Feeding Calcium-Ammonium Nitrate to Lactating Dairy Goats: Milk Quality and Ruminal Fermentation Responses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Diets
2.2. Sample Collection and Chemical Analyses
2.3. Milk Collection and Laboratory Assays
2.4. Blood and Ruminal Fluid Collections
2.5. Statistical Analyses
3. Results
3.1. Dry Matter Intake and Nutrient Digestibility
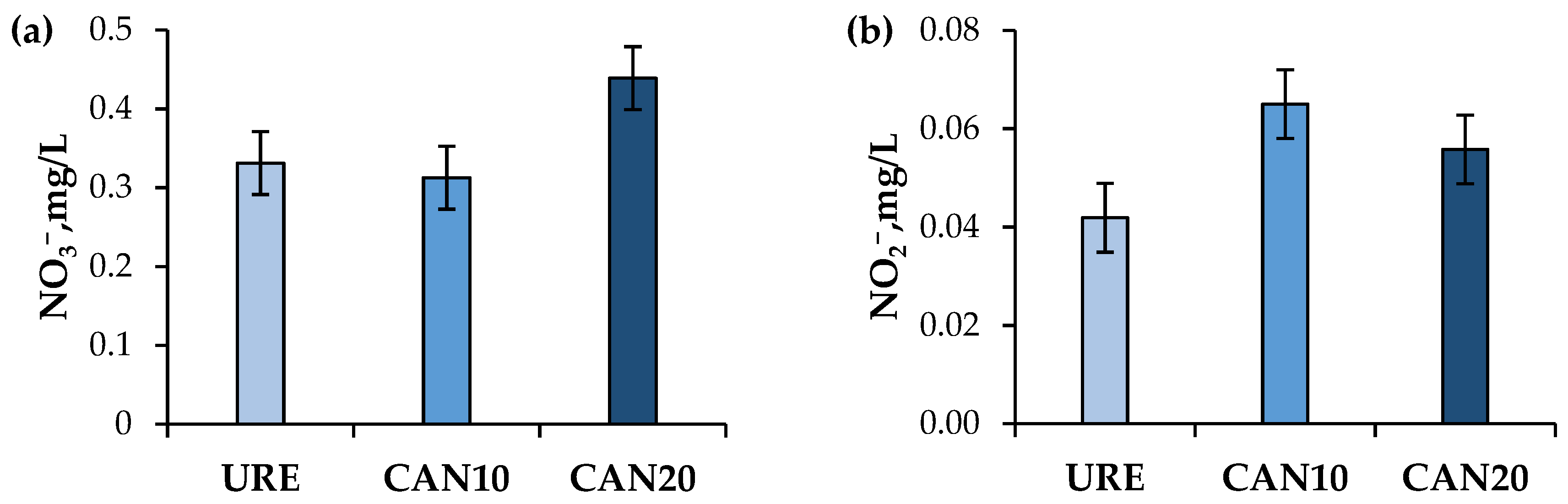
3.2. Yield, Composition, Antioxidant Capacity, and Nitrate and Nitrite Residues in Milk
4. Discussion
4.1. Feed Intake and Nutrient Digestibility
4.2. Milk Quality and Nitrate and Nitrite Residues in Milk
4.3. Plasma Urea Nitrogen and Ruminal Fermentation Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X.Y.; Dijkstra, J.; Bannink, A.; van Gastelen, S.; France, J.; Kebreab, E. Antimethanogenic effects of nitrate supplementation in cattle: A meta-analysis. J. Dairy Sci. 2020, 103, 11375–11385. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.; Ungerfeld, E.M.; Zhang, X.M.; Long, D.L.; Mao, H.X.; Deng, J.P.; Bannink, A.; Tan, Z.L. Nitrate improves ammonia incorporation into rumen microbial protein in lactating dairy cows fed a low-protein diet. J. Dairy Sci. 2018, 101, 9789–9799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, R.A. The potential of feeding nitrate to reduce enteric methane production in ruminants. In A Report to The Department of Climate Change; Commonwealth Government of Australia: Canberra, Australia, 2008; pp. 1–90. [Google Scholar]
- Popova, M.; Guyader, J.; Silberberg, M.; Seradj, A.R.; Saro, C.; Bernard, A.; Gérard, C.; Martin, C.; Morgavi, D.P. Changes in the Rumen Microbiota of Cows in Response to Dietary Supplementation with Nitrate, Linseed, and Saponin Alone or in Combination. Appl. Environ. Microbiol. 2019, 85, e02657-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latham, E.A.; Anderson, R.C.; Pinchak, W.E.; Nisbet, D.J. Insights on alterations to the rumen ecosystem by nitrate and nitrocompounds. Front. Microbiol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.J.; Simon, J.; Rowley, G.; Bedmar, E.J.; Richardson, D.J.; Gates, A.J.; Delgado, M.J. Nitrous Oxide Metabolism in Nitrate-Reducing Bacteria: Physiology and Regulatory Mechanisms, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 68, ISBN 9780128048238. [Google Scholar]
- Lee, C.; Beauchemin, K.A. A review of feeding supplementary nitrate to ruminant animals: Nitrate toxicity, methane emissions, and production performance. Can. J. Anim. Sci. 2014, 94, 557–570. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, S2–S16. [Google Scholar] [CrossRef] [Green Version]
- Villar, M.L.; Godwin, I.R.; Hegarty, R.S.; Erler, D.V.; Farid, H.T.; Nolan, J.V. Nitrate and nitrite absorption, recycling and retention in tissues of sheep. Small Rumin. Res. 2021, 200, 106392. [Google Scholar] [CrossRef]
- Guyader, J.; Doreau, M.; Morgavi, D.P.; Gérard, C.; Loncke, C.; Martin, C. Long-term effect of linseed plus nitrate fed to dairy cows on enteric methane emission and nitrate and nitrite residuals in milk. Animal 2016, 10, 1173–1181. [Google Scholar] [CrossRef] [Green Version]
- Olijhoek, D.W.; Hellwing, A.L.F.; Brask, M.; Weisbjerg, M.R.; Højberg, O.; Larsen, M.K.; Dijkstra, J.; Erlandsen, E.J.; Lund, P. Effect of dietary nitrate level on enteric methane production, hydrogen emission, rumen fermentation, and nutrient digestibility in dairy cows. J. Dairy Sci. 2016, 99, 6191–6205. [Google Scholar] [CrossRef] [Green Version]
- Meller, R.A.; Wenner, B.A.; Ashworth, J.; Gehman, A.M.; Lakritz, J.; Firkins, J.L. Potential roles of nitrate and live yeast culture in suppressing methane emission and influencing ruminal fermentation, digestibility, and milk production in lactating Jersey cows. J. Dairy Sci. 2019, 102, 6144–6156. [Google Scholar] [CrossRef]
- Clark, S.; Mora García, M.B. A 100-Year Review: Advances in goat milk research. J. Dairy Sci. 2017, 100, 10026–10044. [Google Scholar] [CrossRef]
- Silanikove, N.; Fira, S.; Mayan, S.; Uzi, M.; Gabriel, L. Hydrogen peroxide-dependent conversion of nitrite to nitrate as a crucial feature of bovine milk catalase. J. Agric. Food Chem. 2009, 57, 8018–8025. [Google Scholar] [CrossRef]
- Silanikove, N.; Rauch-Cohen, A.; Shapiro, F.; Arieli, A.; Merin, U.; Leitner, G. Lipopolysaccharide challenge of the mammary gland in cows induces nitrosative stress that impairs milk oxidative stability. Animal 2012, 6, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Klop, G.; Hatew, B.; Bannink, A.; Dijkstra, J. Feeding nitrate and docosahexaenoic acid affects enteric methane production and milk fatty acid composition in lactating dairy cows. J. Dairy Sci. 2016, 99, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Villar, M.L.; Hegarty, R.S.; Nolan, J.V.; Godwin, I.R.; McPhee, M. The effect of dietary nitrate and canola oil alone or in combination on fermentation, digesta kinetics and methane emissions from cattle. Anim. Feed Sci. Technol. 2020, 259, 114294. [Google Scholar] [CrossRef]
- De Raphélis-Soissan, V.; Li, L.; Godwin, I.R.; Barnett, M.C.; Perdok, H.B.; Hegarty, R.S. Use of nitrate and Propionibacterium acidipropionici to reduce methane emissions and increase wool growth of Merino sheep. Anim. Prod. Sci. 2014, 54, 1860–1866. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007; p. 384. [Google Scholar]
- AOAC International. Official Methods of Analysis, 19th ed.; AOAC Int.: Arlington, VA, USA, 2012. [Google Scholar]
- Cochran, R.C.; Adams, D.C.; Wallace, J.D. Predicting digestibility of different diets with internal markers: Evaluation of four potential markers. J. Anim. Sci. 1986, 63, 1476–1483. [Google Scholar] [CrossRef]
- De Andrade, P.V.D.; De Souza, M.R.; Penna, C.F.A.D.M.; Ferreira, J.M. Características microbiológicas e físico- químicas do leite de cabra submetido à pasteurização lenta pós-envase e ao congelamento. Cienc. Rural 2008, 38, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Arcuri, E.F.; da Silva, P.D.L.; Brito, J.R.F.; Silva, M.R.; Souza, G.N. Emprego do Somacount 300, calibrado com leite de vaca, na contagem de células somáticas no leite de cabra. Ciência Rural 2004, 34, 1497–1500. [Google Scholar] [CrossRef]
- Sklan, D.; Ashkenazi, R.; Braun, A.; Devorin, A.; Tabori, K. Fatty Acids, Calcium Soaps of Fatty Acids, and Cottonseeds Fed to High Yielding Cows. J. Dairy Sci. 1992, 75, 2463–2472. [Google Scholar] [CrossRef]
- Sjaunja, L.O.; Bævre, L.; Junkkarinen, L.; Pedersen, J.; Setälä, J. A nordic proposal for an energy corrected milk (ECM) formula. Perform. Rec. Anim. State Art 1990, 1990, 156–157. [Google Scholar]
- Kiokias, S.N.; Dimakou, C.P.; Tsaprouni, I.V.; Oreopoulou, V. Effect of compositional factors against the thermal oxidative deterioration of novel food emulsions. Food Biophys. 2006, 1, 115–123. [Google Scholar] [CrossRef]
- Vyncke, W. Direct determination of the thiobarbituric acid value in trichoracetic acid extracts of fish as a measure of oxidative rancidity. Fette Seifen Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Osorio, J.A.C.; Daniel, J.L.P.; Cabral, J.F.; Almeida, K.V.; Guimarães, K.L.; Sippert, M.R.; Lourenço, J.C.S.; De Marchi, F.E.; Velho, J.P.; Santos, G.T. Annatto seeds as antioxidants source with linseed oil for dairy cows. Animals 2021, 11, 1465. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS.+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Cortas, N.K.; Wakid, N.W. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin. Chem. 1990, 36, 1440–1443. [Google Scholar] [CrossRef]
- Murphy, J.J.; Connolly, J.F.; McNeill, G.P. Effects on milk fat composition and cow performance of feeding concentrates containing full fat rapeseed and maize distillers grains on grass-silage based diets. Livest. Prod. Sci. 1995, 44, 1–11. [Google Scholar] [CrossRef]
- ISO 5509; ISO Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 1978; Volume 2000, pp. 1–6.
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Cox, G.E.P.; Box, D.R. An analysis of transformations revisited. J. R. Stat. Soc. 1964, 26, 211–252. [Google Scholar]
- Jorgensen, E.; Pedersen, A.R. How to obtain those nasty standard errors from transformed data—And why they should not be used. Biometry Res. Unit Dan. Inst. Agric. Sci. 1998, 7, 1–20. [Google Scholar]
- Silveira, R.F.; Fernandes, M.H.M.R.; Almeida, A.K.; Araujo, R.C.; Biagioli, B.; Lima, A.R.C.; Teixeira, I.A.M.A.; Resende, K.T. Energy partition and nitrogen utilization by male goats fed encapsulated calcium nitrate as a replacement for soybean meal. Anim. Feed Sci. Technol. 2019, 248, 67–76. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, Z.; Meng, Q. Effects of nitrate on methane production, fermentation, and microbial populations in in vitro ruminal cultures. Bioresour. Technol. 2012, 103, 173–179. [Google Scholar] [CrossRef] [PubMed]
- van Zijderveld, S.M.; Gerrits, W.J.J.; Dijkstra, J.; Newbold, J.R.; Hulshof, R.B.A.; Perdok, H.B. Persistency of methane mitigation by dietary nitrate supplementation in dairy cows. J. Dairy Sci. 2011, 94, 4028–4038. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils. Food Chem. 2002, 77, 503–510. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Rossell, J.B.; Hudson, B.J.F.; Löliger, J. Chemistry of rancidity in foods. In Chemistry of Rancidity in Foods; Allen, J.C., Hamilton, R.J., Eds.; Applied Science Publishers Ltd.: London, UK, 1983. [Google Scholar]
- Jadhav, S.J.; Nimbalkar, S.S.; Kulkarni, A.D. Lipid Oxidation in Biological and Food Systems. In Food Antioxidants: Technological, Toxicological, and Health Perspectives; Madhavi, D.L., Deshpande, S.S., Salunkhe, D.K., Eds.; CRC Press: New York, NY, USA, 1996. [Google Scholar]
- Almeida, K.V.; Santos, G.T.; Daniel, J.L.P.; Osorio, J.A.C.; Yamada, K.L.G.; Sippert, M.R.; Cabral, J.F.; Marchi, F.E.; Araujo, R.C.; Vyas, D. Effects of calcium ammonium nitrate fed to dairy cows on nutrient intake and digestibility, milk quality, microbial protein synthesis, and ruminal fermentation parameters. J. Dairy Sci. 2022, 105, 2228–2241. [Google Scholar] [CrossRef]
- Silanikove, N.; Merin, U.; Shapiro, F.; Leitner, G. Subclinical mastitis in goats is associated with upregulation of nitric oxide-derived oxidative stress that causes reduction of milk antioxidative properties and impairment of its quality. J. Dairy Sci. 2014, 97, 3449–3455. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Beghelli, D.; Lupidi, G.; Damiano, S.; Cavallucci, C.; Bistoni, O.; De Cosmo, A.; Polidori, P. Rapid Assay to Evaluate the Total Antioxidant Capacity in Donkey Milk and in more Common Animal Milk for Human Consumption. Austin Food Sci. 2016, 1, 1003. [Google Scholar]
- WHO. Chemical Fact Sheets, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Hord, N.G.; Ghannam, J.S.; Garg, H.K.; Berens, P.D.; Bryan, N.S. Nitrate and Nitrite Content of Human, Formula, Bovine, and Soy Milks: Implications for Dietary Nitrite and Nitrate Recommendations. Breastfeed. Med. 2011, 6, 393–399. [Google Scholar] [CrossRef]
- Cockburn, A.; Brambilla, G.; Fernández-Cruz, M.L.; Arcella, D.; Bordajandi, L.R.; Cottrill, B.; van Peteghem, C.; Dorne, J. Lou Nitrite in feed: From animal health to human health. Toxicol. Appl. Pharmacol. 2013, 270, 209–217. [Google Scholar] [CrossRef]
- Parodi, P.W. Milk fat in human nutrition. Aust. J. Dairy Technol. 2004, 59, 3–59. [Google Scholar]
- Lindmark Månsson, H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giger-Reverdin, S.; Domange, C.; Broudiscou, L.P.; Sauvant, D.; Berthelot, V. Rumen function in goats, an example of adaptive capacity. J. Dairy Res. 2020, 87, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Fievez, V.; Colman, E.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Anim. Feed Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Yang, C.; McKain, N.; McCartney, C.A.; Wallace, R.J. Consequences of inhibiting methanogenesis on the biohydrogenation of fatty acids in bovine ruminal digesta. Anim. Feed Sci. Technol. 2019, 254, 114189. [Google Scholar] [CrossRef]
- Van Zijderveld, S.M.; Gerrits, W.J.J.; Apajalahti, J.A.; Newbold, J.R.; Dijkstra, J.; Leng, R.A.; Perdok, H.B. Nitrate and sulfate: Effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J. Dairy Sci. 2010, 93, 5856–5866. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, N.; Yokoyama, S.; Hino, T. Effects of nitrate addition to a diet on fermentation and microbial populations in the rumen of goats, with special reference to Selenomonas ruminantium having the ability to reduce nitrate and nitrite. Anim. Sci. J. 2015, 86, 378–384. [Google Scholar] [CrossRef]

| Item | Treatment 1 | ||
|---|---|---|---|
| URE | CAN10 | CAN20 | |
| Ingredient proportion, g/kg DM | |||
| Corn silage | 450 | 450 | 450 |
| Ground corn | 382 | 381 | 381 |
| Soybean meal | 139 | 139 | 139 |
| Urea 2 | 7.32 | 3.66 | 0.00 |
| Calcium-ammonium nitrate 3 | 0.00 | 10.0 | 20.0 |
| Limestone 4 | 11.5 | 5.77 | 0.00 |
| Mineral supplement 5 | 10.0 | 10.0 | 10.0 |
| Nutritional composition, g/kg DM 6 | |||
| Dry matter, as-fed basis | 505 | 504 | 504 |
| Organic matter | 944 | 941 | 939 |
| Crude protein | 160 | 160 | 160 |
| Rumen degradable protein | 107 | 107 | 107 |
| Neutral detergent fiber | 299 | 299 | 299 |
| Ether extract | 32.3 | 32.3 | 32.3 |
| Nitrate | 0.00 | 7.65 | 15.3 |
| Item | Treatment 1 | SEM | p-Value 2 | |||
|---|---|---|---|---|---|---|
| URE | CAN10 | CAN20 | Lin | Quad | ||
| BW, kg | 53.2 | 53.3 | 53.0 | 1.833 | 0.81 | 0.73 |
| DMI, kg/d | 1.73 | 1.78 | 1.76 | 0.059 | 0.33 | 0.21 |
| Digestibility, g/kg DM | ||||||
| DM | 589 | 618 | 553 | 20.93 | 0.23 | 0.08 |
| OM | 609 | 635 | 574 | 20.27 | 0.23 | 0.09 |
| CP | 591 | 649 | 613 | 19.89 | 0.44 | 0.06 |
| EE | 710 | 728 | 685 | 16.21 | 0.26 | 0.13 |
| NDF | 445 | 460 | 417 | 15.68 | 0.19 | 0.11 |
| Item | Treatment 9 | SEM | p-Value 10 | |||
|---|---|---|---|---|---|---|
| URE | CAN10 | CAN20 | Lin | Quad | ||
| Production, kg/d | ||||||
| Milk yield | 2.04 | 2.14 | 2.13 | 0.123 | 0.16 | 0.41 |
| 3.5% FCM 1 | 1.98 | 2.06 | 2.05 | 0.145 | 0.29 | 0.36 |
| ECM 2 | 1.92 | 2.00 | 1.99 | 0.136 | 0.26 | 0.40 |
| FE 3 | 1.12 | 1.14 | 1.14 | 0.076 | 0.52 | 0.65 |
| Composition, % | ||||||
| Fat | 3.26 | 3.26 | 3.19 | 0.177 | 0.48 | 0.68 |
| True protein | 2.76 | 2.73 | 2.75 | 0.071 | 0.92 | 0.61 |
| Lactose | 4.09 | 4.08 | 4.07 | 0.067 | 0.70 | 0.98 |
| Yield, kg/d | ||||||
| Fat | 0.067 | 0.070 | 0.069 | 0.006 | 0.49 | 0.39 |
| True protein | 0.056 | 0.058 | 0.059 | 0.003 | 0.20 | 0.66 |
| Lactose | 0.084 | 0.087 | 0.087 | 0.005 | 0.23 | 0.37 |
| Antioxidant capacity | ||||||
| TAC 4 | 202 | 207 | 200 | 8.211 | 0.68 | 0.14 |
| TBARS 5 | 9.74 | 7.00 | 7.34 | 0.797 | 0.01 | 0.06 |
| CD 6 | 47.1 | 55.7 | 66.0 | 4.898 | 0.01 | 0.78 |
| MUN 7, mg/dL | 22.8 | 22.4 | 23.3 | 2.063 | 0.67 | 0.55 |
| SCC 8, 1000/mL | 1570 | 2172 | 1417 | 666.7 | 0.86 | 0.37 |
| Log10 SCC | 2.98 | 3.00 | 2.89 | 0.143 | 0.63 | 0.64 |
| Item 1 | Treatment 5 | SEM | p-Value 6 | |||
|---|---|---|---|---|---|---|
| URE | CAN10 | CAN20 | Lin | Quad | ||
| FA proportions | ||||||
| 6:0 | 0.57 | 0.50 | 0.49 | 0.143 | 0.69 | 0.87 |
| 8:0 | 1.41 | 1.32 | 1.25 | 0.222 | 0.54 | 0.98 |
| 10:0 | 8.81 | 8.61 | 8.03 | 0.898 | 0.37 | 0.80 |
| 11:0 | 0.26 | 0.22 | 0.21 | 0.026 | 0.08 | 0.44 |
| 12:0 | 5.63 | 5.30 | 4.96 | 0.431 | 0.04 | 0.99 |
| 13:0 | 0.20 | 0.18 | 0.18 | 0.017 | 0.25 | 0.48 |
| 14:0 | 14.37 | 14.28 | 14.10 | 0.328 | 0.34 | 0.85 |
| 14:1 | 0.88 | 0.86 | 0.82 | 0.061 | 0.31 | 0.92 |
| 15:0 | 1.26 | 1.23 | 1.36 | 0.085 | 0.34 | 0.40 |
| 15:1 | 0.26 | 0.27 | 0.27 | 0.030 | 0.88 | 0.79 |
| 16:0 | 36.8 | 36.9 | 38.0 | 1.255 | 0.12 | 0.39 |
| 16:1 | 0.62 | 0.59 | 0.66 | 0.031 | 0.21 | 0.07 |
| 17:0 | 0.78 | 0.81 | 0.87 | 0.038 | 0.09 | 0.71 |
| 17:1 | 0.15 | 0.16 | 0.17 | 0.021 | 0.49 | 0.91 |
| 18:0 | 8.27 | 9.24 | 8.58 | 0.601 | 0.56 | 0.08 |
| trans-9 18:1 | 4.70 | 5.37 | 5.47 | 0.237 | 0.03 | 0.33 |
| cis-9 18:1 | 12.9 | 12.2 | 12.4 | 0.490 | 0.37 | 0.33 |
| trans-6 18:2 | 0.53 | 0.52 | 0.54 | 0.034 | 0.80 | 0.74 |
| cis-6 18:2 | 0.94 | 0.94 | 0.89 | 0.069 | 0.50 | 0.74 |
| cis-9, trans-11 CLA | 0.11 | 0.09 | 0.14 | 0.055 | 0.84 | 0.68 |
| 20:0 | 0.14 | 0.14 | 0.14 | 0.006 | 0.55 | 0.97 |
| 20:2 | 0.04 | 0.05 | 0.05 | 0.008 | 0.15 | 0.46 |
| 21:0 | 0.17 | 0.20 | 0.30 | 0.048 | 0.05 | 0.59 |
| FA groups | ||||||
| SFA 2 | 76.8 | 77.1 | 76.8 | 0.517 | 0.92 | 0.47 |
| MUFA 3 | 19.6 | 19.5 | 19.8 | 0.594 | 0.75 | 0.69 |
| PUFA 4 | 1.59 | 1.56 | 1.56 | 0.104 | 0.78 | 0.85 |
| Item | Treatment 1 | SEM | p-Value 2 | |||
|---|---|---|---|---|---|---|
| URE | CAN10 | CAN20 | Lin | Quad | ||
| pH | 6.90 | 7.17 | 7.00 | 0.270 | 0.54 | 0.13 |
| NH3-N, mM | 16.0 | 15.3 | 14.7 | 3.772 | 0.77 | 0.98 |
| Total VFA, mM | 49.5 | 50.5 | 57.0 | 6.885 | 0.34 | 0.68 |
| Individual VFA, mol/100 mol | ||||||
| Acetate | 62.3 | 63.1 | 65.5 | 1.643 | 0.14 | 0.62 |
| Propionate | 22.7 | 21.8 | 19.2 | 1.769 | 0.01 | 0.39 |
| Isobutyrate | 0.82 | 0.86 | 0.71 | 0.128 | 0.32 | 0.31 |
| Butyrate | 12.2 | 12.6 | 12.9 | 1.000 | 0.60 | 0.99 |
| Isovalerate | 0.85 | 0.71 | 0.71 | 0.091 | 0.14 | 0.33 |
| Valerate | 1.07 | 0.93 | 0.85 | 0.113 | 0.08 | 0.76 |
| Acetate:Propionate | 2.76 | 2.91 | 3.61 | 0.356 | <0.01 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, K.V.; Santos, G.T.; Osorio, J.A.C.; Lourenço, J.C.S.; Figueiredo, M.; Durman, T.; Marchi, F.E.; Alcalde, C.R.; Silva-Junior, R.C.; Itavo, C.C.B.F.; et al. Feeding Calcium-Ammonium Nitrate to Lactating Dairy Goats: Milk Quality and Ruminal Fermentation Responses. Animals 2022, 12, 983. https://doi.org/10.3390/ani12080983
Almeida KV, Santos GT, Osorio JAC, Lourenço JCS, Figueiredo M, Durman T, Marchi FE, Alcalde CR, Silva-Junior RC, Itavo CCBF, et al. Feeding Calcium-Ammonium Nitrate to Lactating Dairy Goats: Milk Quality and Ruminal Fermentation Responses. Animals. 2022; 12(8):983. https://doi.org/10.3390/ani12080983
Chicago/Turabian StyleAlmeida, Kleves V., Geraldo T. Santos, Jesus A. C. Osorio, Jean C. S. Lourenço, Monique Figueiredo, Thomer Durman, Francilaine E. Marchi, Claudete R. Alcalde, Ranulfo C. Silva-Junior, Camila C. B. F. Itavo, and et al. 2022. "Feeding Calcium-Ammonium Nitrate to Lactating Dairy Goats: Milk Quality and Ruminal Fermentation Responses" Animals 12, no. 8: 983. https://doi.org/10.3390/ani12080983
APA StyleAlmeida, K. V., Santos, G. T., Osorio, J. A. C., Lourenço, J. C. S., Figueiredo, M., Durman, T., Marchi, F. E., Alcalde, C. R., Silva-Junior, R. C., Itavo, C. C. B. F., Araujo, R. C., & Brito, A. F. (2022). Feeding Calcium-Ammonium Nitrate to Lactating Dairy Goats: Milk Quality and Ruminal Fermentation Responses. Animals, 12(8), 983. https://doi.org/10.3390/ani12080983







