Modeling Paratuberculosis Transmission in a Small Dairy Herd Typical of Slovenia Suggests That Different Models Should Be Used to Study Disease Spread in Herds of Different Sizes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
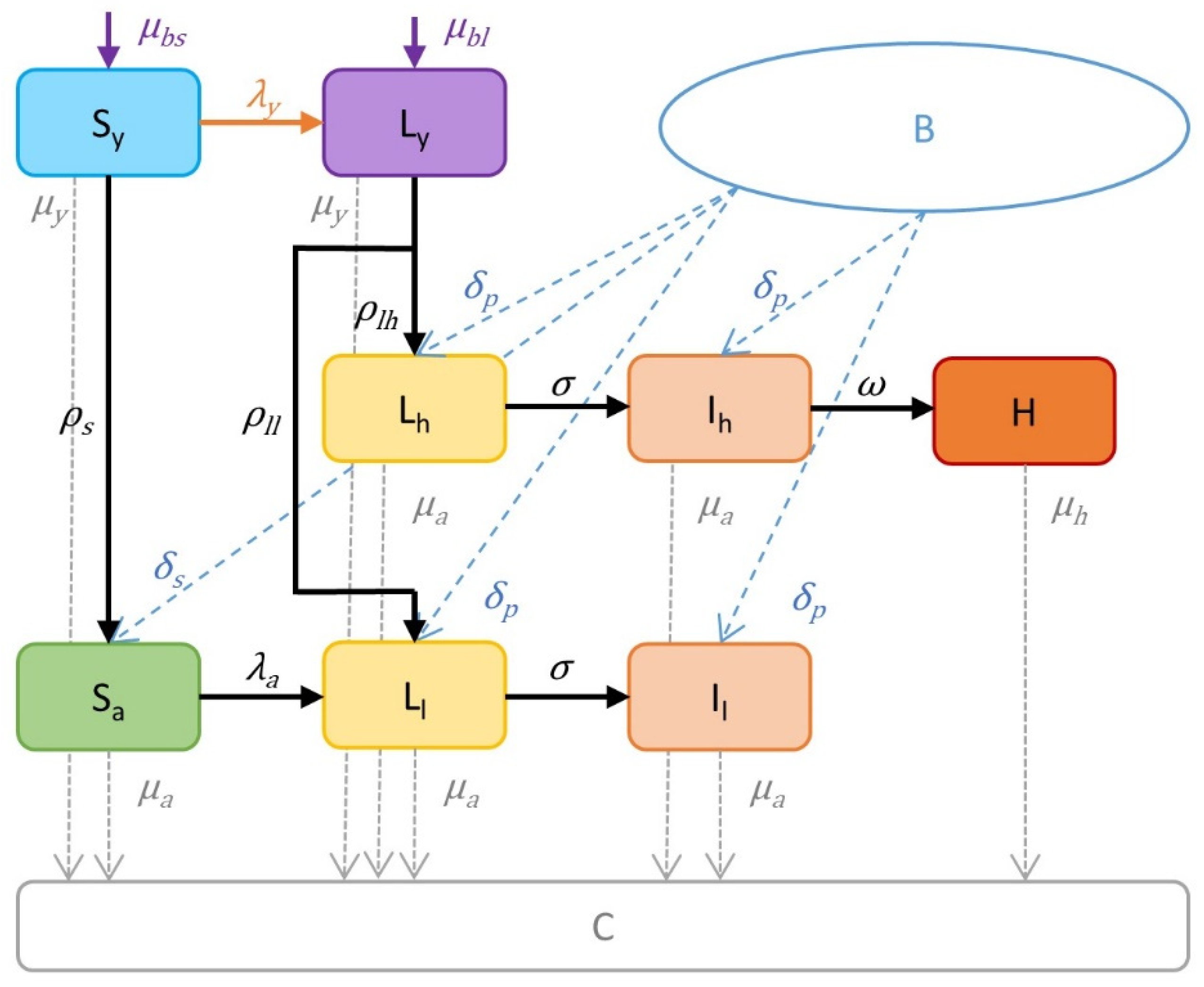
2.1. Structure of the Model
2.2. Model Parameters, Calibration and Simulation
| Symbol | Description | Value | Source |
|---|---|---|---|
| Ni | Initial number of adult animals; dairy cows | 17 | [35] |
| Yi | Initial number of young animals; calves and heifers | 15 | [35] |
| Transition rate from latent to infected cows (/day) | 0.00274 | [9,41] | |
| Progression rate from infected cows on high shedding pathway to high shedding cows (/day) | 0.00137 | [9,41] | |
| Transmission rate for latently infected cows (/day) | 0.000027 | [42,43] | |
| Transmission rate for infected cows (/day) | 0.000274 | [43] | |
| Transmission rate for high shedding cows (/day) | 0.00274 | [43] | |
| Culling rate for all cows except high shedding cows (/day) | 0.000457 | Expert opinion | |
| Culling rate for high shedding cows (/day) | 0.003653 | Expert opinion | |
| Culling rate or sale of surplus young animals (/day) | 0.000322 | Expert opinion | |
| Rate of buying in new animals (/day) | 0.000009 | [34] | |
| Prevalence at animal level | 0.08 | [4,29]; expert opinion | |
| Birth rate for all cows except high shedding cows (/day) | 0.001315 | [36] | |
| Birth rate for high shedding cows (/day) | 0.000411 | [44] | |
| Proportion of latent calves per latently infected and infected cows | 0.25 | [45] | |
| Proportion of latent calves per high shedding cows | 0.65 | [41] | |
| Transmission rate from latently infected calves to susceptible calves (/day) | 0.000007 | [43] | |
| Transmission rate from latently infected cows to susceptible calves (/day) | 0.00021 | [43] | |
| Transmission rate from infected cows to susceptible calves (/day) | 0.002099 | [43] | |
| Transmission rate from high shedding cows to susceptible calves (/day) | 0.020986 | [43] | |
| Transmission rate from latently infected heifers to susceptible heifers (/day) | 0.000003 | [43] | |
| Proportion of latently infected young animals entering high shedding pathway | 0.335 | [44] |
| Symbol | Description | Calculation |
|---|---|---|
| Number of adult animals; dairy cows | ||
| Number of young animals; calves and heifers | ||
| Force of infection for adult animals (/day) | ||
| Transition rate from bought to susceptible cow compartment | ||
| Transition rate from bought to latently infected and infected cow compartments | ||
| Birth rate of latently infected female calves and direct transmission from mothers | ||
| Birth rate of susceptible female calves | ||
| Force of infection for young animals (/day) | ||
| Replacement of culled animals with own animals; from Sy to Sa | ||
| Replacement of culled animals with own animals; from Ly to Ll | ||
| Replacement of culled animals with own animals; from Ly to Lh | ||
| Culling, mortality or sale of young animals (/day) |
2.3. Model Outputs and Validation
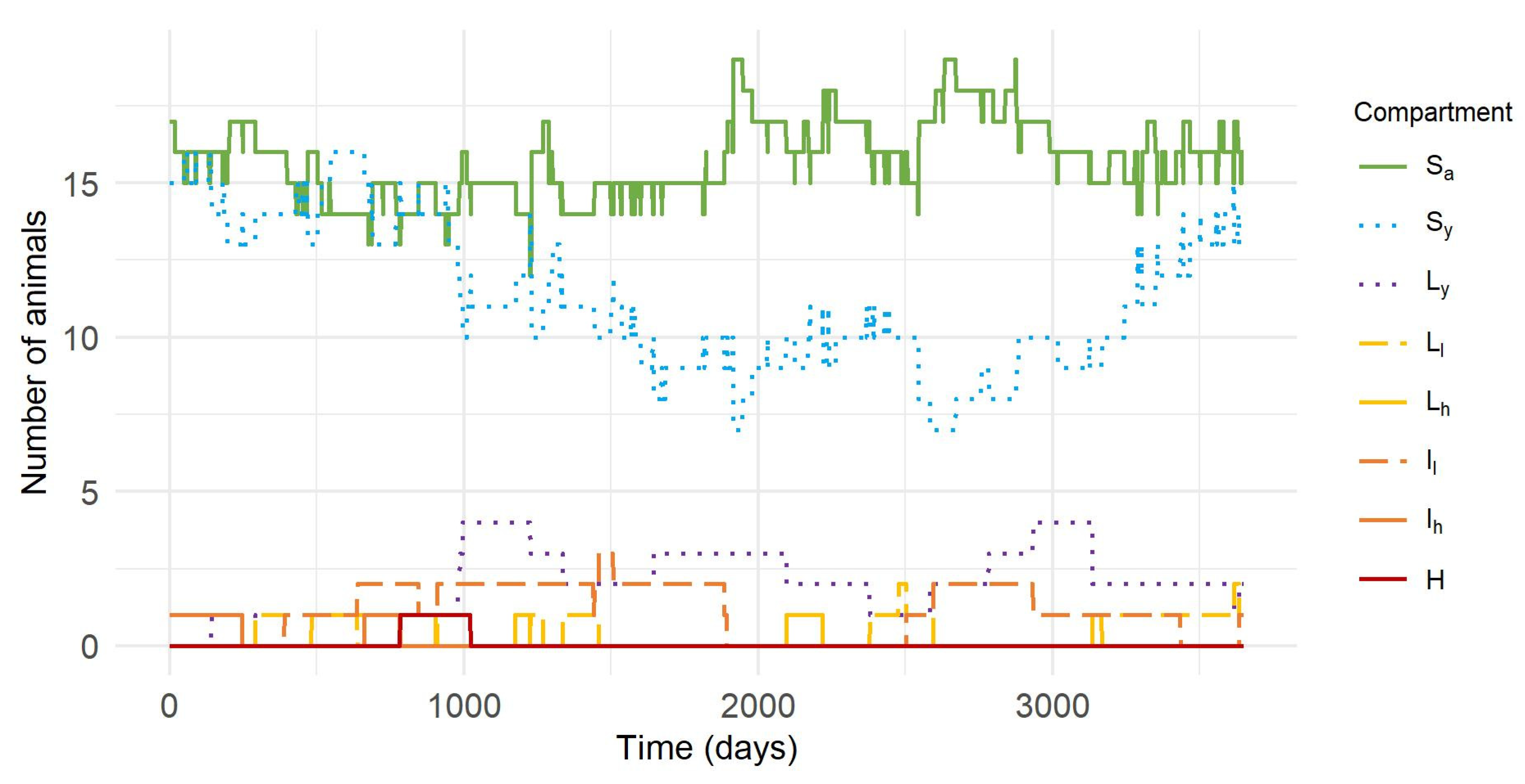
3. Results
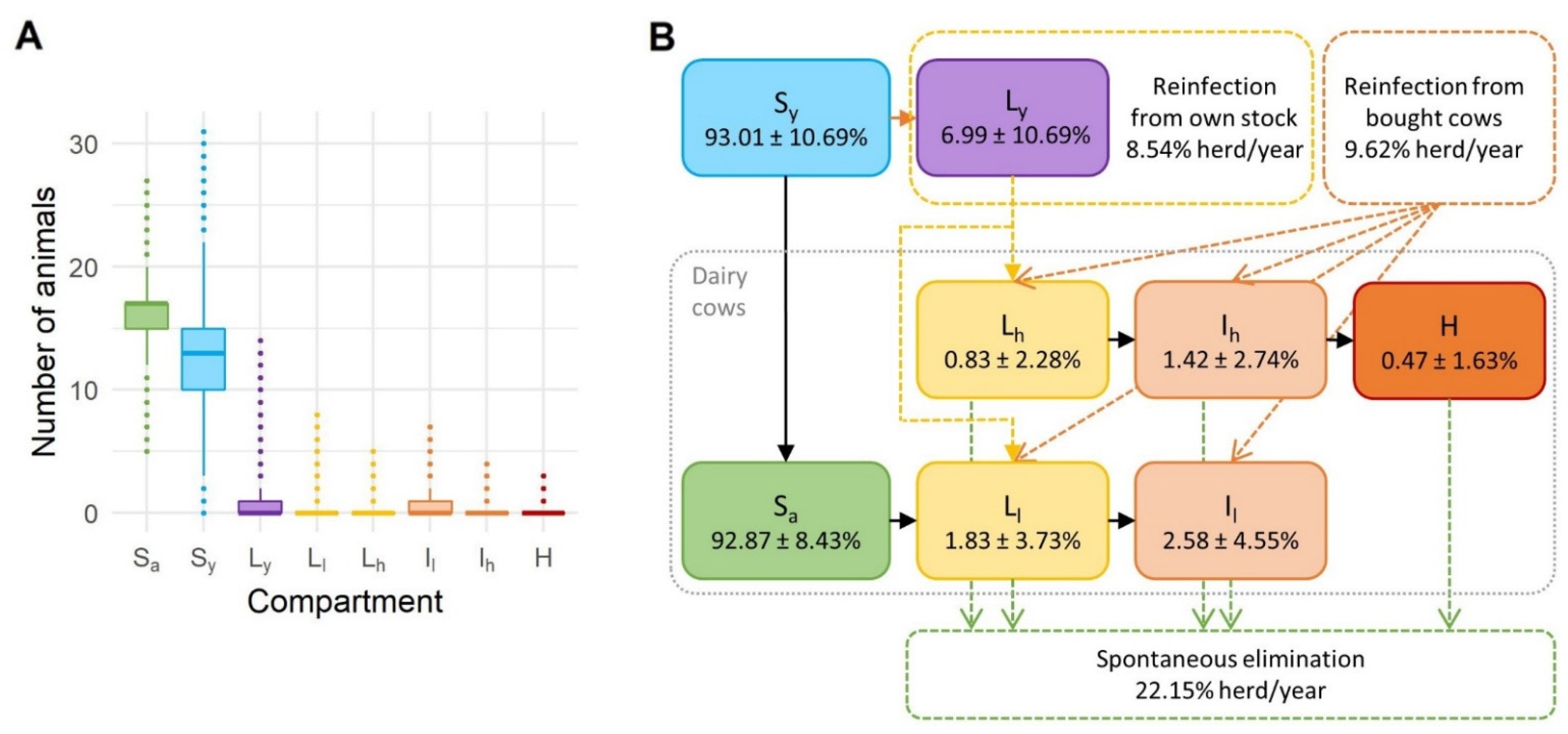
3.1. Distribution of Animals in Different Health States
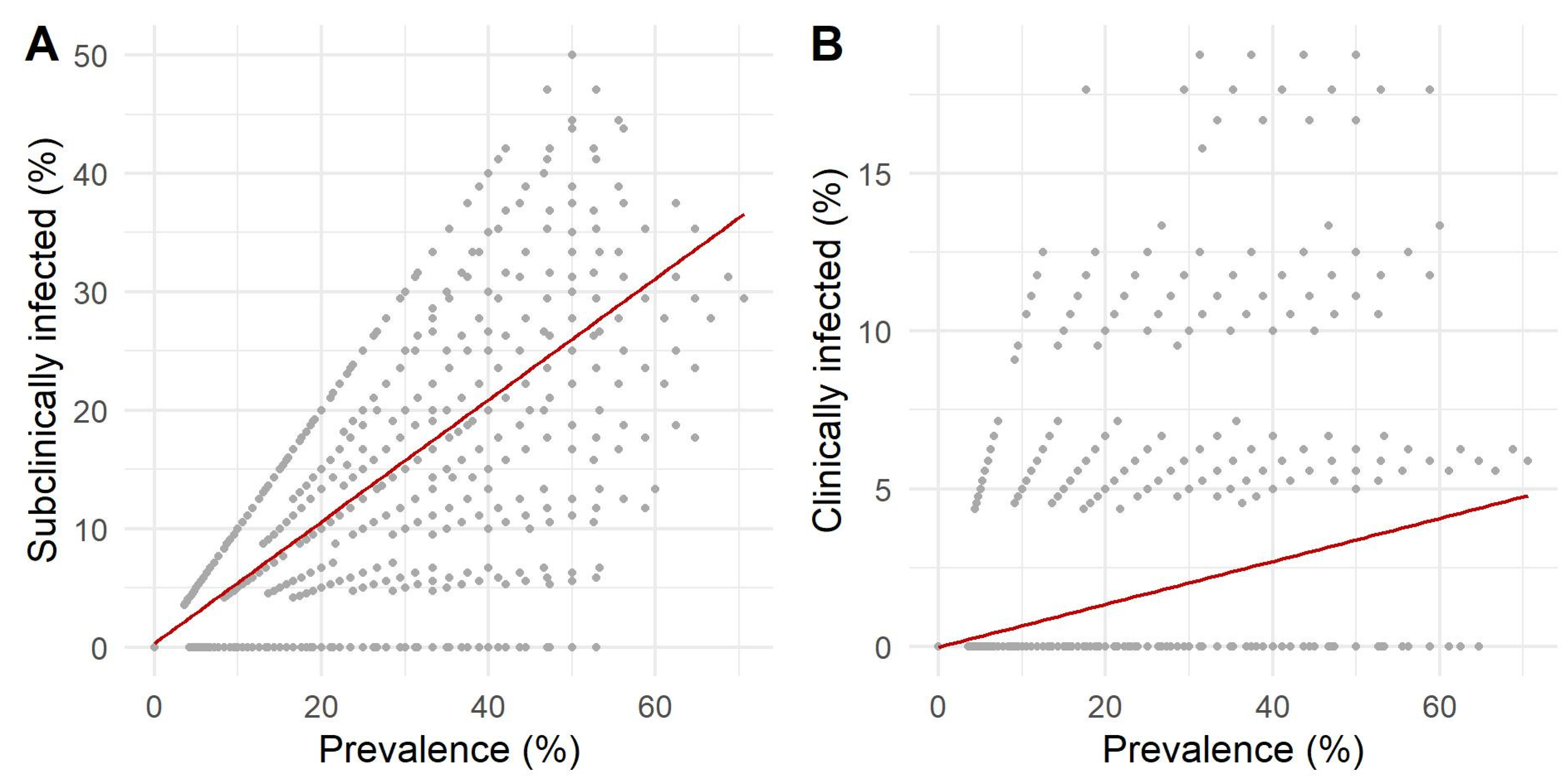
3.2. Occurrence of Spontaneous Elimination and Reinfection
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barkema, H.W.; Hesselink, J.W.; McKenna, S.L.B.; Benedictus, G.; Groenendaal, H. Global Prevalence and Economics of Infection with Mycobacterium avium subsp. Paratuberculosis in Ruminants. In Paratuberculosis: Organism, Disease, Control; Behr, M.A., Collins, D.M., Eds.; CAB International: Cambridge, UK, 2010; pp. 10–21. ISBN 9781845936136. [Google Scholar]
- Sweeney, R.W. Transmission of paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 305–312. [Google Scholar] [CrossRef]
- Smith, R.L.; Strawderman, R.L.; Schukken, Y.H.; Wells, S.J.; Pradhan, A.K.; Espejo, L.A.; Whitlock, R.H.; Van Kessel, J.S.; Smith, J.M.; Wolfgang, D.R.; et al. Effect of Johne’s disease status on reproduction and Culling in Dairy Cattle. J. Dairy Sci. 2010, 93, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Logar, K.; Kopinč, R.; Bandelj, P.; Starič, J.; Lapanje, A.; Ocepek, M. Evaluation of combined high-efficiency DNA extraction and real-time PCR for detection of Mycobacterium avium subsp. paratuberculosis in subclinically infected dairy cattle: Comparison with faecal culture, milk real-time PCR and milk ELISA. BMC Vet. Res. 2012, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitlock, R.H.; Buergelt, C. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 345–356. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Bjerre, H.; Toft, N. Colostrum and milk as risk factors for infection with Mycobacterium avium subspecies paratuberculosis in dairy cattle. J. Dairy Sci. 2008, 91, 4610–4615. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.L.; Schukken, Y.H.; Gröhn, Y.T. A new compartmental model of Mycobacterium avium subsp. paratuberculosis infection dynamics in cattle. Prev. Vet. Med. 2015, 122, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.M.; Schukken, Y.; Koets, A.; Weber, M.; Bakker, D.; Stabel, J.; Whitlock, R.H.; Louzoun, Y. Differences in intermittent and continuous fecal shedding patterns between natural and experimental Mycobacterium avium subspecies paratuberculosis infections in cattle. Vet. Res. 2015, 46, 66. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.M.; Whitlock, R.H.; Gröhn, Y.T.; Schukken, Y.H. Back to the real world: Connecting models with data. Prev. Vet. Med. 2015, 118, 215–225. [Google Scholar] [CrossRef]
- Espejo, L.A.; Kubat, N.; Godden, S.M.; Wells, S.J. Effect of delayed exposure of cattle to Mycobacterium avium subsp paratuberculosis on the development of subclinical and clinical Johne’s disease. Am. J. Vet. Res. 2013, 74, 1304–1310. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Whitlock, R.H.; Wolfgang, D.; Grohn, Y.; Beaver, A.; VanKessel, J.; Zurakowski, M.; Mitchell, R. Longitudinal data collection of Mycobacterium avium subspecies paratuberculosis infections in dairy herds: The value of precise field data. Vet. Res. 2015, 46, 65. [Google Scholar] [CrossRef] [Green Version]
- Nigsch, A.; Robbe-Austerman, S.; Stuber, T.P.; Bitar, P.D.P.; Gröhn, Y.T.; Schukken, Y.H. Who infects whom?—Reconstructing infection chains of Mycobacterium avium ssp. paratuberculosis in an endemically infected dairy herd by use of genomic data. PLoS ONE 2021, 16, e0246983. [Google Scholar] [CrossRef] [PubMed]
- Benedictus, A.; Mitchell, R.M.; Linde-Widmann, M.; Sweeney, R.; Fyock, T.; Schukken, Y.H.; Whitlock, R.H. Transmission parameters of Mycobacterium avium subspecies paratuberculosis infections in a dairy herd going through a control program. Prev. Vet. Med. 2008, 83, 215–227. [Google Scholar] [CrossRef] [PubMed]
- van Roermund, H.J.W.; de Koeijer, A.A.; Velthuis, A.G.J.; Weber, M.F.; Saatkamp, H.W.; de Jong, M.C.M. Quality Assurance Program for Paratuberculosis; Reports of the Animal Sciences Group; Animal Sciences Group: Lelystad, The Netherlands, 2004. [Google Scholar]
- Corn, J.L.; Manning, E.J.B.; Sreevatsan, S.; Fischer, J.R. Isolation of Mycobacterium avium subsp. paratuberculosis from free-ranging birds and mammals on livestock premises. Appl. Environ. Microbiol. 2005, 71, 6963–6967. [Google Scholar] [CrossRef] [Green Version]
- Nugent, G.; Whitford, E.J.; Hunnam, J.C.; Wilson, P.R.; Cross, M.L.; de Lisle, G.W. Mycobacterium avium subsp. paratuberculosis infection in wildlife on three deer farms with a history of Johne’s disease. N. Z. Vet. J. 2011, 59, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, M.E.; Whitlock, R.H. Paratuberculosis in Cattle. In Paratuberculosis: Organism, Disease, Control; Behr, M.A., Collins, D.M., Eds.; CAB International: Cambridge, UK, 2010; pp. 144–156. ISBN 9781845936136. [Google Scholar]
- Fecteau, M.E. Paratuberculosis in cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 209–222. [Google Scholar] [CrossRef] [PubMed]
- ACMSF. ACM/486: Heat Resistance of Mycobacterium Avium subsp. Paratuberculosis (MAP) in Milk at Pasteurisation Temperatures. In Proceedings of the Advisory Committee on the Microbiological Safety of Food 38th Meeting, London, UK, 19 September 2000; pp. 1–7. Available online: https://acmsf.food.gov.uk/sites/default/files/mnt/drupal_data/sources/files/multimedia/pdfs/ACM486.PDF (accessed on 16 June 2019).
- Cho, J.; Tauer, L.W.; Schukken, Y.H.; Smith, R.L.; Lu, Z.; Grohn, Y.T. Cost-effective control strategies for Johne’s disease in dairy herds. Can. J. Agric. Econ. 2013, 61, 583–608. [Google Scholar] [CrossRef]
- McAloon, C.G.; Whyte, P.; More, S.J.; Green, M.J.; O’Grady, L.; Garcia, A.; Doherty, M.L. The effect of paratuberculosis on milk yield—A systematic review and meta-analysis. J. Dairy Sci. 2016, 99, 1449–1460. [Google Scholar] [CrossRef] [Green Version]
- Chi, J.; Van Leeuwen, J.; Weersink, A.; Keefe, G.P. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev. Vet. Med. 2002, 55, 137–153. [Google Scholar] [CrossRef]
- Stott, A.W.; Jones, G.M.; Humphry, R.W.; Gunn, G.J. Financial incentive to control paratuberculosis (Johne’s disease) on dairy farms in the United Kingdom. Vet. Rec. 2005, 156, 825–831. [Google Scholar] [CrossRef]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999, 40, 179–192. [Google Scholar] [CrossRef]
- Hermon-Taylor, J. Mycobacterium avium subspecies paratuberculosis as a cause of Crohn’s disease. Gut 2001, 49, 755–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, I.R.; Ball, H.J.; Neill, S.D.; Rowe, M.T. Inactivation of Mycobacterium paratuberculosis in cows’ milk at pasteurization temperatures. Appl. Environ. Microbiol. 1996, 62, 631–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Animal Health and Welfare (AHAW); More, S.; Bøtner, A.; Butterworth, A.; Calistri, P.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Gortázar Schmidt, C.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Paratuberculosis. EFSA J. 2017, 15, e04960. [Google Scholar] [CrossRef] [PubMed]
- Ocepek, M.; Posedi, J.; Ljubljana, M.P. Prevalence of bovine paratuberculosis in Slovenia in 1997 and 1998. Zb. Vet. Fak. Univ. 1999, 36, 111–119. [Google Scholar]
- Kušar, D.; Ocepek, M.; Logar, K.; Pate, M.; Krt, B. Seroprevalence of cattle paratuberculosis in Slovenia in 2008 and a comparison of data from current and previous studies. Slov. Vet. Res. 2011, 48, 39–44. [Google Scholar]
- Ocepek, M.; Krt, B.; Pate, M.; Pogacnik, M. Seroprevalence of paratuberculosis in Slovenia between 1999 and 2001. Slov. Vet. Res. 2002, 39, 179–185. [Google Scholar]
- Nielsen, S.S.; Toft, N. A review of prevalences of paratuberculosis in farmed animals in Europe. Prev. Vet. Med. 2009, 88, 1–14. [Google Scholar] [CrossRef]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef] [Green Version]
- Collins, M. Paratuberculosis in Ruminants—MSD Veterinary Manual. Available online: https://www.msdvetmanual.com/digestive-system/intestinal-diseases-in-ruminants/paratuberculosis-in-ruminants?redirectid=3156?ruleredirectid=30 (accessed on 10 March 2022).
- Knific, T.; Ocepek, M.; Kirbiš, A.; Lentz, H.H.K. Implications of cattle trade for the spread and control of infectious diseases in Slovenia. Front. Vet. Sci. 2020, 6, 454. [Google Scholar] [CrossRef] [Green Version]
- Agricultural Institute of Slovenia. Govedoreja v Sloveniji, GOVEDO.si. Available online: https://www.govedo.si/ (accessed on 10 June 2019).
- Sadar, M.; Jenko, J.; Jeretina, J.; Logar, B.; Perpar, T.; Podgoršek, P. Rezultati Kontrole Prireje Mleka in Mesa: Slovenija 2018 [Results of Dairy and Beef Recording: Slovenia 2018]; Agricultural Institute of Slovenia: Ljubljana, Slovenia, 2019; pp. 17–43. Available online: https://www.govedo.si/files/cpzgss/knjiznica/porocila/kontrola_porocila/REZULTATI_KONTROLE_2018.pdf (accessed on 10 March 2020).
- Archive: Small and Large Farms in the EU–Statistics from the Farm Structure Survey. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Small_and_large_farms_in_the_EU_-_statistics_from_the_farm_structure_survey&oldid=406560 (accessed on 17 April 2022).
- Hodnik, J.J.; Knific, T.; Starič, J.; Toplak, I.; Ocepek, M.; Hostnik, P.; Ježek, J. Overview of Slovenian control programmes for selected cattle diseases, listed under category C, D or E of the European Animal Health Law. Front. Vet. Sci. 2021, 8, 674515. [Google Scholar] [CrossRef]
- Picault, S.; Huang, Y.L.; Sicard, V.; Arnoux, S.; Beaunée, G.; Ezanno, P. Emulsion: Transparent and flexible multiscale stochastic models in human, animal and plant epidemiology. PLoS Comput. Biol. 2019, 15, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistical Office of the Republic of Slovenia. Sistat Database. Available online: https://pxweb.stat.si/sistat/en/Podrocja/Index/85/agriculture-forestry-and-fishery (accessed on 6 June 2019).
- Marcé, C.; Ezanno, P.; Seegers, H.; Pfeiffer, D.; Fourichon, C. Predicting fadeout versus persistence of paratuberculosis in a dairy cattle herd for management and control purposes: A modelling study. Vet. Res. 2011, 42, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robins, J.; Bogen, S.; Francis, A.; Westhoek, A.; Kanarek, A.; Lenhart, S.; Eda, S. Agent-based model for Johne’s disease dynamics in a dairy herd modeling Johne’s disease: From the inside out Dr Ad Koets and Prof Yrjo Grohn. Vet. Res. 2015, 46, 68. [Google Scholar] [CrossRef] [Green Version]
- Al-Mamun, M.A.; Smith, R.L.; Schukken, Y.H.; Gröhn, Y.T. Use of an individual-based model to control transmission pathways of Mycobacterium avium subsp. paratuberculosis infection in cattle herds. Sci. Rep. 2017, 7, 11845. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Al-Mamun, M.A.; Gröhn, Y.T. Economic consequences of paratuberculosis control in dairy cattle: A stochastic modeling study. Prev. Vet. Med. 2017, 138, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martcheva, M.; Lenhart, S.; Eda, S.; Klinkenberg, D.; Momotani, E.; Stabel, J. An immuno-epidemiological model for Johne’s disease in cattle modeling Johne’s disease: From the inside out Dr Ad Koets and Prof Yrjo Grohn. Vet. Res. 2015, 46, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 1 February 2019).
- Marcé, C.; Ezanno, P.; Seegers, H.; Pfeiffer, D.U.; Fourichon, C. Within-herd contact structure and transmission of Mycobacterium avium subspecies paratuberculosis in a persistently infected dairy cattle herd. Prev. Vet. Med. 2011, 100, 116–125. [Google Scholar] [CrossRef]
- Beaunée, G.; Vergu, E.; Ezanno, P. Modelling of paratuberculosis spread between dairy cattle farms at a regional scale. Vet. Res. 2015, 46, 111. [Google Scholar] [CrossRef] [Green Version]


| Symbol | Description | Value Used in the Basic Model | Minimum Value | Maximum Value |
|---|---|---|---|---|
| Transition rate from latent to infected cows (/day) | 0.00274 | 0 | 0.000685 | |
| Progression rate from infected cows on high shedding pathway to high shedding cows (/day) | 0.00137 | 0 | 0.000548 | |
| Transmission rate for infected cows (/day) | 0.000274 | 0 | 0.00274 | |
| Transmission rate for high shedding cows (/day) | 0.00274 | 0 | 0.027398 | |
| Prevalence at animal level | 0.08 | 0.01 | 0.2 | |
| Transmission rate from infected cows to susceptible calves (/day) | 0.002099 | 0 | 0.013699 | |
| Transmission rate from high shedding cows to susceptible calves (/day) | 0.020986 | 0.00274 | 0.068494 | |
| Proportion of latently infected young animals entering high shedding pathway | 0.335 | 0.1 | 0.5 |
| Parameter | Min./Max. Value | 5th PC | 1st Q | Median | Mean | SD | 3rd Q | 95th PC |
|---|---|---|---|---|---|---|---|---|
| Basic model | / | 0 | 0 | 5.56 | 7.13 | 8.43 | 11.11 | 23.53 |
| Min. | 0 | 0 | 5.56 | 6.24 | 7.31 | 10.53 | 22.22 | |
| Max. | 0 | 0 | 5.56 | 6.53 | 7.63 | 11.11 | 22.22 | |
| Min. | 0 | 0 | 5.56 | 5.38 | 6.28 | 5.88 | 17.65 | |
| Max. | 0 | 0 | 5.56 | 6.63 | 7.85 | 11.11 | 23.53 | |
| Min. | 0 | 0 | 5.56 | 6.83 | 8.37 | 11.11 | 23.53 | |
| Max. | 0 | 5.26 | 11.76 | 16.88 | 17.32 | 27.78 | 52.94 | |
| Min. | 0 | 0 | 5.56 | 6.49 | 7.47 | 11.11 | 22.22 | |
| Max. | 0 | 0 | 5.88 | 12.46 | 15.43 | 17.65 | 47.06 | |
| Min. | 0 | 0 | 0 | 4.08 | 6.89 | 5.88 | 17.65 | |
| Max. | 0 | 5.56 | 11.11 | 12.73 | 11.30 | 17.65 | 35.29 | |
| Min. | 0 | 0 | 5.56 | 5.60 | 6.98 | 5.88 | 17.65 | |
| Max. | 0 | 5.88 | 16.67 | 20.22 | 17.88 | 31.58 | 55.56 | |
| Min. | 0 | 0 | 5.56 | 5.26 | 6.38 | 5.88 | 17.65 | |
| Max. | 0 | 0 | 5.88 | 12.09 | 13.85 | 17.65 | 41.18 | |
| Min. | 0 | 0 | 5.88 | 7.32 | 8.22 | 11.76 | 23.53 | |
| Max. | 0 | 0 | 5.88 | 7.54 | 8.93 | 11.76 | 26.32 |
| Parameter | Min./Max. Value | 5th PC | 1st Q | Median | Mean | SD | 3rd Q | 95th PC |
|---|---|---|---|---|---|---|---|---|
| Basic model | / | 0 | 0 | 0 | 4.00 | 5.42 | 5.88 | 15.79 |
| Min. | 0 | 0 | 0 | 3.05 | 4.41 | 5.88 | 11.76 | |
| Max. | 0 | 0 | 0 | 3.46 | 4.8 | 5.88 | 11.76 | |
| Min. | 0 | 0 | 0 | 3.82 | 4.91 | 5.88 | 11.76 | |
| Max. | 0 | 0 | 0 | 4.17 | 5.47 | 5.88 | 15.79 | |
| Min. | 0 | 0 | 0 | 3.98 | 5.46 | 5.88 | 15.79 | |
| Max. | 0 | 0 | 5.88 | 9.34 | 10.84 | 14.29 | 31.58 | |
| Min. | 0 | 0 | 0 | 3.76 | 4.99 | 5.88 | 11.76 | |
| Max. | 0 | 0 | 0 | 3.76 | 4.99 | 5.88 | 11.76 | |
| Min. | 0 | 0 | 0 | 2.32 | 4.33 | 5.56 | 11.76 | |
| Max. | 0 | 0 | 5.88 | 7.41 | 7.49 | 11.76 | 23.53 | |
| Min. | 0 | 0 | 0 | 3.40 | 4.75 | 5.88 | 11.76 | |
| Max. | 0 | 0 | 5.88 | 10.55 | 10.83 | 17.65 | 33.33 | |
| Min. | 0 | 0 | 0 | 3.19 | 4.50 | 5.88 | 11.76 | |
| Max. | 0 | 0 | 5.56 | 6.68 | 8.36 | 11.11 | 23.53 | |
| Min. | 0 | 0 | 4.76 | 4.39 | 5.63 | 5.88 | 16.67 | |
| Max. | 0 | 0 | 0 | 4.09 | 5.50 | 5.88 | 16.67 |
| Parameter | Min./Max. Value | 5th PC | 1st Q | Median | Mean | SD | 3rd Q | 95th PC |
|---|---|---|---|---|---|---|---|---|
| Basic model | / | 0 | 0 | 0 | 0.47 | 1.63 | 0 | 5.56 |
| Min. | 0 | 0 | 0 | 0.25 | 1.17 | 0 | 0 | |
| Max. | 0 | 0 | 0 | 0.33 | 1.34 | 0 | 5.56 | |
| Min. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Max. | 0 | 0 | 0 | 0.24 | 1.19 | 0 | 0 | |
| Min. | 0 | 0 | 0 | 0.45 | 1.61 | 0 | 5.56 | |
| Max. | 0 | 0 | 0 | 0.54 | 1.73 | 0 | 5.88 | |
| Min. | 0 | 0 | 0 | 0.42 | 1.54 | 0 | 5.56 | |
| Max. | 0 | 0 | 0 | 0.42 | 1.54 | 0 | 5.56 | |
| Min. | 0 | 0 | 0 | 0.27 | 1.23 | 0 | 0 | |
| Max. | 0 | 0 | 0 | 0.71 | 2.02 | 0 | 5.88 | |
| Min. | 0 | 0 | 0 | 0.40 | 1.49 | 0 | 5.56 | |
| Max. | 0 | 0 | 0 | 0.88 | 2.28 | 0 | 5.88 | |
| Min. | 0 | 0 | 0 | 0.39 | 1.49 | 0 | 5.56 | |
| Max. | 0 | 0 | 0 | 0.65 | 1.96 | 0 | 5.88 | |
| Min. | 0 | 0 | 0 | 0.36 | 1.42 | 0 | 5.56 | |
| Max. | 0 | 0 | 0 | 0.57 | 1.80 | 0 | 5.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knific, T.; Kirbiš, A.; Gethmann, J.M.; Prezelj, J.; Krt, B.; Ocepek, M. Modeling Paratuberculosis Transmission in a Small Dairy Herd Typical of Slovenia Suggests That Different Models Should Be Used to Study Disease Spread in Herds of Different Sizes. Animals 2022, 12, 1150. https://doi.org/10.3390/ani12091150
Knific T, Kirbiš A, Gethmann JM, Prezelj J, Krt B, Ocepek M. Modeling Paratuberculosis Transmission in a Small Dairy Herd Typical of Slovenia Suggests That Different Models Should Be Used to Study Disease Spread in Herds of Different Sizes. Animals. 2022; 12(9):1150. https://doi.org/10.3390/ani12091150
Chicago/Turabian StyleKnific, Tanja, Andrej Kirbiš, Jörn M. Gethmann, Jasna Prezelj, Branko Krt, and Matjaž Ocepek. 2022. "Modeling Paratuberculosis Transmission in a Small Dairy Herd Typical of Slovenia Suggests That Different Models Should Be Used to Study Disease Spread in Herds of Different Sizes" Animals 12, no. 9: 1150. https://doi.org/10.3390/ani12091150
APA StyleKnific, T., Kirbiš, A., Gethmann, J. M., Prezelj, J., Krt, B., & Ocepek, M. (2022). Modeling Paratuberculosis Transmission in a Small Dairy Herd Typical of Slovenia Suggests That Different Models Should Be Used to Study Disease Spread in Herds of Different Sizes. Animals, 12(9), 1150. https://doi.org/10.3390/ani12091150






