Nesting Site and Plumage Color Are the Main Traits Associated with Bird Species Presence in Urban Areas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Bird Surveys
2.3. Environmental Characteristics
2.4. Bird Species Urbanness
2.5. Life History Traits
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. The World’s Cities in 2018; Department of Economic and Social, Affairs, United Nations: New York, NY, USA, 2018. [Google Scholar]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proceed. R. Soc. B 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, D.F.; Strohbach, M.W.; Warren, P.S.; Fuller, R.A. The challenges of urban living. In Avian Urban Ecology Behavioural and Physiological Adaptations; Gil, D., Brumm, H., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 3–20. [Google Scholar]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A metaanalysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Croci, S.; Butet, A.; Clergeau, P. Does urbanization filter birds on the basis of their biological traits. Condor 2008, 110, 223–240. [Google Scholar] [CrossRef]
- Silva, C.P.; Sepúlveda, R.D.; Barbosa, O. Nonrandom filtering effect on birds: Species and guilds response to urbanization. Ecol. Evol. 2016, 6, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.S.; Reitsma, R.; Hurlbert, A.H.; Marra, P.P. Environmental filtering of avian communities along a rural-to-urban gradient in Greater Washington, DC, USA. Ecosphere 2018, 911, e02402. [Google Scholar] [CrossRef] [Green Version]
- Hensley, C.B.; Trisos, C.H.; Warren, P.S.; MacFarland, J.; Blumenshine, S.; Reece, J.; Katti, M. Effects of urbanization on native bird species in three southwestern US Cities. Front. Ecol. Evol. 2019, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Puga-Caballero, A.; Arizmendi MD, C.; Sánchez-González, L.A. Phylogenetic and phenotypic filtering in hummingbirds from urban environments in Central Mexico. Evol. Ecol. 2020, 34, 525–541. [Google Scholar] [CrossRef]
- Leveau, L.M. Consistency in bird community assembly over medium-term along rural-urban gradients in Argentina. Ecol. Process. 2021, 10, 34. [Google Scholar] [CrossRef]
- Patankar, S.; Jambhekar, R.; Suryawanshi, K.R.; Nagendra, H. Which traits influence bird survival in the city? A review. Land 2021, 10, 92. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Couvet, D.; Lee, A.; Jiguet, F. Functional homogenization effect of urbanization on bird communities. Conserv. Biol. 2007, 21, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Bonier, F.; Martin, P.R.; Wingfield, J.C. Urban birds have broader environmental tolerance. Biol. Lett. 2007, 3, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.L.; Chamberlain, D.E.; Hatchwell, B.J.; Gregory, R.D.; Gaston, K.J. What makes an urban bird? Glob. Chang. Biol. 2011, 17, 32–44. [Google Scholar] [CrossRef]
- Sol, D.; González-Lagos, C.; Moreira, D.; Maspons, J.; Lapiedra, O. Urbanisation tolerance and the loss of avian diversity. Ecol. Lett. 2014, 17, 942–950. [Google Scholar] [CrossRef]
- Pagani-Núñez, E.; Liang, D.; He, C.; Zhou, X.; Luo, X.; Liu, Y.; Goodale, E. Niches in the Anthropocene: Passerine assemblages show niche expansion from natural to urban habitats. Ecography 2019, 42, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, C.T.; Major, R.E.; Wilshire, J.H.; Martin, J.M.; Kingsford, R.T.; Cornwell, W.K. Generalists are the most urban-tolerant of birds: A phylogenetically controlled analysis of ecological and life history traits using a novel continuous measure of bird responses to urbanization. Oikos 2019, 128, 845–858. [Google Scholar] [CrossRef]
- Palacio, F.X. Urban exploiters have broader dietary niches than urban avoiders. Ibis 2020, 162, 42–49. [Google Scholar] [CrossRef]
- Leveau, C.M.; Leveau, L.M. Avian community response to urbanization in the Pampean region, Argentina. Ornitol. Neotrop. 2005, 16, 503–510. [Google Scholar]
- Blair, R.B.; Johnson, E.M. Suburban habitats and their role for birds in the urban–rural habitat network: Points of local invasion and extinction? Landsc. Ecol. 2008, 23, 1157–1169. [Google Scholar] [CrossRef]
- Conole, L.E.; Kirkpatrick, J.B. Functional and spatial differentiation of urban bird assemblages at the landscape scale. Landsc. Urban Plan. 2011, 100, 11–23. [Google Scholar] [CrossRef]
- Cardoso, G.C. Nesting and acoustic ecology, but not phylogeny, influence passerine urban tolerance. Glob. Chang. Biol. 2014, 20, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Dale, S.; Lifjeld, J.T.; Rowe, M. Commonness and ecology, but not bigger brains, predict urban living in birds. BMC Ecol. 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokimäki, J.; Suhonen, J.; Jokimäki-Kaisanlahti, M.L.; Carbó-Ramírez, P. Effects of urbanization on breeding birds in European towns: Impacts of species traits. Urban Ecosyst. 2016, 19, 1565–1577. [Google Scholar] [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the city: Can anyone become an ‘urban exploiter’? J. Biogeogr. 2007, 34, 638–651. [Google Scholar] [CrossRef]
- Dale, J.; Dey, C.; Delhey, K.; Kempenaers, B.; Valcu, M. The effects of life-history and social selection on male and female plumage coloration. Nature 2015, 527, 367–370. [Google Scholar] [CrossRef]
- Leveau, L.M. Bird traits in urban–rural gradients: How many functional groups are there? J. Ornithol. 2013, 154, 655–662. [Google Scholar] [CrossRef]
- Leveau, L.M. Urbanization induces bird color homogenization. Landsc. Urban Plan. 2019, 192, 103645. [Google Scholar] [CrossRef]
- Møller, A.P. Behavioural and ecological predictors of urbanization. In Avian Urban Ecology: Behavioural and Physiological Adaptations; Oxford University Press: Oxford, UK, 2014; pp. 54–68. [Google Scholar]
- Iglesias-Carrasco, M.; Duchêne, D.A.; Head, M.L.; Møller, A.P.; Cain, K. Sex in the city: Sexual selection and urban colonization in passerines. Biol. Lett. 2019, 15, 20190257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delhey, K. Darker where cold and wet: Australian birds follow their own version of Gloger’s rule. Ecography 2018, 41, 673–683. [Google Scholar] [CrossRef]
- Delhey, K.; Dale, J.; Valcu, M.; Kempenaers, B. Reconciling ecogeographical rules: Rainfall and temperature predict global colour variation in the largest bird radiation. Ecol. Lett. 2019, 22, 726–736. [Google Scholar] [CrossRef]
- Marcondes, R.S.; Nations, J.A.; Seeholzer, G.F.; Brumfield, R.T. Rethinking Gloger’s rule: Climate, light environments, and color in a large family of tropical birds (Furnariidae). Am. Nat. 2021, 197, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Leveau, L.M. United colours of the city: A review about urbanisation impact on animal colours. Austral Ecol. 2021, 46, 670–679. [Google Scholar] [CrossRef]
- Marchetti, K. Dark habitats and bright birds illustrate the role of the environment in species divergence. Nature 1993, 362, 149–152. [Google Scholar] [CrossRef]
- Meneses-Giorgi, M.A.; Cadena, C.D. Plumage convergence resulting from social mimicry in birds? A tetrachromatic view. Anim. Behav. 2021, 180, 337–361. [Google Scholar] [CrossRef]
- Stevens, M.; Troscianko, J.; Wilson-Aggarwal, J.K.; Spottiswoode, C.N. Improvement of individual camouflage through background choice in ground-nesting birds. Nat. Ecol. Evol. 2017, 1, 1325–1333. [Google Scholar] [CrossRef]
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Blumstein, D.T. Flight-initiation distance in birds is dependent on intruder starting distance. J. Wildl. Manag. 2003, 67, 852–857. [Google Scholar] [CrossRef]
- Valcarcel, A.; Fernández-Juricic, E. Antipredator strategies of house finches: Are urban habitats safe spots from predators even when humans are around? Behav. Ecol. Sociobiol. 2009, 63, 673–685. [Google Scholar] [CrossRef]
- Blair, R.B. Land use and avian species diversity along an urban gradient. Ecol. Appl. 1996, 6, 506–519. [Google Scholar] [CrossRef]
- Møller, A.P. Successful city dwellers: A comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 2009, 159, 849–858. [Google Scholar] [CrossRef]
- McNaught, M.K.; Owens, I.P. Interspecific variation in plumage colour among birds: Species recognition or light environment? J. Evol. Biol. 2002, 15, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Leveau, L.M. Primary productivity and habitat diversity predict bird species richness and composition along urban-rural gradients of central Argentina. Urban For. Urban Green. 2019, 43, 126349. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancements and Retrogradation of Natural Vegetation; Final Report; NASA/GSFC: Greenbelt, MD, USA, 1974; pp. 1–137.
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, A.H.; Haskell, J.P. The effect of energy and seasonality on avian species richness and community composition. Am. Nat. 2003, 161, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Justice, C.O.; Townshend, J.R.G.; Vermote, E.F.; Masuoka, E.; Wolfe, R.E.; Saleous, N.; Morisette, J.T. An overview of MODIS Land data processing and product status. Remote Sens. Environ. 2002, 83, 3–15. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S. The Vegan Package. Community Ecology Package. 2007. Available online: http://vegan.r-forge.r-project.org/ (accessed on 15 January 2019).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation Project; GNU Project: Boston, MA, USA, 2019. [Google Scholar]
- Härdle, W.K.; Simar, L. Applied Multivariate Statistical Analysis; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals: Ecological Archives E095-178. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef] [Green Version]
- De Caceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- De Caceres, M.; Jansen, F. Package Indicspecies: Relationship Between Species and Groups of Sites. R Package Version 1.7.6. 2015. Available online: https://cran.r-project.org/web/packages/indicspecies/index.html (accessed on 7 May 2020).
- Narosky, T.; Di Giacomo, A. Las Aves de la Provincia de Buenos Aires: Distribución y Estatus; Asociación Ornitológica del Plata, L.O.L.A., Vazquez Mazzini Editores: Buenos Aires, Argentina, 1993. [Google Scholar]
- de la Pena, M.R. Nidos y Reproducción de las Aves Argentinas; Serie Naturaleza, Conservación y Sociedad N° 8; Ediciones Biológica: Santa Fe, Argentina, 2013. [Google Scholar]
- de la Peña, M.R. Aves Argentinas: Incluye Nidos y Huevos; Tomo 2; Ediciones UNL y EUDEBA: Buenos Aires, Argentina, 2015. [Google Scholar]
- Rasband, W. ImageJ 1.53a; National Institute of Health: Bethesda, MD, USA, 2020.
- Canevari, M.; Manzione, M. Aves Argentinas, Guía de Campo Digital; Aves Argentinas/Asociación Ornitológica del Plata: Buenos Aires, Argentina, 2017. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘nlme’. Linear and Nonlinear Mixed Effects Models, Version 3.1-157; 2017. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 7 May 2020).
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Redding, D.W.; Hartmann, K.; Mooers, A.O. Global distribution and conservation of evolutionary distinctness in birds. Curr. Biol. 2014, 24, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Ericson, P.G.P.; Zuccon, D.; Ohlson, J.I.; Johansson, U.S.; Alvarenga, H.; Prum, R.O. Higher-level phylogeny and morphological evolution of tyrant flycatchers, cotingas, manakins, and their allies (Aves: Tyrannida). Mol. Phylogenet. Evol. 2006, 40, 471–483. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J. TreeAnnotator v1.10.4. 2002–2015. Available online: http://beast.bio.ed.ac.uk/ (accessed on 25 October 2018).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFadden, D. Conditional logit analysis of qualitative choice behavior. In Frontiers in Econometrics; Zarembka, P., Ed.; Academic Press: New York, NY, USA, 1973; pp. 105–142. [Google Scholar]
- Breheny, P.; Burchett, W. Visualizing Regression Models Using Visreg. Available online: http://myweb.uiowa.edu/pbreheny/publications/visreg.pdf/ (accessed on 7 May 2020).
- Jokimäki, J.; Huhta, E. Artificial nest predation and abundance of birds along an urban gradient. Condor 2000, 102, 838–847. [Google Scholar] [CrossRef]
- Jokimäki, J.; Kaisanlahti-Jokimäki, M.L.; Sorace, A.; Fernández-Juricic, E.; Rodriguez-Prieto, I.; Jimenez, M.D. Evaluation of the “safe nesting zone” hypothesis across an urban gradient: A multi-scale study. Ecography 2005, 28, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Troscianko, J.; Lown, A.E.; Hughes, A.E.; Stevens, M. Defeating crypsis: Detection and learning of camouflage strategies. PLoS ONE 2013, 8, e73733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, D.; Théry, M. Influence of ambient light on the evolution of colour signals: Comparative analysis of a Neotropical rainforest bird community. Ecol. Lett. 2004, 7, 279–284. [Google Scholar] [CrossRef]
- Gomez, D.; Théry, M. Simultaneous crypsis and conspicuousness in color patterns: Comparative analysis of a neotropical rainforest bird community. Am. Nat. 2007, 169, S42–S61. [Google Scholar] [CrossRef]
- Barreira, A.S.; García, N.C. Visual and acoustic communication in Neotropical birds: Diversity and evolution of signals. In Behavioral Ecology of Neotropical Birds; Springer: Cham, The Netherlands, 2019; pp. 155–183. [Google Scholar]
- Li, Q.; Gao, K.Q.; Meng, Q.; Clarke, J.A.; Shawkey, M.D.; D’Alba, L.; Pei, R.; Ellison, M.; Norell, M.A.; Vinther, J. Reconstruction of Microraptor and the evolution of iridescent plumage. Science 2012, 335, 1215–1219. [Google Scholar] [CrossRef] [Green Version]
- Sicsú, P.; Manica, L.T.; Maia, R.; Macedo, R.H. Here comes the sun: Multimodal displays are associated with sunlight incidence. Behav. Ecol. Sociobiol. 2013, 67, 1633–1642. [Google Scholar] [CrossRef]
- Dalrymple, R.L.; Flores-Moreno, H.; Kemp, D.J.; White, T.E.; Laffan, S.W.; Hemmings, F.A.; Hitchcock, T.D.; Moles, A.T. Abiotic and biotic predictors of macroecological patterns in bird and butterfly coloration. Ecol. Monogr. 2018, 88, 204–224. [Google Scholar] [CrossRef]
- Kinnunen, R.P.; Fraser, K.C.; Schmidt, C.; Garroway, C.J. The socioeconomic status of cities covaries with avian life-history strategies. Ecosphere 2022, 13, e3918. [Google Scholar] [CrossRef]
- Hu, Y.; Cardoso, G.C. Are bird species that vocalize at higher frequencies preadapted to inhabit noisy urban areas? Behav. Ecol. 2009, 20, 1268–1273. [Google Scholar] [CrossRef] [Green Version]
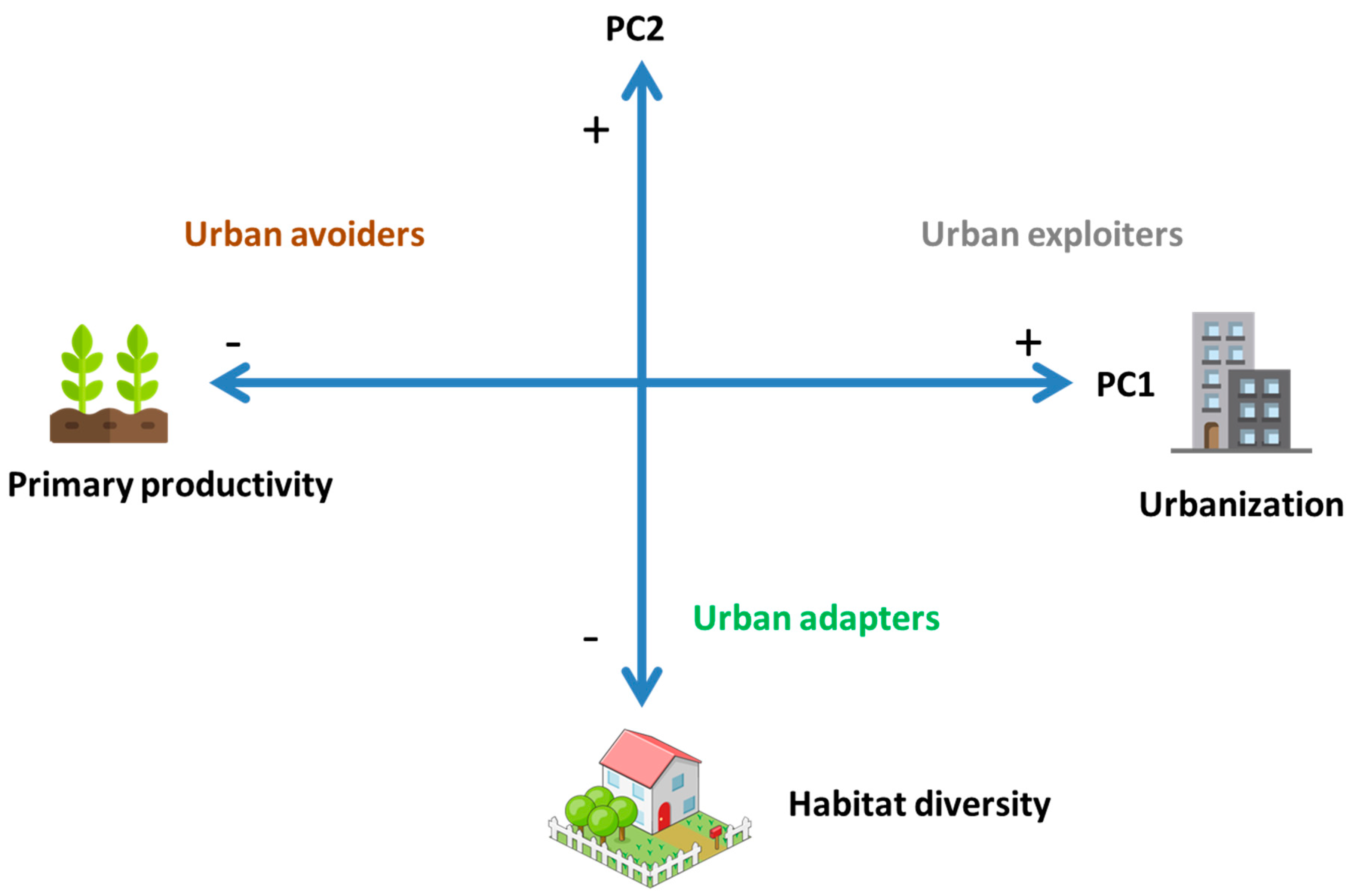
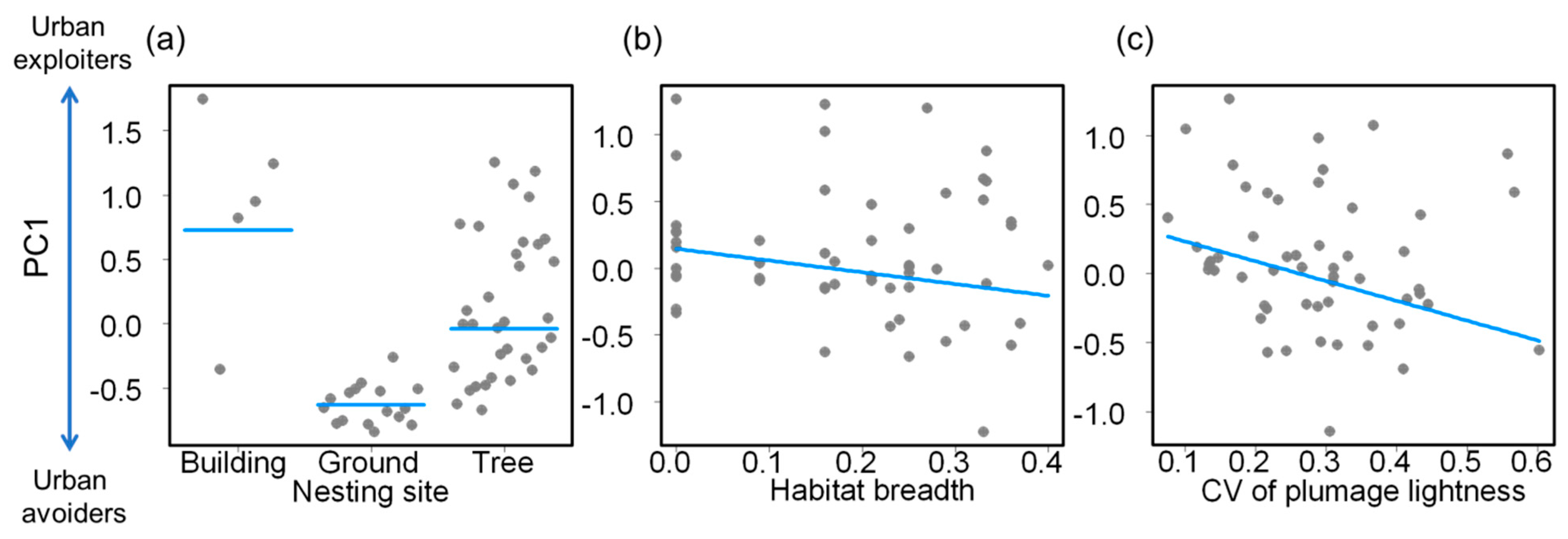
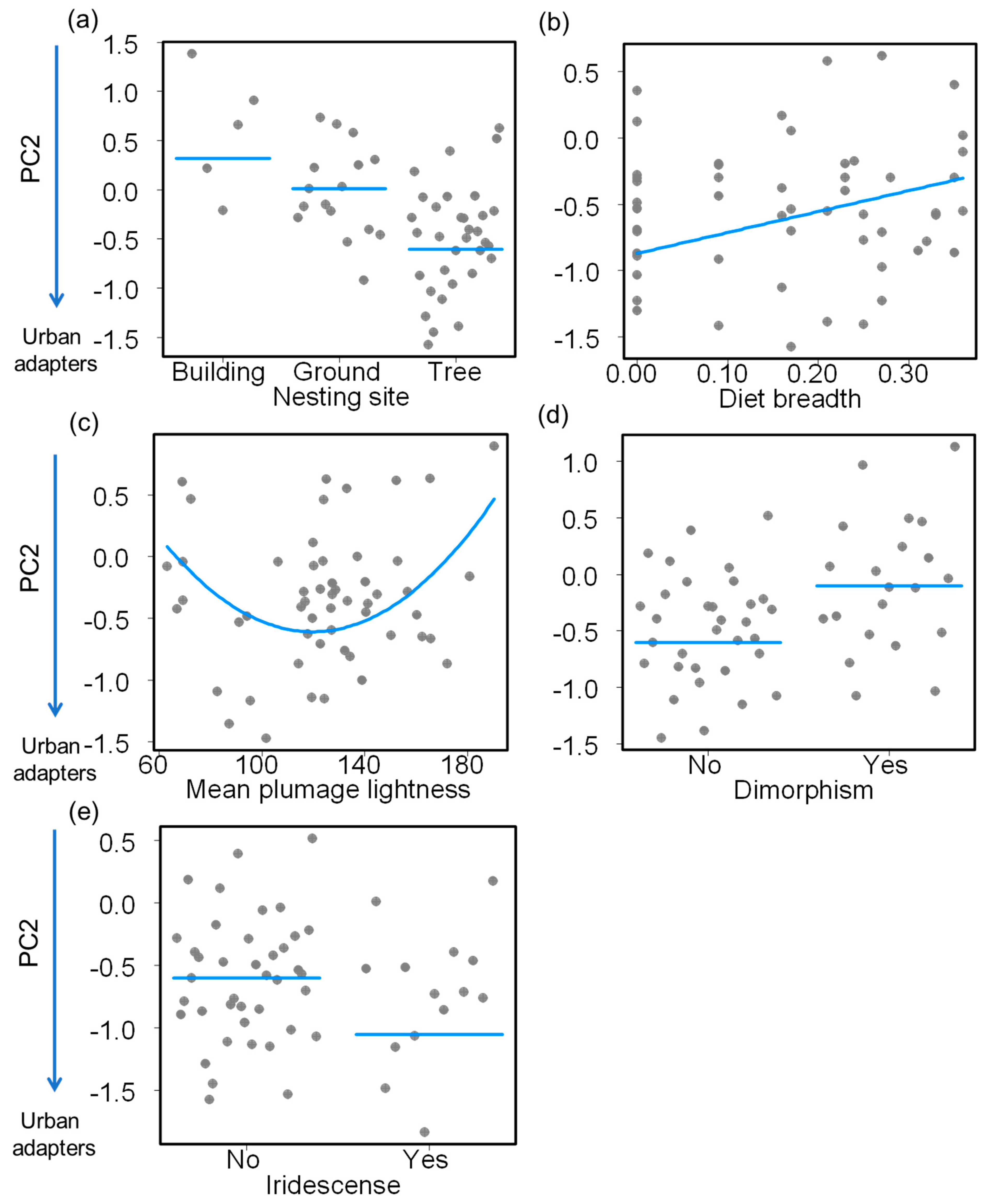
| Environmental Variables | PC1 | PC2 |
|---|---|---|
| Impervious cover (%) | 0.95 | −0.15 |
| Primary productivity (NDVI) | −0.94 | 0.06 |
| Habitat diversity (H index) | 0.23 | −0.95 |
| Car traffic | 0.75 | 0.25 |
| Pedestrian traffic | 0.87 | 0.19 |
| Bicycle traffic | 0.84 | 0.07 |
| Motorcycle traffic | 0.73 | 0.14 |
| Minimum distance to rural areas (m) | 0.86 | −0.11 |
| Eigenvalues | 5.12 | 1.07 |
| Proportion of variance explained | 0.64 | 0.13 |
| Value | Std. Error | t-Value | p-Value | |
|---|---|---|---|---|
| (a) PC1 | ||||
| Intercept | 1.325 | 0.614 | 2.158 | 0.036 |
| Nest—ground | −1.359 | 0.303 | −4.490 | <0.001 |
| Nest—tree | −0.765 | 0.265 | −2.888 | 0.006 |
| Habitat breadth | −0.880 | 0.460 | −1.914 | 0.062 |
| CV of plumage lightness | −1.429 | 0.490 | −2.916 | 0.005 |
| (b) PC2 | ||||
| Intercept | 3.129 | 1.192 | 2.625 | 0.012 |
| Nest—ground | −0.307 | 0.414 | −0.740 | 0.463 |
| Nest—tree | −0.921 | 0.370 | −2.488 | 0.017 |
| Diet breadth | 1.579 | 0.692 | 2.282 | 0.027 |
| Mean plumage lightness | −0.052 | 0.016 | −3.212 | 0.002 |
| Mean plumage lightness2 | <0.001 | <0.001 | 2.935 | 0.005 |
| Plumage dimorphism—presence | 0.503 | 0.188 | 2.681 | 0.010 |
| Iridescence—presence | −0.452 | 0.231 | −1.954 | 0.057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leveau, L.M.; Ibáñez, I. Nesting Site and Plumage Color Are the Main Traits Associated with Bird Species Presence in Urban Areas. Animals 2022, 12, 1148. https://doi.org/10.3390/ani12091148
Leveau LM, Ibáñez I. Nesting Site and Plumage Color Are the Main Traits Associated with Bird Species Presence in Urban Areas. Animals. 2022; 12(9):1148. https://doi.org/10.3390/ani12091148
Chicago/Turabian StyleLeveau, Lucas M., and Isis Ibáñez. 2022. "Nesting Site and Plumage Color Are the Main Traits Associated with Bird Species Presence in Urban Areas" Animals 12, no. 9: 1148. https://doi.org/10.3390/ani12091148
APA StyleLeveau, L. M., & Ibáñez, I. (2022). Nesting Site and Plumage Color Are the Main Traits Associated with Bird Species Presence in Urban Areas. Animals, 12(9), 1148. https://doi.org/10.3390/ani12091148





