Current Knowledge on the Bioavailability of Thymol as a Feed Additive in Humans and Animals with a Focus on Rabbit Metabolic Processes
Abstract
:Simple Summary
Abstract
1. Introduction
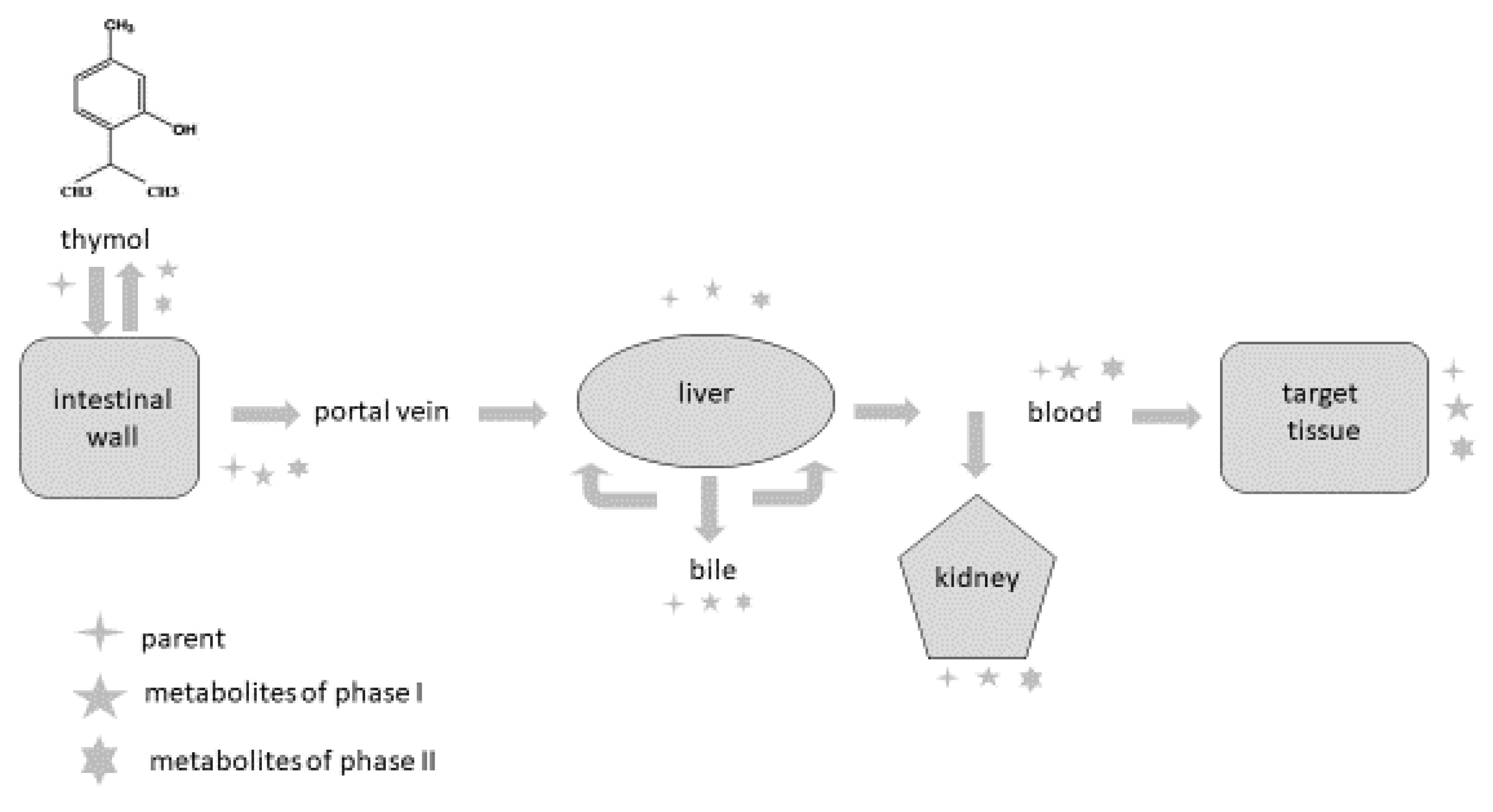
2. Detection of Thymol and Its Metabolites in Humans and Animals
| Animal Species | Applied Form | Detectable Compounds | Samples | References |
|---|---|---|---|---|
| human | thymol/orally | thymol glucuronide thymol sulphate thymohydroquinone sulphate thymol | urine | [59] |
| human | Bronchipret® TP/orally (equivalent to 1.08 mg thymol) | thymol sulphate | plasma, urine | [55] |
| thymol glucuronide | urine | |||
| human | thymol/orally | p-cymene-2,5-diol p-cymene-2,3-diol p-cymene-3-ol-8-ene | urine | [62] |
| human | dried thyme/orally | thymol sulphate caffeic acid sulphate hydroxyphenylpropionic acid sulphate | plasma | [67] |
| thymol sulphate caffeic acid sulphate hydroxyphenylpropionic acid sulphate thymol glucuronide | urine | |||
| rabbit | thymol/orally | glucuronic acid ethereal sulphuric acid thymol | urine | [59] |
| rabbit | thymol/orally | thymol | plasma, small intestinal wall, liver, kidney, spleen, caecum, colon, muscle, faeces | [58] |
| rat | thymol/orally | p-cymene-2,5-diol p-cymene-3,9-diol p-cymene-3,7-diol thymol | urine | [60] |
| rat | thyme extract/orally | thymol sulphate | plasma | [68] |
| laying hen | thyme extract/orally | p-cymene-2,3-diol thymol | egg yolk | [61] |
| Japanese quail | thymol/orally | thymol | egg yolk, faeces | [74] |
| broiler chicken | dried Thymi herba/orally | thymol | plasma, small intestine, caecum, liver, muscle | [70] |
| broiler chicken | thyme essential oil/orally | thymol | plasma, liver, kidney, muscle, duodenal wall, gut content | [63] |
| broiler chicken | thyme essential oil/orally | thymol sulphate thymol glucuronide | plasma, duodenal wall, liver | [76] |
| piglet | essential oil/orally (carvacrol, thymol, eugenol and trans-cinnamaldehyde) | carvacrol thymol eugenol | plasma | [64] |
| carvacrol thymol eugenol trans-cinnamaldehyde | small intestine | |||
| carvacrol thymol eugenol trans-cinnamaldehyde | bile | |||
| carvacrol thymol eugenol | urine | |||
| piglet | Biomin® P.E.P 1000 (main compounds thymol and carvacrol)/orally | thymol | plasma, kidney, faeces | [72] |
| piglet | Thymi herba/orally | thymol | plasma | [73] |
| bovine | Phyto-Mast (essential oil of Thymus vulgaris and oregano)/intramammary | thymol | milk, plasma, liver, kidney, fat | [65] |
| dairy cattle | Phyto-Mast (essential oil of Thymus vulgaris and oregano)/intramammary | thymol | milk, plasma, liver, kidney, fat | [66] |
| horse | Bronchipret (equivalent to 2–4 g thyme extract)/orally | thymol | plasma | [69] |
3. Bioavailability of Thymol Generally and in Rabbits as Model Animal
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacometti, J.; Kovacevic, D.B.; Putnik, P.; Gabric, D.; Bilusic, T.; Kresic, G.; Stulic, V.; Barba, F.J.; Chemat, F.; Barbosa-Canovas, G.; et al. Extraction of bioactive compounds and essential oils from Mediterranesn herbs by conventional and green innovative techniques: A review. Food. Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Almeida, M.M.; Salazar, M.L.A.R.; Pires, F.C.S.; Bezzera, F.W.F.; Cunha, V.M.B.; Cordeiro, R.M.; Urbina, G.R.O.; Silva, A.P.S.; Pinto, R.H.H.; et al. Potential of medicinal use of essential oils from aromatic plants. In Potential of Essential Oils; El-Shemy, H., Ed.; IntechOpen: London, UK, 2018; pp. 1–19. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.; Ribnicky, D.M.; Poulev, A.; Logendra, S.; Cefalu, W.T.; Raskin, I. A natural history of botanical therapeutics. Metab. Clin. Exp. 2008, 57 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives, Aromatic Plants and Herbs in Animal Nutrition and Health; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Elsevier Academic Press, Inc.: London, UK, 2020; pp. 1–15. [Google Scholar]
- Genser, D. Food and drug Interaction: Consequences for the nutrition/health status. Ann. Nutr. Metab. 2008, 52 (Suppl. S1), 29–32. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro dos Santos, R.; Andrade, M.; Sanches-Silva, A.; De Melo, N.R. Essential oils for food application: Natural substances with established biological activities. Food Bioprocess. Technol. 2017, 11, 43–71. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial drugs discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Tortorella, E.; Tedesco, P.; Esposito, P.F.; January, G.G.; Fani, R.; Jaspars, M.; de Pascale, D. Antibiotics from deep-sea microorganisms: Current discoveries and perspectives. Mar. Drugs 2018, 16, 355. [Google Scholar] [CrossRef] [Green Version]
- Penesyan, A.; Khelleberg, S.; Egan, S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs 2010, 8, 438–459. [Google Scholar] [CrossRef] [Green Version]
- Chavan, S.S.; Damale, M.G.; Devanand, B.S.; Sangshetti, J.N. Antibacterial and antifungal drugs from natural source: A review of clinical development. Nat. Prod. Clin. Trials 2018, 1, 114–164. [Google Scholar] [CrossRef]
- Boucher, H.W.; Ambrose, P.G.; Chambers, H.F.; Ebright, R.H.; Jezek, A.; Murray, B.E.; Newland, J.G.; Ostrowsky, B.; Rex, J.H. White paper: Developing antimicrobial drugs for resistane pathogens, narrow-spectrum indivations, and unmet needs. J. Infect. Dis. 2017, 216, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Song, J.; Sun, C.; Xu, J.; Zhu, Y.; Verpoorte, R.; Fan, T.P. Herbal genomics: Examining the biology of traditional medicines. Science 2015, 347, S27–S29. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicicnal plants as antimicrobial therapeutics: Potential avenues of biocompatible grug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA) 2014. Sales of Veterinary Antimicrobial Agents in 26 EU/EEA Countries in 2012. Available online: http://www.ema.europa.eu/docs/enGB/document_libraryReport/2014/10/WC500175671.pdf (accessed on 1 March 2022).
- Federal Food Safety and Veterinary Office 2019. Statistics and Reports. Swiss Antibiotic Resistance Report 2018. Available online: www.blv.admin.ch/blv/en/home/tiere/publikationen-und-forschung/statistiken-berichte-tiere (accessed on 1 March 2022).
- Bennett, R.M.; Christiansen, K.; Clifton-Hadley, R.S. Direct costs of endemic diseases of farm animals in Great Britain. Vet. Rec. 1999, 145, 376–377. [Google Scholar] [CrossRef]
- Bradley, A. Bovine mastitis: An evolving disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [CrossRef]
- Mertenat, D.; Dal Cero, M.; Vogl, C.R.; Ivemeyer, S.; Meier, B.; Maeschli, A.; Hamburger, M.; Walkenhorst, M. Ethnoveterinary knowledge of farmers in bilingual regions of Switzerland – is there potential to extend veterinary options to reduce antimicrobial use? J. Ethnopharmacol. 2020, 246, 112184. [Google Scholar] [CrossRef]
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 1670. [Google Scholar] [CrossRef]
- Second Joint FAO/OIE/WHO Expert Workshop on Non-human Antimicrobial Usage and Antimicrobial Resistance: Management Options, Oslo, Norway, 15–18 March 2004. Available online: www.oie.int/doc/ged/D895.PDF (accessed on 1 March 2022).
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Maeschli, A.; Schmidt, A.; Ammann, W.; Schurtenberger, P.; Maurer, E.; Walkenhorst, M. Einfluss eines komplementarmedizinischen telefonischen Beratungssystems auf den Antibiotikaeinsatz bei Nutztieren in der Schweiz. Complement. Med. Res. 2019, 26, 174–181. [Google Scholar] [CrossRef]
- Ayrle, H.; Mevissen, M.; Kaske, M.; Nathues, H.; Grutzner, N.; Melzig, M.; Walkenhorst, M. Medicinal plants – prophylactic and therapeutic options for gastrointestinal and respiratory diseases in calves and piglets? A systematic review. BMC Vet. Res. 2016, 12, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupper, J.; Walkenhorst, M.; Ayrle, H.; Mevissen, M.; Demuth, D.; Naegeli, H. Online-Informationssystem für die Phytotherapie bei Tieren. Schweiz. Arch. Tierheilkd. 2018, 10, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.N.; Hsu, Y.S.; Ho, C.T. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J. Food Drug Anal. 2014, 22, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.M.; Chao, T.Y.; Chang, W.C.; Chang, M.J.; Lee, M.F. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J. Food Drug Anal. 2016, 24, 556–563. [Google Scholar] [CrossRef] [Green Version]
- Meeran, M.F.N.; Javed, H.; Taee, H.A.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Espaňa, P.; Cisneros-Estrada, A.; Garduno-Ramirez, M.L.; Hernandez-Abreu, O.; Ramirez, R.; Estrada-Soto, S. Preliminary ethnopharmacological survey of plants used in Mexico for the treatment of hypertension. Phcog. Rev. 2009, 3, 41–65. [Google Scholar] [CrossRef]
- Giordani, R.; Hadef, Y.; Kaloustian, J. Compositions and antifungal activities of essential oils of some Algerian aromatic plants. Fitoterapia 2008, 79, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnophamacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calheha, R.; Fernandes, A.; Markovič, T.; Markovič, D.; Giweli, A.; Sokovič, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Cros. Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J.L.; Phil, D. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural drugs a treatment strategy for cardiovascular disease through the regulation of oxidative stress. Oxid. Med. Cell. Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrient 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Costa, G.; Fortuna, A.; Gonçalves, D.; Figueiredo, I.V.; Falcão, A.; Batista, M.T. Pharmacokinetics of Cymbopogon citratus infusion in rats after single oral dose administration. SOJ Pharm. Pharm. Sci. 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Suen, J.; Thomas, J.; Kranz, A.; Vun, S.; Miller, M. Effect of flavonoids on oxidative stress and inflammation in adults at risk of cardiovascular disease: A systematic review. Healthcare 2016, 4, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rawi, N.H.; Shahid, A.M. Oxidative stress, antioxidants, and lipid profile in the serum and saliva of individuals with coronary heart disease: Is there a link with periodontal health? Minerva Stomatol. 2017, 66, 212–225. [Google Scholar] [CrossRef]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7A–11A. [Google Scholar] [CrossRef]
- de Brito Alves, J.L.; de Sousa, V.P.; Cavalcanti Neto, M.P.; Magnani, M.; de Andrade Braga, V.; da Costa-Silva, J.H.; Leandro, C.G.; Vidal, H.; Pirola, L. New insights on the use of dietary polyphenols or probiotics for the management of arterial hypertension. Front. Physiol. 2016, 7, 448. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Pérez, L.; Gosalbes, M.J.; Yuste, S.; Valls, R.M.; Pedret, A.; Llauradó, E.; Jimenez-Hernandez, N.; Artacho, A.; Pla-Paga, L.; Companys, J.; et al. Gut metagenomic and short chain fatty acids signature in hypertension: A cross-sectional study. Sci. Rep. 2020, 10, 6436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Paga, L.; Pedret, A.; Rubió, L.; Gosalbes, M.J.; Yuste, S.; Sola, R.; Valls, R.M. Interplay between dietary phenolic compound intake and the human gut microbiome in hypertension: A cross-sectional study. Food Chem. 2021, 344, 128567. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Komaki, A.; Hoseini, F.; Shahidi, S.; Baharlouei, H. Study of the effect of extract of Thymus vulgaris on anxiety in male rats. J. Tradit. Complem. Med. 2016, 6, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Asadbegi, M.; Yaghmaei, P.; Salehi, I.; Komaki, A.; Ebrahim-Habibi, A. Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta peptide in high fat diet-fed rats. Metab. Brain Dis. 2017, 32, 827–839. [Google Scholar] [CrossRef]
- Kohlert, C.; van Rensen, I.; März, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Rubió, L.; Macià, A.; Castell-Auví, A.; Pinent, M.; Blay, M.T.; Ardévol, A.; Romero, M.P.; Motilva, M.J. Effect of the co-occurring olive oil and thyme extracts on the phenolic bioaccessibility and bioavailability assessed by in vitro digestion and cell models. Food Chem. 2014, 149, 277–284. [Google Scholar] [CrossRef]
- Kohlert, C.; Schindler, G.; März, R.W.; Abel, G.; Brinkhaus, B.; Derendorf, H.; Gräfe, E.U.; Veit, M. Systemic availability and pharmacokinetics of thymol in humans. J. Clin. Pharmacol. 2002, 42, 731–737. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacova, K.; Zitterl-Eglseer, K.; Chrastinova, L.; Laukova, A.; Madarova, M.; Gancarcikova, S.; Sopkova, D.; Andrejcakova, Z.; Placha, I. Effect of thymol addition and withdrawal on some blood parameters, antioxidative defence system and fatty acid profile in rabbit muscle. Animals 2020, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Bacova, K.; Zitterl Eglseer, K.; Karas Räuber, G.; Chrastinova, L.; Laukova, A.; Takacsova, M.; Pogany Simonova, M.; Placha, I. Effect of sustained administration of thymol on its bioaccessibility and bioavailability in rabbits. Animals 2021, 11, 2595. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Agata, I.; Sakamoto, M.; Yagi, N.; Hayashi, N. On the metabolic detoxication of thymol in rabbit and man. J. Toxicol. Sci. 1979, 4, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austgulen, L.; Solheim, E.; Scheline, R. Metabolism in rats of p-cymene derivatives: Carvacrol and thymol. Pharmacol. Toxicol. 1987, 61, 98–102. [Google Scholar] [CrossRef]
- Krause, E.L.; Ternes, W. Bioavailability of the antioxidative thyme compounds thymol and p-cymene-2,3-diol in egg. Eur. Food Res. Technol. 1999, 209, 140–144. [Google Scholar] [CrossRef]
- Thalhamer, B.; Buchberger, W.; Waser, M. Identification of thymol phase I metabolites in human urine by headspace sorptive extraction combined with thermal desorption and gas chromatography mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 64–69. [Google Scholar] [CrossRef]
- Oceľová, V. Plant additives in relation to the animal gastrointestinal tract and metabolism of their main compounds. Master’s Thesis, Institute of Animal Physiology, Slovak Academy of Sciences, Košice, Slovakia, 2017. [Google Scholar]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2008, 88, 2371–2381. [Google Scholar] [CrossRef]
- Armorini, S.; Yeatts, J.E.; Mullen, K.A.E.; Mason, S.E.; Mehmeti, E.; Anderson, K.L.; Washburn, S.P.; Baynes, R.E. Development of a HS-SPME-GC-MS/MS method for the quantitation of thymol and carvacrol in bovine matrices and to determine residue depletion in milk and tissues. J. Agric. Food Chem. 2016, 64, 7856–7865. [Google Scholar] [CrossRef]
- Mason, S.E.; Mullen, K.A.E.; Anderson, K.L.; Washburn, S.P.; Yeatts, J.L.; Baynes, R.E. Pharmacokinetic analysis of thymol, carvacrol and diallyl disulfide after intramammary and topical applications in healthy organic dairy cattle. Food Addit. Contam. Part A 2017, 34, 740–749. [Google Scholar] [CrossRef]
- Rubió, L.; Farràs, M.; de la Torre, R.; Macià, A.; Romero, M.P.; Valls, R.M.; Solà, R.; Farré, M.; Fitó, M.; Motilva, M.J. Metabolite profiling of olive oil and thyme phenols after a sustained intake of two phenol-enriched olive oils by humans: Identification of compliance markers. Food Res. Int. 2014, 65, 59–68. [Google Scholar] [CrossRef]
- Rubió, L.; Serra, A.; Chen, C.Y.O.; Macià, A.; Romero, M.P.; Covas, M.I.; Solà, R.; Motilva, M.J. Effects of co-occurring components from olive oil and thyme extracts on antioxidant status and their bioavailability in acute ingestion in rats. Food Funct. 2014, 5, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoven, R.; Zappe, H.; Zitterl-Eglseer, K.; Jugl, M.; Franz, C. Study of the effect of Bronchipret on the lung function of five Austrian saddle horses suffering recurrent airway obstruction (heaves). Vet. Rec. 2003, 152, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Haselmeyer, A.; Zentek, J.; Chizzola, R. Effects of thyme as a feed additive in broiler chickens on thymol in gut contents, blood plasma, liver and muscle. J. Sci. Food Agric. 2015, 95, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Haselmeyer, A. Wirkung von Thymian als Futterzusatz beim Broiler. Ph.D. Thesis, Veterinärmedizinischen Universität, Wien, Austria, 2007. [Google Scholar]
- Zitterl-Eglseer, K.; Wetscherek, W.; Stoni, A.; Kroismayr, A.; Windisch, W. Bioavailability of essential oils of a phytobiotic feed additive and impact of performance and nutrient digestibility in weaned piglets. Bodenkult. J. Land Manag. Food Environ. 2008, 59, 121–129. [Google Scholar]
- Hagmüller, W.; Jugl-Chizzola, M.; Zitterl-Eglseer, K.; Gabler, C.; Spergser, J.; Chizzola, R.; Franz, C. The use of Thymi Herba as feed additive (0.1%, 0.5%, 1.0%) in weanling piglets with assessment of the shedding of haemolysing E. coli and the detection of thymol in the blood plasma. Berl. Münch. Tierärztl. Wochenschr. 2005, 119, 50–54. [Google Scholar]
- Fernandez, M.E.; Palacio, M.A.; Labaque, M.C. Thymol detection by solid-phase microextraction in faeces and egg yolk of Japanese quail. J. Chromatogr. B 2017, 1044, 39–46. [Google Scholar] [CrossRef]
- Oceľová, V.; Chizzola, R.; Battelli, G.; Pisarcikova, J.; Faix, S.; Gai, F.; Placha, I. Thymol in the intestinal tract of broiler chickens after sustained administration of thyme essential oil in feed. J. Anim. Physiol. Anim. Nutr. 2019, 103, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Pisarčíková, J.; Oceľová, V.; Faix, Š.; Plachá, I.; Calderón, A.I. Identification and quantification of thymol metabolites in plasma, liver and duodenal wall of broiler chickens using UHPLC-ESI-QTOF-MS. Biomed. Chromatogr. 2017, 31, e3881. [Google Scholar] [CrossRef]
- Oceľová, V.; Chizzola, R.; Pisarčíková, J.; Novak, J.; Ivanišinová, O.; Faix, Š. Effect of thyme essential oil supplementation on thymol content in blood plasma, liver, kidney and muscle in broiler chickens. Nat. Prod. Commun. 2016, 11, 1545–1550. [Google Scholar] [CrossRef]
- Singh, R.; Hu, M. Drug metabolism in gastroinestinal tract. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Li, X., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 91–109. [Google Scholar]
- Hu, M.; Li, X. Barriers to oral bioavailability-an overview. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Li, X., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–5. [Google Scholar]
- Parkinson, A. Biotransformation of Xenobiotics. In Cassaret & Doull’s Toxicology: The Basic Science of Poisons, 6th ed.; Klaassen, C.D., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2001; pp. 133–224. [Google Scholar]
- Rozman, K.K.; Klaassen, C.D. Absorption, distribution, and excretion of toxicants. In Cassaret & Doull’s Toxicology: The Basic Science of Poisons, 6th ed.; Klaassen, C.D., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2001; pp. 107–132. [Google Scholar]
- Timbrell, J.A. Factors Affecting Toxic Responses: Disposition. Principles of Biochemical Toxicology, 4th ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2009; pp. 35–74. [Google Scholar]
- Davies, R.R.; Davies, J.A.E.R. Rabbit gastrointestinal physiology. Vet. Clin. North Am. Exot. Anim. Pract. 2003, 6, 139–153. [Google Scholar] [CrossRef]
- Campbell-Ward, M.L. Gastrointestinal physiology and nutrition. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 3rd ed.; Quesenberry, K.E., Carpenter, J.W., Eds.; Elsevier: Saint-Louis, MI, USA, 2012; pp. 183–192. [Google Scholar]
- Placha, I.; Bacova, K.; Zitterl-Eglseer, K.; Laukova, A.; Chrastinova, L.; Madarova, M.; Zitnan, R.; Strkolcova, G. Thymol in fattening rabbit diet, its bioavailability and effects on intestinal morphology, microbiota from caecal content and immunity. J. Anim. Physiol. Anim. Nutr. 2021, 106, 368–377. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Placha, I.; Bacova, K.; Plachy, L. Current Knowledge on the Bioavailability of Thymol as a Feed Additive in Humans and Animals with a Focus on Rabbit Metabolic Processes. Animals 2022, 12, 1131. https://doi.org/10.3390/ani12091131
Placha I, Bacova K, Plachy L. Current Knowledge on the Bioavailability of Thymol as a Feed Additive in Humans and Animals with a Focus on Rabbit Metabolic Processes. Animals. 2022; 12(9):1131. https://doi.org/10.3390/ani12091131
Chicago/Turabian StylePlacha, Iveta, Kristina Bacova, and Lukas Plachy. 2022. "Current Knowledge on the Bioavailability of Thymol as a Feed Additive in Humans and Animals with a Focus on Rabbit Metabolic Processes" Animals 12, no. 9: 1131. https://doi.org/10.3390/ani12091131
APA StylePlacha, I., Bacova, K., & Plachy, L. (2022). Current Knowledge on the Bioavailability of Thymol as a Feed Additive in Humans and Animals with a Focus on Rabbit Metabolic Processes. Animals, 12(9), 1131. https://doi.org/10.3390/ani12091131






