differently.html#:~:text=Remember%2C%20the%20dress%20is%20actually,Because%20shadows%20overrepresent%20blue%20light (accessed on 16 November 2022).
- Müller-Lyer, F.C. Optische Urteilstäuschungen. Arch. Anat. Physiol. Physiol. Abt. 1889, 2, 263–270. [Google Scholar]
- Thompson, P. Margaret Thatcher: A New Illusion. Perception 1980, 9, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, A.; Ashida, H. Phenomenal characteristics of the peripheral drift illusion. Vision 2003, 15, 261–262. [Google Scholar]
- Carbon, C.-C. Understanding human perception by human-made illusions. Front. Hum. Neurosci. 2014, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Necker, L.A. Observations on some remarkable phenomena seen in Switzerland; And an optical phenomenon which occurs on viewing of a crystal or geometrical solid. Phil. Mag. 1832, 3, 329–337. [Google Scholar] [CrossRef]
- Hering, E. Beitrage zur Physiologie; W. Engelmann: Leipzig, Germany, 1861. [Google Scholar]
- Zöllner, F. Ueber eine neue Art von Pseudoskopie und ihre Beziehungen zu den von Plateau und Oppel beschriebenen Bewegungsphänomenen. Ann. Phys. 1860, 186, 500–523. [Google Scholar] [CrossRef]
- Gregory, R.L. Perceptual illusions and brain models. Proc. R. Soc. Lond. Ser. B Boil. Sci. 1968, 171, 279–296. [Google Scholar] [CrossRef]
- Winslow, C.N. Visual illusions in the chick. Arch. Psychol. 1933, 153, 80. [Google Scholar]
- Dominguez, K.E. A Study of Visual Illusions in the Monkey. J. Genet. Psychol. 1954, 85, 105–127. [Google Scholar] [CrossRef]
- Benhar, E.; Samuel, D. Visual illusions in the baboon (Papio anubis). Anim. Learn. Behav. 1982, 10, 115–118. [Google Scholar] [CrossRef]
- Lõoke, M.; Marinelli, L.; Eatherington, C.J.; Agrillo, C.; Mongillo, P. Do Domestic Dogs (Canis lupus familiaris) Perceive Numerosity Illusions? Animals 2020, 10, 2304. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, C.; Santacà, M.; Pecunioso, A.; Petrazzini, M.E.M. Everything is subjective under water surface, too: Visual illusions in fish. Anim. Cogn. 2020, 23, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Cappellato, A.; Petrazzini, M.E.M.; Bisazza, A.; Dadda, M.; Agrillo, C. Susceptibility to Size Visual Illusions in a Non-Primate Mammal (Equus caballus). Animals 2020, 10, 1673. [Google Scholar] [CrossRef] [PubMed]
- Petrazzini, M.E.M.; Bisazza, A.; Agrillo, C. Do domestic dogs (Canis lupus familiaris) perceive the Delboeuf illusion? Anim. Cogn. 2017, 20, 427–434. [Google Scholar] [CrossRef]
- Byosiere, S.-E.; Feng, L.C.; Woodhead, J.K.; Rutter, N.J.; Chouinard, P.A.; Howell, T.J.; Bennett, P.C. Visual perception in domestic dogs: Susceptibility to the Ebbinghaus–Titchener and Delboeuf illusions. Anim. Cogn. 2017, 20, 435–448. [Google Scholar] [CrossRef]
- Byosiere, S.; Chouinard, P.A.; Howell, T.J.; Bennett, P.C. Illusion susceptibility in domestic dogs. Ethology 2020, 126, 949–965. [Google Scholar] [CrossRef]
- Santacà, M.; Petrazzini, M.E.M.; Agrillo, C.; Wilkinson, A. Can reptiles perceive visual illusions? Delboeuf illusion in red-footed tortoise (Chelonoidis carbonaria) and bearded dragon (Pogona vitticeps). J. Comp. Psychol. 2019, 133, 419–427. [Google Scholar] [CrossRef]
- Fujita, K. Perception of the Ponzo illusion by rhesus monkeys, chimpanzees, and humans: Similarity and difference in the three primate species. Percept. Psychophys. 1997, 59, 284–292. [Google Scholar] [CrossRef]
- Fagot, J.; Tomonaga, M. Effects of element separation on perceptual grouping by humans (Homo sapiens) and chimpanzees (Pan troglodytes): Perception of Kanizsa illusory figures. Anim. Cogn. 2001, 4, 171–177. [Google Scholar] [CrossRef]
- Nakamura, N.; Watanabe, S.; Fujita, K. Pigeons perceive the Ebbinghaus-Titchener circles as an assimilation illusion. J. Exp. Psychol. Anim. Behav. Process. 2008, 34, 375–387. [Google Scholar] [CrossRef]
- Fujita, K.; Blough, D.S.; Blough, P.M. Pigeons see the Ponzo illusion. Anim. Learn. Behav. 1991, 19, 283–293. [Google Scholar] [CrossRef]
- Santaca, M.; Regaiolli, B.; Petrazzini, M.E.M.; Spiezio, C.; Agrillo, C. Preliminary study to investigate the Delboeuf illusion in ring-tailed lemurs (Lemur catta): Methodological Challenges. Anim. Behav. Cogn. 2017, 4, 365–377. [Google Scholar] [CrossRef][Green Version]
- Santacà, M.; Petrazzini, M.E.M.; Agrillo, C.; Wilkinson, A. Exploring the Müller-Lyer illusion in a nonavian reptile (Pogona vitticeps). J. Comp. Psychol. 2020, 134, 391–400. [Google Scholar] [CrossRef] [PubMed]
- McGurk, E. Susceptibility to Visual Illusions. J. Psychol. 1865, 61, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.L. Cross-Cultural Differences in Visual Perception of Color, Illusions, Depth, and Pictures. Cross-Cult. Psychol. Contemp. Themes Perspect. 2019, 287–308. [Google Scholar] [CrossRef]
- Dawson, J.L. Cultural and Physiological Influences Upon Spatial-Perceptual Processes in West Africa. Part I. Int. J. Psychol. 1967, 2, 115–128. [Google Scholar] [CrossRef]
- de Fockert, J.; Davidoff, J.; Fagot, J.; Parron, C.; Goldstein, J. More accurate size contrast judgments in the Ebbinghaus Illusion by a remote culture. J. Exp. Psychol. Hum. Percept. Perform. 2007, 33, 738–742. [Google Scholar] [CrossRef]
- Berry, J.W. Ecology, perceptual development and the Mueller-Lyer illusion. Br. J. Psychol. 1968, 59, 205–210. [Google Scholar] [CrossRef]
- Happé, F.G. Studying Weak Central Coherence at Low Levels: Children with Autism do not Succumb to Visual Illusions. A Research Note. J. Child Psychol. Psychiatry 1996, 37, 873–877. [Google Scholar] [CrossRef]
- Happe, F.; Briskman, J.; Frith, U. Exploring the Cognitive Phenotype of Autism: Weak “Central Coherence” in Parents and Siblings of Children with Autism: I. Experimental Tests. J. Child Psychol. Psychiatry 2001, 42, 299–307. [Google Scholar] [CrossRef]
- Witkin, H.A. A Cognitive-Style Approach to Cross-Cultural Research. Int. J. Psychol. 1967, 2, 233–250. [Google Scholar] [CrossRef]
- Hanus, D.; Truppa, V.; Call, J. Are you as fooled as I am? Visual illusions in human (Homo) and nonhuman (Sapajus, Gorilla, Pan, Pongo) primate species. J. Comp. Psychol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.E.; Agrillo, C.; Perdue, B.M.; Beran, M.J. The elusive illusion: Do children (Homo sapiens) and capuchin monkeys (Cebus apella) see the Solitaire illusion? J. Exp. Child Psychol. 2016, 142, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Cretenoud, A.F.; Grzeczkowski, L.; Bertamini, M.; Herzog, M.H. Individual differences in the Müller-Lyer and Ponzo illusions are stable across different contexts. J. Vis. 2020, 20, 4. [Google Scholar] [CrossRef]
- Cretenoud, A.F.; Grzeczkowski, L.; Kunchulia, M.; Herzog, M.H. Individual differences in the perception of visual illusions are stable across eyes, time, and measurement methods. J. Vis. 2021, 21, 26. [Google Scholar] [CrossRef]
- Grzeczkowski, L.; Clarke, A.M.; Francis, G.; Mast, F.W.; Herzog, M.H. About individual differences in vision. Vis. Res. 2017, 141, 282–292. [Google Scholar] [CrossRef]
- Coren, S.; Porac, C. Individual differences in visual-geometric illusions: Predictions from measures of spatial cognitive abilities. Percept. Psychophys. 1987, 41, 211–219. [Google Scholar] [CrossRef]
- Agrillo, C.; Parrish, A.E.; Beran, M. Do rhesus monkeys (Macaca mulatta) perceive the Zöllner illusion? Psychon. Bull. Rev. 2014, 21, 986–994. [Google Scholar] [CrossRef]
- Frith, C.D.; Frit, U. The solitaire illusion: An illusion of numerosity. Percept. Psychophys. 1972, 11, 409–410. [Google Scholar] [CrossRef]
- Agrillo, C.; Parrish, A.E.; Beran, M.J. Do primates see the solitaire illusion differently? A comparative assessment of humans (Homo sapiens), chimpanzees (Pan troglodytes), rhesus monkeys (Macaca mulatta), and capuchin monkeys (Cebus apella). J. Comp. Psychol. 2014, 128, 402–413. [Google Scholar] [CrossRef]
- Agrillo, C.; Gori, S.; Beran, M.J. Do rhesus monkeys (Macaca mulatta) perceive illusory motion? Anim. Cogn. 2015, 18, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.E.; Brosnan, S.F.; Beran, M.J. Do you see what I see? A comparative investigation of the Delboeuf illusion in humans (Homo sapiens), rhesus monkeys (Macaca mulatta), and capuchin monkeys (Cebus apella). J. Exp. Psychol. Anim. Learn. Cogn. 2015, 41, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.E.; James, B.T.; Beran, M.J. Exploring whether nonhuman primates show a bias to overestimate dense quantities. J. Comp. Psychol. 2017, 131, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.E.; French, K.A.; Guild, A.S.; Creamer, C.L.; Rossettie, M.S.; Beran, M.J. The density bias: Capuchin monkeys (Sapajus apella) prefer densely arranged items in a food-choice task. J. Comp. Psychol. 2020, 134, 232–240. [Google Scholar] [CrossRef]
- Parrish, A.E.; Beran, M.J.; Agrillo, C. Linear numerosity illusions in capuchin monkeys (Sapajus apella), rhesus macaques (Macaca mulatta), and humans (Homo sapiens). Anim. Cogn. 2019, 22, 883–895. [Google Scholar] [CrossRef]
- Jastrow, J. On the judgment of angles and positions of lines. A. On the judgment of angles. Am. J. Psychol. 1892, 5, 214–217. [Google Scholar] [CrossRef]
- Agrillo, C.; Beran, M.J.; Parrish, A.E. Exploring the Jastrow Illusion in Humans (Homo sapiens), Rhesus Monkeys (Macaca mulatta), and Capuchin Monkeys (Sapajus apella). Perception 2019, 48, 367–385. [Google Scholar] [CrossRef]
- Barra, J.; Pallier, C.; New, B. The black superiority effect: Black is taller than gray. Acta Psychol. 2020, 202, 102958. [Google Scholar] [CrossRef]
- Parrish, A.E.; Beran, M.J. Children and monkeys overestimate the size of high-contrast stimuli. Atten. Percept. Psychophys. 2021, 83, 2123–2135. [Google Scholar] [CrossRef]
- McKeon, E.J.; Beran, M.J.; Parrish, A.E. Children (Homo sapiens), but not rhesus monkeys (Macaca mulatta), perceive the one-is-more illusion. J. Comp. Psychol. 2022, 136, 270–278. [Google Scholar] [CrossRef]
- Yousif, S.R.; Scholl, B.J. The one-is-more illusion: Sets of discrete objects appear less extended than equivalent continuous entities in both space and time. Cognition 2019, 185, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Delboeuf, J.L.R. Sur une nouvelle illusion d’optique [On a new optical illusion]. Acad. R. Sci. Lett. Beaux Arts Belg. Bull. 1892, 24, 545–558. [Google Scholar]
- Ebbinghaus, H. The Principles of Psychology: Vol. I. II; Viet: Leipzig, Germany, 1892. [Google Scholar]
- Santacà, M.; Agrillo, C.; Petrazzini, M.M. The Challenge of Illusory Perception of Animals: The Impact of Methodological Variability in Cross-Species Investigation. Animals 2021, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.E. Visual illusions: Insights from comparative cognition. In Comparative Cognition: Commonalities and Diversity; Anderson, J.R., Kuroshima, H., Eds.; Springer: Singapore, 2021; pp. 15–30. [Google Scholar]
- Parrish, A.E.; Agrillo, C. Current perspectives on primate perception. In Primate Cognitive Studies; Schwartz, B.L., Beran, M.J., Eds.; Cambridge University Press: Cambridge, UK, 2022; pp. 115–134. [Google Scholar]
- Berry, J.W. Müller-Lyer Susceptibility: Culture, Ecology or Race? Int. J. Psychol. 1971, 6, 193–197. [Google Scholar] [CrossRef]
- Dakin, S.; Frith, U. Vagaries of Visual Perception in Autism. Neuron 2005, 48, 497–507. [Google Scholar] [CrossRef]
- Fagot, J.; Deruelle, C. Processing of global and local visual information and hemispheric specialization in humans (Homo sapiens) and baboons (Papio papio). J. Exp. Psychol. Hum. Percept. Perform. 1997, 23, 429–442. [Google Scholar] [CrossRef]
- Parron, C.; Fagot, J. Comparison of grouping abilities in humans (Homo sapiens) and baboons (Papio papio) with the Ebbinghaus illusion. J. Comp. Psychol. 2007, 121, 405–411. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Washburn, D.A. Matching visual stimuli on the basis of global and local features by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta). Anim. Cogn. 2002, 5, 27–31. [Google Scholar] [CrossRef]
- De Lillo, C.; Palumbo, M.; Spinozzi, G.; Giustino, G. Effects of pattern redundancy and hierarchical grouping on global–local visual processing in monkeys (Cebus apella) and humans (Homo sapiens). Behav. Brain Res. 2012, 226, 445–455. [Google Scholar] [CrossRef]
- Spinozzi, G.; De Lillo, C.; Salvi, V. Local advantage in the visual processing of hierarchical stimuli following manipulations of stimulus size and element numerosity in monkeys (Cebus apella). Behav. Brain Res. 2006, 166, 45–54. [Google Scholar] [CrossRef]
- Parrish, A.E.; Beran, M. When less is more: Like humans, chimpanzees (Pan troglodytes) misperceive food amounts based on plate size. Anim. Cogn. 2014, 17, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, E.; Call, J.; Hernàndez-Lloreda, M.V.; Hare, B.; Tomasello, M. Humans Have Evolved Specialized Skills of Social Cognition: The Cultural Intelligence Hypothesis. Science 2007, 317, 1360–1366. [Google Scholar] [CrossRef]
- Shaw, R.C.; Schmelz, M. Cognitive test batteries in animal cognition research: Evaluating the past, present and future of comparative psychometrics. Anim. Cogn. 2017, 20, 1003–1018. [Google Scholar] [CrossRef]
- Krasheninnikova, A.; Berardi, R.; Lind, M.-A.; O’Neill, L.; von Bayern, A.M. Primate cognition test battery in parrots. Behaviour 2019, 156, 721–761. [Google Scholar] [CrossRef]
| Monkey | Agrillo et al., 2014a | Agrillo et al., 2014b Exp 2 | Agrillo et al., 2014b Exp 3 | Agrillo et al., 2015 Exp 1 | Agrillo et al., 2015 Exp 4 | Parrish et al., 2015 Exp 1 | Parrish et al., 2015 Exp 2 | Parrish et al., 2016 Exp 1 | Parrish et al., 2019 | Agrillo et al., 2019 | Parrish et al., 2020 | Parrish & Beran 2021 Exp 1 | Parrish & Beran 2021 Exp 2 | McKeon et al., 2022 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macaques | ||||||||||||||
| Chewie | YES | NO | NO | YES | YES | No | YES | NO | NO | |||||
| Gale | YES | NO | YES | NO | YES | YES | ||||||||
| Han | YES | YES | NO | YES | YES | No | NO ^ | NO ^ | NO | |||||
| Hank | NO | NO | YES | NO | NO | NO ^ | NO | NO | NO | |||||
| Lou | YES | YES | YES | NO | YES | NO | NO | YES | YES | NO | ||||
| Luke | YES | NO | NO | YES | YES | NO | NO | NO | ||||||
| Murph | YES | NO | YES | NO | YES | NO | YES | NO | NO | YES | YES | NO | ||
| Obi | NO | NO | NO | YES | YES | NO | YES | YES | NO | |||||
| Capuchins | ||||||||||||||
| Gonzo * | NO | YES | YES | NO | NO | |||||||||
| Gretel * | NO | YES | YES | NO | NO | |||||||||
| Griffin | YES | NO | NO | YES | NO | NO | YES | |||||||
| Liam | NO | NO | NO ^ | YES | NO | NO | YES | |||||||
| Logan | YES | NO ^ | NO ^ | YES | YES | NO | NO | |||||||
| Mason | NO ^ | YES | NO | NO | NO | |||||||||
| Nala * | NO | NO | NO ^ | NO ^ | NO | NO | YES | |||||||
| Nkima | YES | NO | NO | YES | NO ^ | NO | NO | |||||||
| Widget * | NO | NO | YES | NO | YES | |||||||||
| Wren * | YES | NO | NO | YES | YES | NO | YES |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beran, M.J.; Parrish, A.E. Consistently Inconsistent Perceptual Illusions in Nonhuman Primates: The Importance of Individual Differences. Animals 2023, 13, 22. https://doi.org/10.3390/ani13010022
Beran MJ, Parrish AE. Consistently Inconsistent Perceptual Illusions in Nonhuman Primates: The Importance of Individual Differences. Animals. 2023; 13(1):22. https://doi.org/10.3390/ani13010022
Chicago/Turabian StyleBeran, Michael J., and Audrey E. Parrish. 2023. "Consistently Inconsistent Perceptual Illusions in Nonhuman Primates: The Importance of Individual Differences" Animals 13, no. 1: 22. https://doi.org/10.3390/ani13010022
APA StyleBeran, M. J., & Parrish, A. E. (2023). Consistently Inconsistent Perceptual Illusions in Nonhuman Primates: The Importance of Individual Differences. Animals, 13(1), 22. https://doi.org/10.3390/ani13010022







