Infection of a Free-Living Wild Boar (Sus scrofa) with a Bacterium from the Mycobacterium kansasii Complex
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Lesion Assessment and Mycobacterial Isolation
2.3. Strain Generic Identification
2.4. Strain Species Identification
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krajewska, M.; Augustynowicz-Kopeć, E.; Orłowska, B.; Welz, M.; Anusz, K.; Szulowski, K. Mycobacterium caprae—Bovine bacillus. Part II. Microbiological diagnostics and veterinary legislation. Życie Weter. 2016, 91, 348–350. [Google Scholar]
- Verdugo-Escárcega, D.A.; Perea-Razo, C.A.; González-Ruíz, S.; Sosa-Gallegos, S.L.; Suazo, F.M.; Cantó-Alarcón, G.J. Analysis of bovine tuberculosis transmission in Jalisco, Mexico through whole-genome sequencing. J. Vet. Res. 2020, 64, 51–61. [Google Scholar] [CrossRef]
- Zhou, L.; Mal, C.; Xiao, T.; Li, M.; Liu, H.; Zhao, X.; Wan, K.; Wang, R. A New Single Gene Differential Biomarker for Mycobacterium tuberculosis Complex and Non-tuberculosis Mycobacteria. Front. Microbiol. 2019, 10, 1887. [Google Scholar] [CrossRef]
- Röltgen, K.; Pluschke, G.; Spencer, J.S.; Brennan, P.J.; Avanzi, C. The immunology of other mycobacteria: M. ulcerans, M. leprae. Semin. Immunopathol. 2020, 42, 333–353. [Google Scholar] [CrossRef]
- Sharma, S.K.; Upadhyay, V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J. Med. Res. 2020, 152, 185–226. [Google Scholar]
- Jagielski, T.; Borówka, P.; Bakuła, Z.; Lach, J.; Marciniak, B.; Brzostek, A.; Dziadek, J.; Dziurzyński, M.; Pennings, L.; van Ingen, J.; et al. Genomic Insights into the Mycobacterium kansasii Complex: An Update. Front. Microbiol. 2020, 10, 2918. [Google Scholar] [CrossRef]
- Ghielmetti, G.; Hilbe, M.; Friedel, U.; Menegatti, C.; Bacciarini, L.; Stephan, R.; Bloemberg, G. Mycobacterial infections in wild boars (Sus scrofa) from Southern Switzerland: Diagnostic improvements, epidemiological situation and zoonotic potential. Transbound. Emerg. Dis. 2021, 68, 573–586. [Google Scholar] [CrossRef]
- Naranjo, V.; Gortazar, C.; Vicente, J.; de la Fuente, J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 2008, 127, 1–9. [Google Scholar] [CrossRef]
- Mycobacterium kansasii—Medscape. Available online: https://emedicine.medscape.com/article/223230-overview (accessed on 4 March 2022).
- Buhler, V.B.; Pollak, A. Human infection with atypical acid-fast organisms; report of two cases with pathologic findings. Am. J. Clin. Pathol. 1953, 23, 363–374. [Google Scholar] [CrossRef]
- Bakuła, Z.; Safianowska, A.; Nowacka-Mazurek, M.; Bielecki, J.; Jagielski, T. Mycobacterium kansasii: Pathogen biology and clinical and epidemiological features of infections. Adv. Microbiol. 2014, 53, 241–254. [Google Scholar]
- Mycobacterium kansasii—StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430906/ (accessed on 4 March 2022).
- Fukanoa, H.; Terazonob, T.; Hirabayashic, A.; Yoshidaa, M.; Suzukic, M.; Wadad, S.; Ishiie, N.; Hoshino, Y. Human pathogenic Mycobacterium kansasii (former subtype I) with zoonotic potential isolated from a diseased indoor pet cat, Japan. Emerg. Microbes Infect. 2021, 10, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.C.; Chiang, L.; Elwood, K. Mycobacterium kansasii. In Tuberculosis and Nontuberculous Mycobacterial Infections, 7th ed.; Schlossberg, D., Ed.; ASM Press: Washington, DC, USA, 2017; pp. 725–734. ISBN 9781555819859. [Google Scholar]
- Nalepa, P.; Strach, M.; Rybak-Bąk, M.; Siedlar, M. Two sibilings with an IL-12 nad INF- production disorder diagnosed with pulmonary mycobacteriosis caused by M. kansasii. Mandelian susceptibility to mycobacterial infection. An overview of literature. Pneumonol. Alergol. Pol. 2011, 79, 428–436. [Google Scholar] [PubMed]
- Guan, Y.; Bai, D.; Wang, C.; Liu, Y. Mycobacterium kansasii Infection Presenting as an Invasive Endobronchial Occupancy. AIDS Res. Hum. Retrovir. 2021, 37, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Min, K.H.; Lee, S.Y.; Shim, J.J.; Lee, J.; Kim, W.J. Invasive Endobronchial Mycobacterium kansasii Infection. Am. J. Respir. Crit. Care Med. 2019, 200, 143–144. [Google Scholar] [CrossRef]
- Breathnach, A.; Levell, N.; Munro, C.; Natarajan, S.; Pedler, S. Cutaneous Mycobacterium kansasii infection: Case report and review. Clin. Infect. Dis. 1995, 20, 812–817. [Google Scholar] [CrossRef]
- Razavi, B.; Cleveland, M.G. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis 2000, 38, 173–175. [Google Scholar] [CrossRef]
- Kaustová, J.; Chmelík, M.; Ettlová, D.; Hudec, V.; Lazarová, H.; Richtrová, S. Disease due to Mycobacterium kansasii in the Czech Republic: 1984–89. Tuberc. Respir. Dis. 1995, 76, 205–209. [Google Scholar] [CrossRef]
- Van Deun, A.; Hossain, M.A.; Gumusboga, M.; Rieder, H.L. Ziehl-Neelsen staining: Theory and practice. Int. J. Tuberc. Lung Dis. 2008, 12, 108–110. [Google Scholar]
- Hain Lifescience. Geno Type MTBC—Manufacturer Instruction for the Test no. 301-11. Available online: https://www.hain-lifescience.de/en/instructions-for-use.html (accessed on 4 March 2022).
- Lévesque, S.; Dufresne, P.J.; Soualhine, H.; Domingo, M.C.; Bekal, S.; Lefebvre, B.; Tremblay, C. Side by Side Comparison of Bruker Biotyperand VITEK MS: Utility of MALDI-TOF MSTechnology for Microorganism Identification in a Public Health Reference Laboratory. PLoS ONE 2015, 10, e0144878. [Google Scholar]
- Mycobacterium kansasii Hauduroy 1955. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=info&id=1768 (accessed on 3 April 2022).
- García-Jiménez, W.L.; Benítez-Medina, J.M.; Martínez, R.; Carranza, J.; Cerrato, R.; García-Sánchez, A.; Risco, D.; Moreno, J.C.; Sequeda, M.; Gómez, L.; et al. Non-tuberculous Mycobacteria in Wild Boar (Sus scrofa) from Southern Spain: Epidemiological, Clinical and Diagnostic Concerns. Transbound. Emerg. Dis. 2015, 62, 72–80. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M. Wild Boar: A Reservoir of Foodborne Zoonoses. Foodborne Pathog. Dis. 2019, 16, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Csivincsik, A.; Rónai, Z.; Nagy, G.; Svéda, G.; Halász, T. Surveillance of Mycobacterium caprae infection in a wild boar. Vet. Arh. 2016, 86, 767–775. [Google Scholar]
- Ghielmetti, G.; Friedel, U.; Scherrer, S.; Sarno, E.; Landolt, P.; Dietz, O.; Hilbe, M.; Zweifel, C.; Stephan, R. Non-tuberculous Mycobacteria isolated from lymph nodes and faecal samples of healthy slaughtered cattle and the abattoir environment. Transbound. Emerg. Dis. 2018, 65, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Whelan, A.O.; Lyashchenko, K.P.; Greenwald, R.; Palmer, M.V.; Harris, B.N.; Hewinson, R.G.; Vordermeier, H.M. Immune Responses in Cattle Inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii. Clin. Vaccine Immunol. 2010, 17, 247–252. [Google Scholar] [CrossRef]
- Briggs Hall, P.; Bender, L.C.; Garner, M.M. Mycobacteriosis in a black-tailed deer (Odocoileus hemionus columbianus) caused by Mycobacterium kansasii. J. Zoo Wildl. Med. 2005, 36, 115–116. [Google Scholar] [CrossRef]
- Rocha, V.C.M.; Ramiro Corrêa, S.H.; Setzer, A.P.; Catão-Dias, J.L.; Ramos, M.C.C.; Fiori, W.; Ikuta, C.Y.; Ferreira Neto, J.S. Mycobacterium kansasii isolation from captive South American coati (Nasua nasua). J. Zoo Wildl. Med. 2013, 44, 167–168. [Google Scholar] [CrossRef]
- Miller, M.; Terrell, S.; Lyashchenko, K.; Greenwald, R.; Harris, B.; Thomsen, B.V.; Fontenot, D.; Stetter, M.; Neiffer, D.; Fleming, G. Mycobacterium kansasii infection in a bontebok (Damaliscus pygaragus dorcas) herd: Diagnostic challenges in differentiating from the Mycobacterium tuberculosis complex. J. Zoo Wildl. Med. 2011, 42, 468–472. [Google Scholar] [CrossRef]
- Schafbuch, R.; Tinkler, S.; Lim, C.K.; Wolking, R.; Ramos-Vara, J. Disseminated mycobacteriosis caused by Mycobacterium kansasii in a pot-bellied pig. J. Vet. Diagn. Investig. 2018, 30, 646–650. [Google Scholar] [CrossRef]
- Murai, A.; Maruyama, S.; Nagata, M.; Yuki, M. Mastitis caused by Mycobacterium kansasii infection in a dog. Vet. Clin. Pathol. 2013, 42, 377–381. [Google Scholar] [CrossRef]
- Ford, A.K.; Niedringhaus, K.D.; Anderson, A.N.; LaCour, J.M.; Nemeth, N.M. Disseminated Mycobacterium kansasii infection in a white-tailed deer and implications for public and livestock health. J. Vet. Diagn. Investig. 2020, 32, 147–151. [Google Scholar] [CrossRef]
- Yoo, E. Mycobacterium kansasii liver abscess in a patient with HIV. Open J. Clin. Med. Case Rep. 2017, 3, 1–4. [Google Scholar]
- Lee, S.H.; Go, D.M.; Woo, S.H.; Park, H.T.; Kim, E.; Yoo, H.S.; Kim, D.Y. Systemic Mycobacterium kansasii Infection in a Domestic Shorthair Cat. J. Comp. Pathol. 2017, 157, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Previtali, M.; Gautschi, A.; Forster, E.; Steininger, K.; Irmer, M.; Reichle, S.; Sydler, T.; Wiederkehr, D.; Ruetten, M.; et al. Sonographic findings in an alpaca with Mycobacterium kansasii infection. Schweiz Arch Tierheilkd 2009, 151, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.S.; Sweeney, S.J. Mycobacterium bovis (bovine tuberculosis) infection in North American wildlife: Current status and opportunities for mitigation of risks of further infection in wildlife populations. Epidemiol. Infect. 2013, 141, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Animal Zoonotic Related Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570559/ (accessed on 4 March 2022).
- Aparecida-Sgarioni, S.; Crespo-Hirata, R.D.; Hiroyuki-Hirata, M.; Fujimura-Leite, C.Q.; Andrade-de Prince, K.; de Andrade-Leite, S.R.; Vedovello-ilho, D.; Dias-Siqueira, V.L.; Rizzieri-Caleffi-Ferracioli, K.; Fressatti-Cardoso, R. Occurrence of Mycobacterium bovis and non-tuberculous mycobacteria (NTM) in raw and pasteurized milk in the northwestern region of Paraná, Brazil. Braz. J. Microbiol. 2014, 45, 707–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vordermeier, H.M.; Brown, J.; Cockle, P.J.; Franken, W.P.J.; Arend, S.M.; Ottenhoff, T.H.M.; Jahans, K.; Hewinson, R.G. Assessment of Cross-Reactivity between Mycobacterium bovis and M. kansasii ESAT-6 and CFP-10 at the T-Cell Epitope Level. Clin. Vaccine Immunol. 2007, 14, 1203–1209. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Oréfice, F. The potential of the IGRA (Interferon Gamma Release Assay) test for the diagnosis of ocular tuberculosis. Review and comparative analysis with the tuberculosis skin test. Rev. Bras. Oftalmol. 2019, 78, 202–209. [Google Scholar]
- Ghielmetti, G.; Landolt, P.; Friedel, U.; Morach, M.; Hartnack, S.; Stephan, R.; Schmitt, S. Evaluation of Three Commercial Interferon γ Assays in a Bovine Tuberculosis Free Population. Front. Vet. Sci. 2021, 8, 68246. [Google Scholar] [CrossRef]
- Ronai, Z.; Eszterbauer, E.; Csivincsik, A.; Guti, C.F.; Dencso, L.; Janosi, S.; Dan, A. Detection of wide genetic diversity and several novel strains among non-avium nontuberculous mycobacteria isolated from farmed and wild animals in Hungary. J. Appl. Microbiol. 2016, 121, 41–54. [Google Scholar] [CrossRef]
- Meng, X.J.; Lindsay, D.S.; Sriranganathan, N. Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2697–2707. [Google Scholar] [CrossRef]
- Casalinuovo, F.; Ciambrone, L.; Grillone, R. Tuberculosis in wild boar and the risk of human infection by Mycobacterium bovis results of a study conducted in Southern Italy. Int. J. Food Microbiol. 2017, 1, 22–26. [Google Scholar]
- Witkowski, L.; Rzewuska, M.; Takai, S.; Kizerwetter-Świda, M.; Kita, J. Molecular epidemiology of Rhodococcus equi in slaughtered swine, cattle and horses in Poland. BMC Microbiol. 2016, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, L.; Rzewuska, M.; Cisek, A.A.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Czopowicz, M.; Welz, M.; Kita, J. Prevalence and genetic diversity of Rhodococcus equi in wild boars (Sus scrofa), roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in Poland. BMC Microbiol. 2015, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Żychska, M.; Witkowski, L.; Klementowska, A.; Rzewuska, M.; Kwiecień, E.; Stefańska, I.; Czopowicz, M.; Szaluś-Jordanow, O.; Mickiewicz, M.; Moroz, A.; et al. Rhodococcus equi—Occurrence in Goats and Clinical Case Report. Pathogens 2021, 10, 1141. [Google Scholar] [CrossRef]
- Dangel, A.; Berger, A.; Rau, J.; Eisenberg, T.; Kämpfer, P.; Margos, G.; Contzen, M.; Busse, H.J.; Konrad, R.; Peters, M.; et al. Corynebacterium silvaticum sp. nov., a unique group of NTTB corynebacteria in wild boar and roe deer. Int. J. Syst. Evol. Microbiol. 2020, 70, 3614–3624. [Google Scholar] [CrossRef]


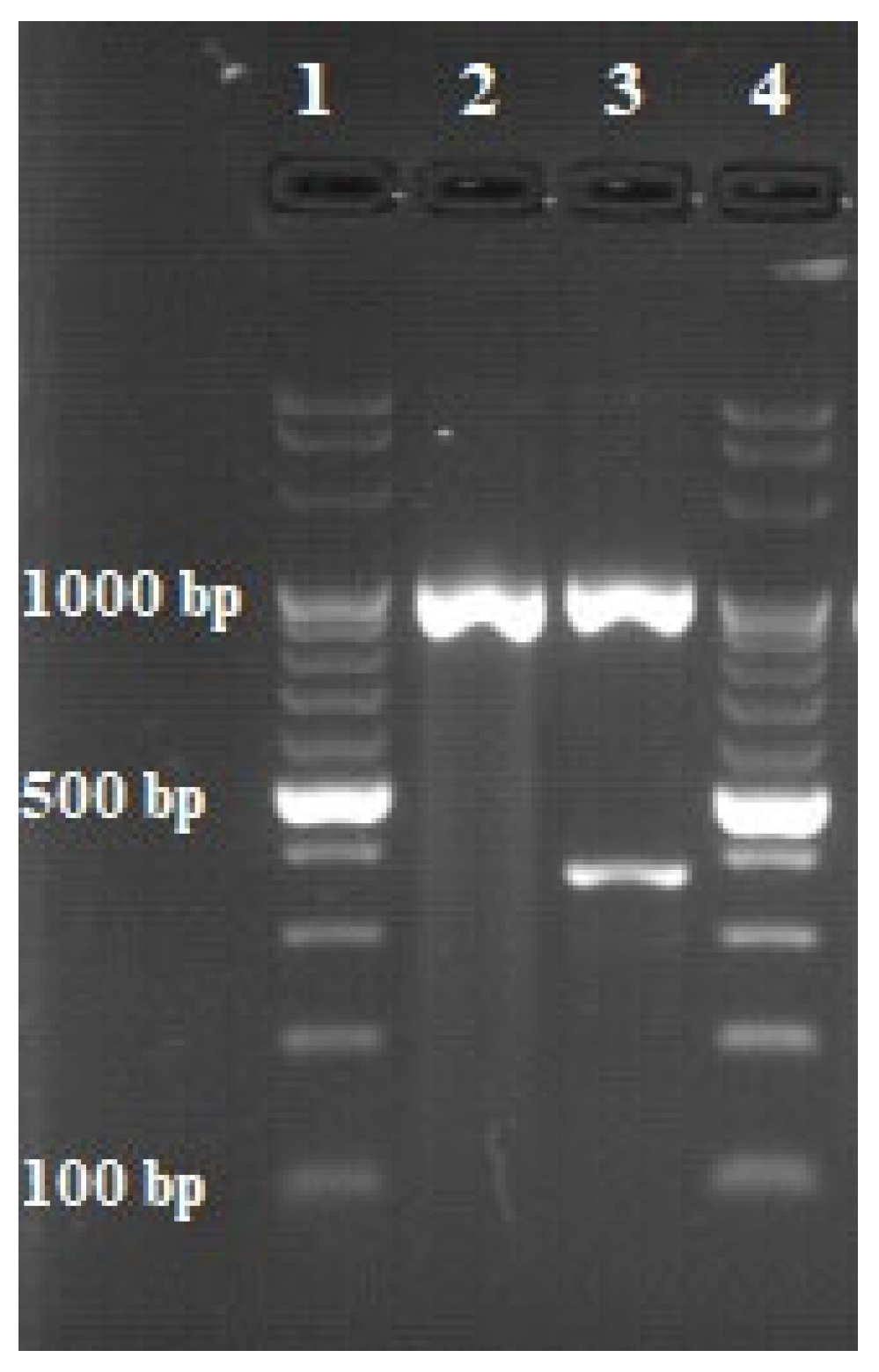

| Identification | Target DNA | Primer | Sequence (5′–3′) * | Sequence Length (bp) |
|---|---|---|---|---|
| Mycobacterium genus | 16 S rRNA | Mycgen-F | AGA GTT TGA TCC TGG CTC AG | 1030 |
| Mycgen-R | TGC ACA CAG GCC ACA AGG GA | |||
| Mycobacterium tuberculosis complex | MPB70 | TB1-F | GAA CAA TCC GGA GTT GAC AA | 372 |
| TB1-R | AGC ACG CTG TCA ATC ATG TA |
| Reagent | Concentration | Volume (µL) |
|---|---|---|
| Nuclease-free water | - | 3.4 |
| Multiplex PCR Master Mix | 2× | 12.5 |
| Mycgen-F and -R primers | 35 ng/µL | 1.5 |
| TB1-F and -R primers | 20 ng/µL | 2.5 |
| Mg2+ | 25 mM | 0.1 |
| DNA | - | 5 |
| Total volume | 25 | |
| Cycle | Time | Temperature |
|---|---|---|
| Initial denaturation | 10 min | 95 °C |
| Denaturation | 30 s | 95 °C |
| Annealing | 2 min | 65 °C |
| Elongation | 3 min | 72 °C |
| Final elongation | 10 min | 72 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulski, Ł.; Krajewska-Wędzina, M.; Lipiec, M.; Szulowski, K. Infection of a Free-Living Wild Boar (Sus scrofa) with a Bacterium from the Mycobacterium kansasii Complex. Animals 2022, 12, 964. https://doi.org/10.3390/ani12080964
Radulski Ł, Krajewska-Wędzina M, Lipiec M, Szulowski K. Infection of a Free-Living Wild Boar (Sus scrofa) with a Bacterium from the Mycobacterium kansasii Complex. Animals. 2022; 12(8):964. https://doi.org/10.3390/ani12080964
Chicago/Turabian StyleRadulski, Łukasz, Monika Krajewska-Wędzina, Marek Lipiec, and Krzysztof Szulowski. 2022. "Infection of a Free-Living Wild Boar (Sus scrofa) with a Bacterium from the Mycobacterium kansasii Complex" Animals 12, no. 8: 964. https://doi.org/10.3390/ani12080964
APA StyleRadulski, Ł., Krajewska-Wędzina, M., Lipiec, M., & Szulowski, K. (2022). Infection of a Free-Living Wild Boar (Sus scrofa) with a Bacterium from the Mycobacterium kansasii Complex. Animals, 12(8), 964. https://doi.org/10.3390/ani12080964






