Cutaneous and Subcutaneous Tumours of Small Pet Mammals—Retrospective Study of 256 Cases (2014–2021)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Guinea Pigs
3.2. Rats
3.3. Rabbits
3.4. Ferrets
3.5. Hamsters
3.6. Other Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Brien, D.J.; Kaneene, J.B.; Poppenga, R.H. The use of mammals as sentinels for human exposure to toxic contaminants in the environment. Environ. Health Perspect. 1993, 99, 351–368. [Google Scholar] [CrossRef]
- Greenacre, C.B. Spontaneous tumors of small mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2004, 7, 627–651. [Google Scholar] [CrossRef]
- Bertram, C.A.; Bertram, B.; Bartel, A.; Ewringmann, A.; Fragoso-Garcia, M.A.; Erickson, N.A.; Müller, K.; Klopfleisch, R. Neoplasia and Tumor-Like Lesions in Pet Rabbits (Oryctolagus cuniculus): A Retrospective Analysis of Cases Between 1995 and 2019. Vet. Pathol. 2021, 58, 901–911. [Google Scholar] [CrossRef]
- Raymond, J.T.; Garner, M.M. Spontaneous tumours in captive African hedgehogs (Atelerix albiventris): A retrospective study. J. Comp. Pathol. 2001, 124, 128–133. [Google Scholar] [CrossRef]
- Williams, B.H.; Wyre, N.R. Neoplasia in Ferrets. In Ferrets, Rabbits, and Rodents, 4th ed.; Quesenberry, K.E., Orcutt, C.J., Mans, C., Carpenter, J.W., Eds.; Elsevier Ltd.: Oxford, UK, 2020; pp. 92–108. [Google Scholar] [CrossRef]
- Mauldin, E.A.; Goldschmidt, M.H. A retrospective study of cutaneous neoplasms in domestic rabbits (1990–2001). Vet. Dermatol. 2002, 13, 214. [Google Scholar] [CrossRef]
- Minarikova, A.; Hauptman, K.; Jeklova, E.; Knotek, Z.; Jekl, V. Diseases in pet guinea pigs: A retrospective study in 1000 animals. Vet. Rec. 2015, 177, 200. [Google Scholar] [CrossRef]
- Okada, K.; Kondo, H.; Sumi, A.; Kagawa, Y. A retrospective study of disease incidence in African pygmy hedgehogs (Atelerix albiventris). J. Vet. Med. Sci. 2018, 80, 1504–1510. [Google Scholar] [CrossRef]
- Greaves, P.; Chouinard, L.; Ernst, H.; Mecklenburg, L.; Pruimboom-Brees, I.M.; Rinke, M.; Rittinghausen, S.; Thibault, S.; Von Erichsen, J.; Yoshida, T. Proliferative and non-proliferative lesions of the rat and mouse soft tissue, skeletal muscle and mesothelium. J. Toxicol. Pathol. 2013, 26, 1S–26S. [Google Scholar] [CrossRef]
- Quesenberry, K.E. Guinea pigs. Vet. Clin. N. Am. Small Anim. Pract. 1994, 24, 67–87. [Google Scholar] [CrossRef]
- Kanfer, S.; Reavill, D.R. Cutaneous neoplasia in ferrets, rabbits, and guinea pigs. Vet. Clin. N. Am. Exot. Anim. Pract. 2013, 16, 579–598. [Google Scholar] [CrossRef]
- Hocker, S.E.; Eshar, D.; Wouda, R.M. Rodent Oncology: Diseases, Diagnostics, and Therapeutics. Vet. Clin. N. Am. Exot. Anim. Pract. 2017, 20, 111–134. [Google Scholar] [CrossRef]
- Garner, M.M. Cytologic diagnosis of diseases of rabbits, guinea pigs, and rodents. Vet. Clin. N. Am. Exot. Anim. Pract. 2007, 10, 25–49. [Google Scholar] [CrossRef]
- Hawkins, M.G.; Bishop, C.R. Disease Problems of Guinea Pigs. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 3rd ed.; Quesenberry, K.E., Carpenter, J.W., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 295–310. [Google Scholar] [CrossRef]
- Meuten, D.J. (Ed.) Tumors in Domestic Animals, 5th ed.; Wiley Blackwell: Ames, IA, USA, 2017; pp. 144–145, 159–162. [Google Scholar]
- Doria-Torra, G.; Martínez, J.; Domingo, M.; Vidaña, B.; Isidoro-Ayza, M.; Casanova, M.I.; Vidal, E. Liposarcoma in animals: Literature review and case report in a domestic pig (Sus scrofa). J. Vet. Diagn. Investig. 2015, 27, 196–202. [Google Scholar] [CrossRef]
- White, S.D.; Bourdeau, P.J.; Brément, T.; Bruet, V.; Gimenez-Acosta, C.; Guzman, D.S.; Paul-Murphy, J.; Hawkins, M.G. Companion rats (Rattus norvegicus) with cutaneous lesions: A retrospective study of 470 cases at two university veterinary teaching hospitals (1985–2018). Vet. Dermatol. 2019, 30, 237-e72. [Google Scholar] [CrossRef]
- Ellis, C.; Mori, M. Skin diseases of rodents and small exotic mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2001, 4, 493–542. [Google Scholar] [CrossRef]
- Barsoum, N.J.; Hanna, W.; Gough, A.W.; Smith, G.S.; Sturgess, J.M.; de la Iglesia, F.A. Histiocytic sarcoma in Wistar rats. A light microscopic, immunohistochemical, and ultrastructural study. Arch. Pathol. Lab. Med. 1984, 108, 802–807. [Google Scholar]
- Frith, C.H.; Ward, J.M.; Chandra, M. The morphology, immunohistochemistry, and incidence of hematopoietic neoplasms in mice and rats. Toxicol. Pathol. 1993, 21, 206–218. [Google Scholar] [CrossRef]
- Kemmochi, Y.; Takahashi, A.; Miyajima, K.; Yasui, Y.; Tanoue, G.; Shoda, T.; Kakimoto, K. Spontaneous histiocytic sarcoma of the popliteal lymph node in a young sprague-dawley rat. J. Toxicol. Pathol. 2010, 23, 161–164. [Google Scholar] [CrossRef][Green Version]
- Kolenda-Roberts, H.M.; Harris, N.; Singletary, E.; Hardisty, J.F. Immunohistochemical characterization of spontaneous and acrylonitrile-induced brain tumors in the rat. Toxicol. Pathol. 2013, 41, 98–108. [Google Scholar] [CrossRef]
- Von Bomhard, W.; Goldschmidt, M.H.; Shofer, F.S.; Perl, L.; Rosenthal, K.L.; Mauldin, E.A. Cutaneous neoplasms in pet rabbits: A retrospective study. Vet. Pathol. 2007, 44, 579–588. [Google Scholar] [CrossRef]
- Baum, B. Not Just Uterine Adenocarcinoma-Neoplastic and Non-Neoplastic Masses in Domestic Pet Rabbits (Oryctolagus cuniculus): A Review. Vet. Pathol. 2021, 58, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Avallone, G.; Forlani, A.; Tecilla, M.; Riccardi, E.; Belluco, S.; Santagostino, S.F.; Grilli, G.; Khadivi, K.; Roccabianca, P. Neoplastic diseases in the domestic ferret (Mustela putorius furo) in Italy: Classification and tissue distribution of 856 cases (2000–2010). BMC Vet. Res. 2016, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, L.; Meléndez-Lazo, A.; Doria, G.; Ramis, A.; Solano-Gallego, L.; Pastor, J.; Martorell, J. Clinical, Cytological, Histological and Immunohistochemical Features of Cutaneous Mast Cell Tumours in Ferrets (Mustela putorius furo). J. Comp. Pathol. 2016, 155, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A.; Picut, C.A. Histopathologic features and post-surgical sequelae of 57 cutaneous neoplasms in ferrets (Mustela putorius furo L.). Vet. Pathol. 1993, 30, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.H.; Turk, M.A.; Foil, C.S. Disseminated cutaneous squamous cell carcinoma in a ferret. J. Am. Vet. Med. Assoc. 1985, 186, 702–703. [Google Scholar] [PubMed]
- Rodrigues, A.; Gates, L.; Payne, H.R.; Kiupel, M.; Mansell, J. Multicentric squamous cell carcinoma in situ associated with papillomavirus in a ferret. Vet. Pathol. 2010, 47, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Stedman, N.L.; Richey, L.J. Histology and immunohistochemistry of seven ferret vaccination-site fibrosarcomas. Vet. Pathol. 2003, 40, 288–293. [Google Scholar] [CrossRef]
- Kondo, H.; Onuma, M.; Shibuya, H.; Sato, T. Spontaneous tumors in domestic hamsters. Vet. Pathol. 2008, 45, 674–680. [Google Scholar] [CrossRef]
- Rother, N.; Bertram, C.A.; Klopfleisch, R.; Fragoso-Garcia, M.; Bomhard, W.V.; Schandelmaier, C.; Müller, K. Tumours in 177 pet hamsters. Vet. Rec. 2021, 188, e14. [Google Scholar] [CrossRef]
- Foster, A.P.; Brown, P.J.; Jandrig, B.; Grosch, A.; Voronkova, T.; Scherneck, S.; Ulrich, R. Polyomavirus infection in hamsters and trichoepitheliomas/cutaneous adnexal tumours. Vet. Rec. 2002, 151, 13–17. [Google Scholar] [CrossRef]
- Baba, Y.; Takahashi, K.; Nakamura, S. Androgen-dependent atypical fibromas spontaneously arising in the skin of Djungarian hamsters (Phodopus sungorus). Comp. Med. 2003, 53, 527–531. [Google Scholar] [PubMed]
- Harvey, R.G.; Whitbread, T.J.; Ferrer, L.; Cooper, J.E. Epidermotropic Cutaneous T-Cell Lymphoma (mycosis fungoides) in Syrian Hamsters (Mesocricetus auratus). A Report of Six Cases and the Demonstration of T-Cell Specificity. Vet. Dermatol. 1992, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Colby, L.A.; Rush, H.G.; Mahoney, M.M.; Lee, T.M. Degu. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 1031–1053. [Google Scholar] [CrossRef]
- Švara, T.; Gombač, M.; Poli, A.; Račnik, J.; Zadravec, M. Spontaneous Tumors and Non-Neoplastic Proliferative Lesions in Pet Degus (Octodon degus). Vet. Sci. 2020, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Heatley, J.J.; Mauldin, G.E.; Cho, D.Y. A Review of Neoplasia in the Captive African Hedgehog (Atelerix albiventris). Semin. Avian Exot. Pet Med. 2005, 14, 182–192. [Google Scholar] [CrossRef]
- Makishima, R.; Kondo, H.; Shibuya, H. Clinical, histopathological, and immunohistochemical studies of histiocytic sarcoma in four-toed hedgehogs (Atelerix albiventris): A retrospective study. J. Vet. Med. Sci. 2021, 83, 419–426. [Google Scholar] [CrossRef]
- Matsuoka, K.; Suzuki, J. Spontaneous tumors in the Mongolian gerbil (Meriones unguiculatus). Exp. Anim. 1995, 43, 755–760. [Google Scholar] [CrossRef]
- Deutschland, M.; Denk, D.; Skerritt, G.; Hetzel, U. Surgical excision and morphological evaluation of altered abdominal scent glands in Mongolian gerbils (Meriones unguiculatus). Vet. Rec. 2011, 169, 636. [Google Scholar] [CrossRef]
- Chen, H.C.; Slone, T.W., Jr.; Frith, C.H. Histiocytic sarcoma in an aging gerbil. Toxicol. Pathol. 1992, 20, 260–263. [Google Scholar] [CrossRef]
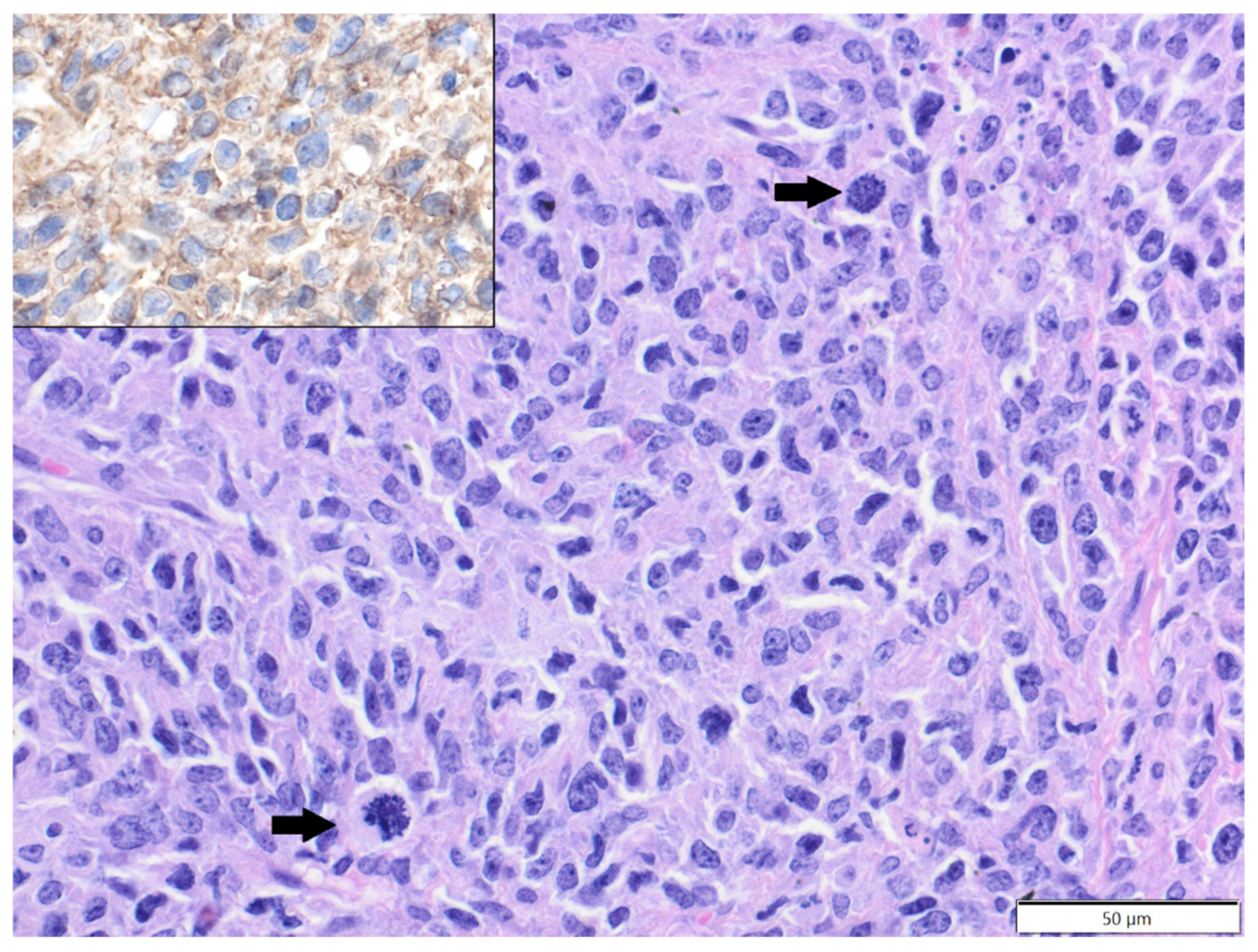
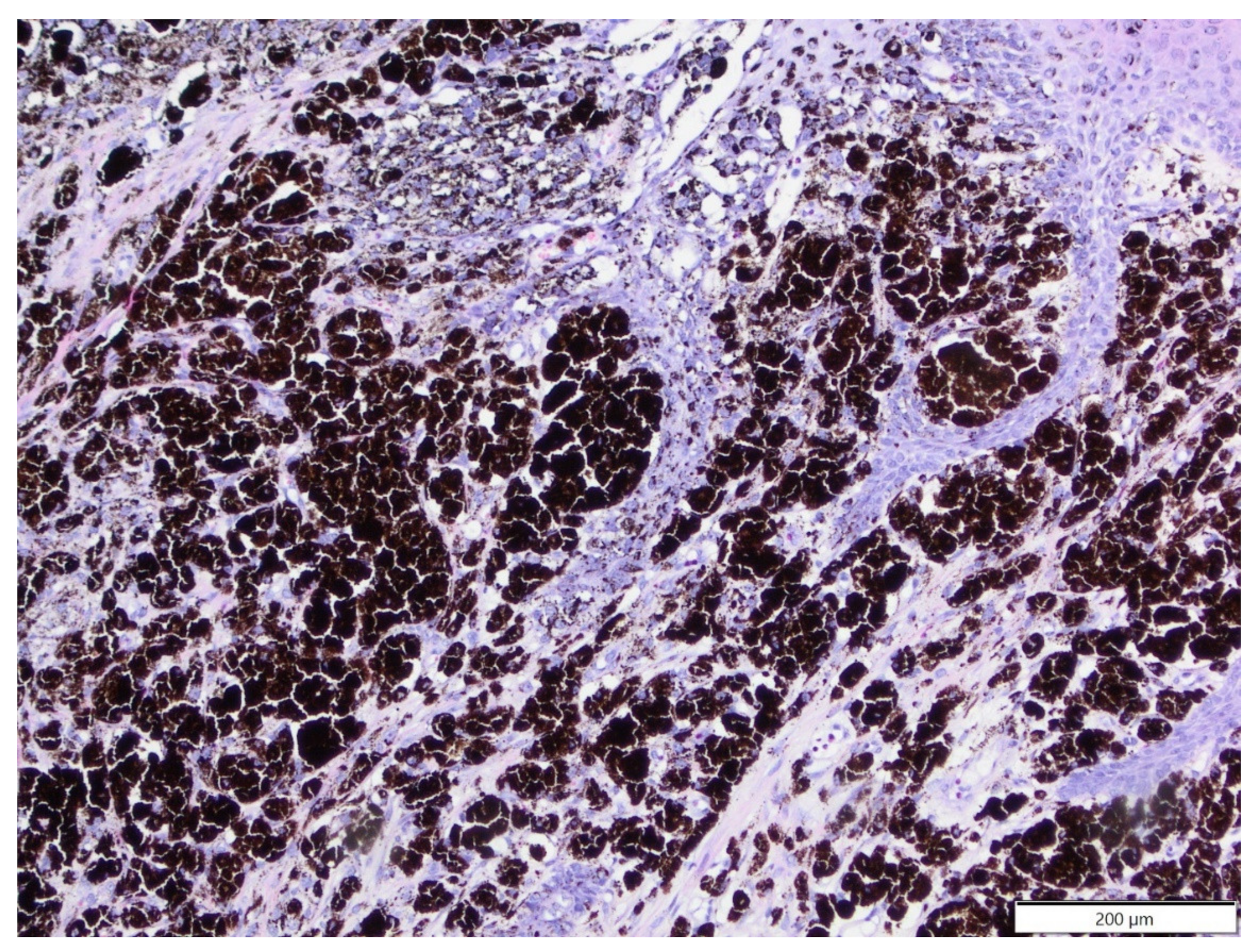
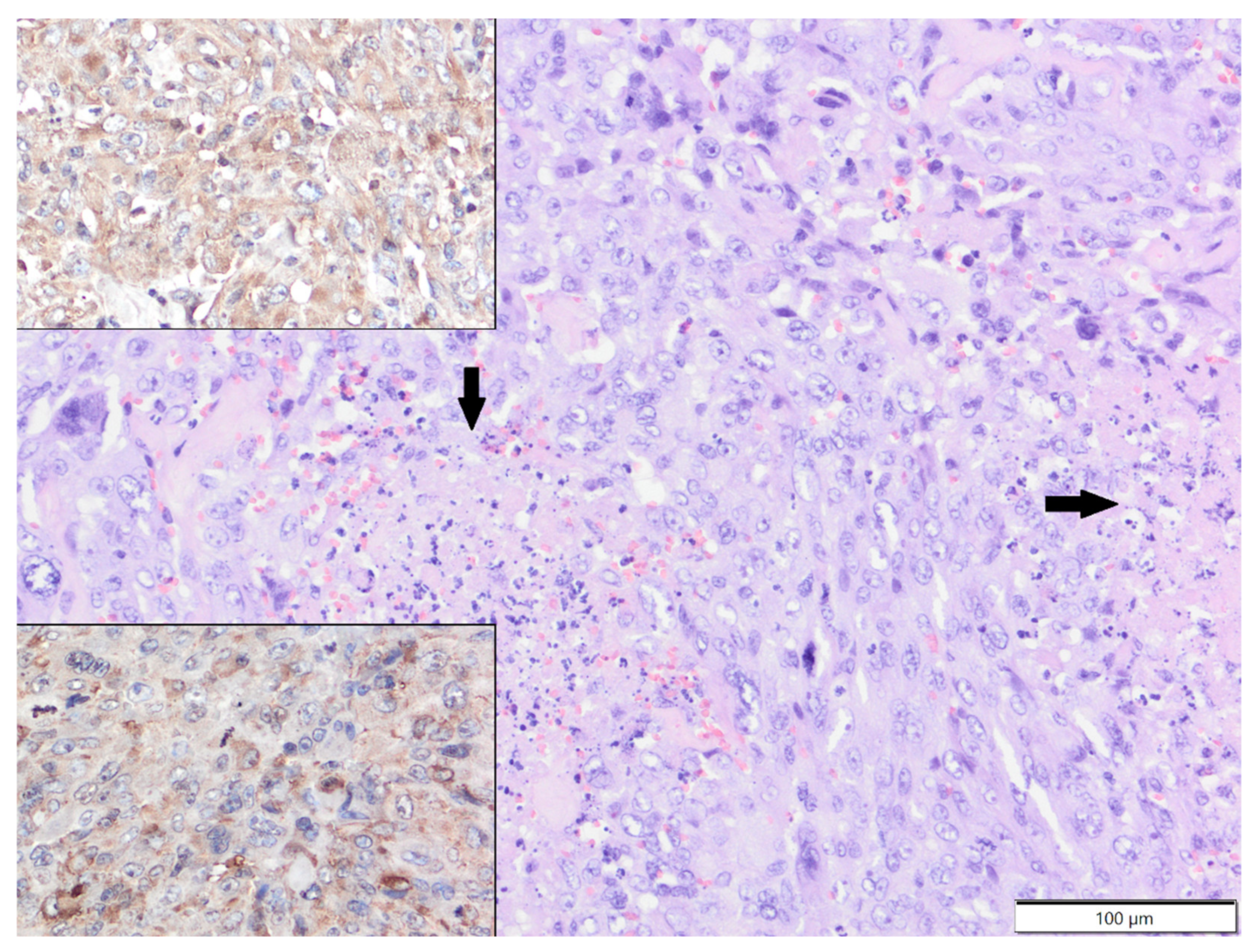

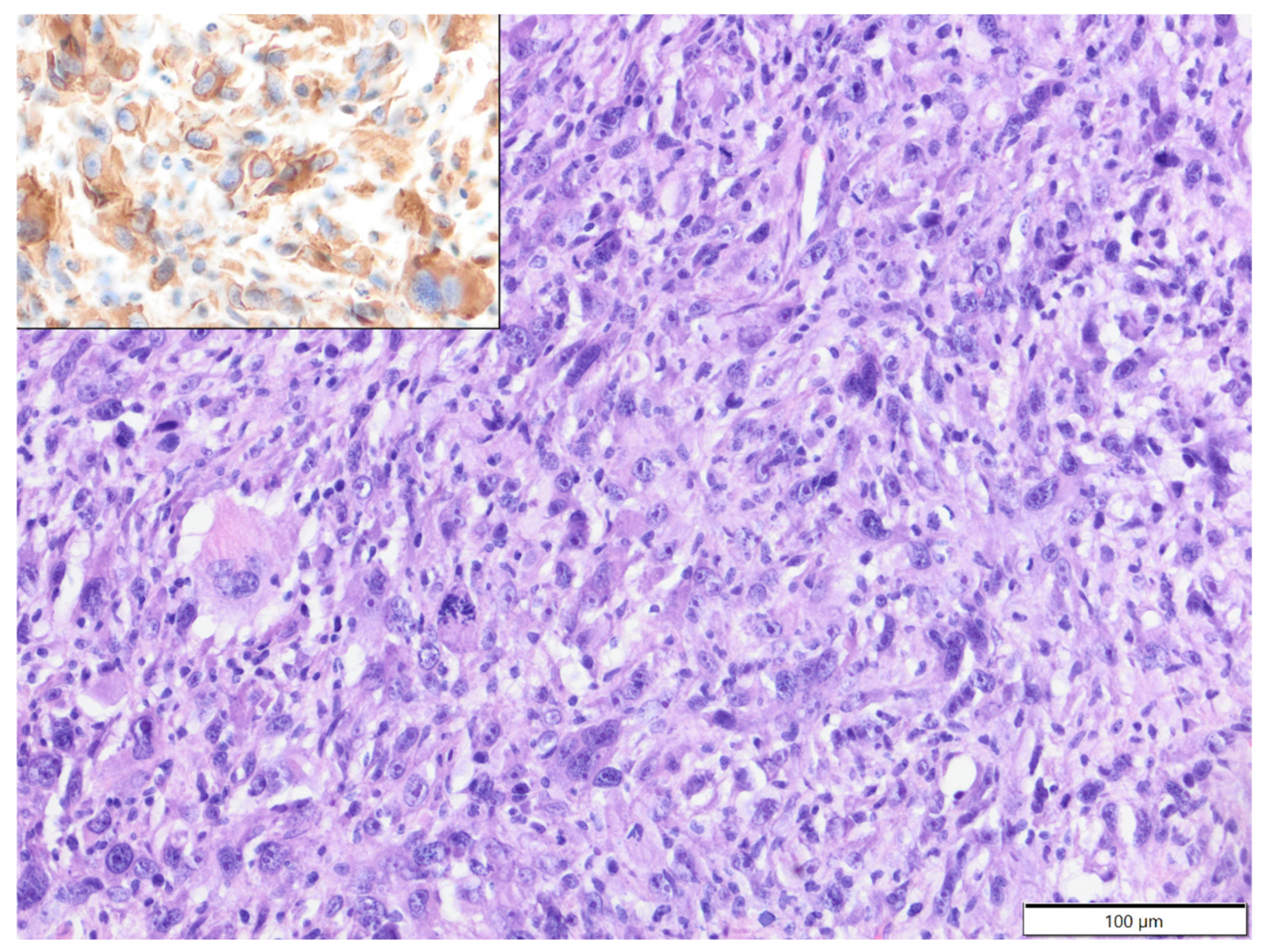
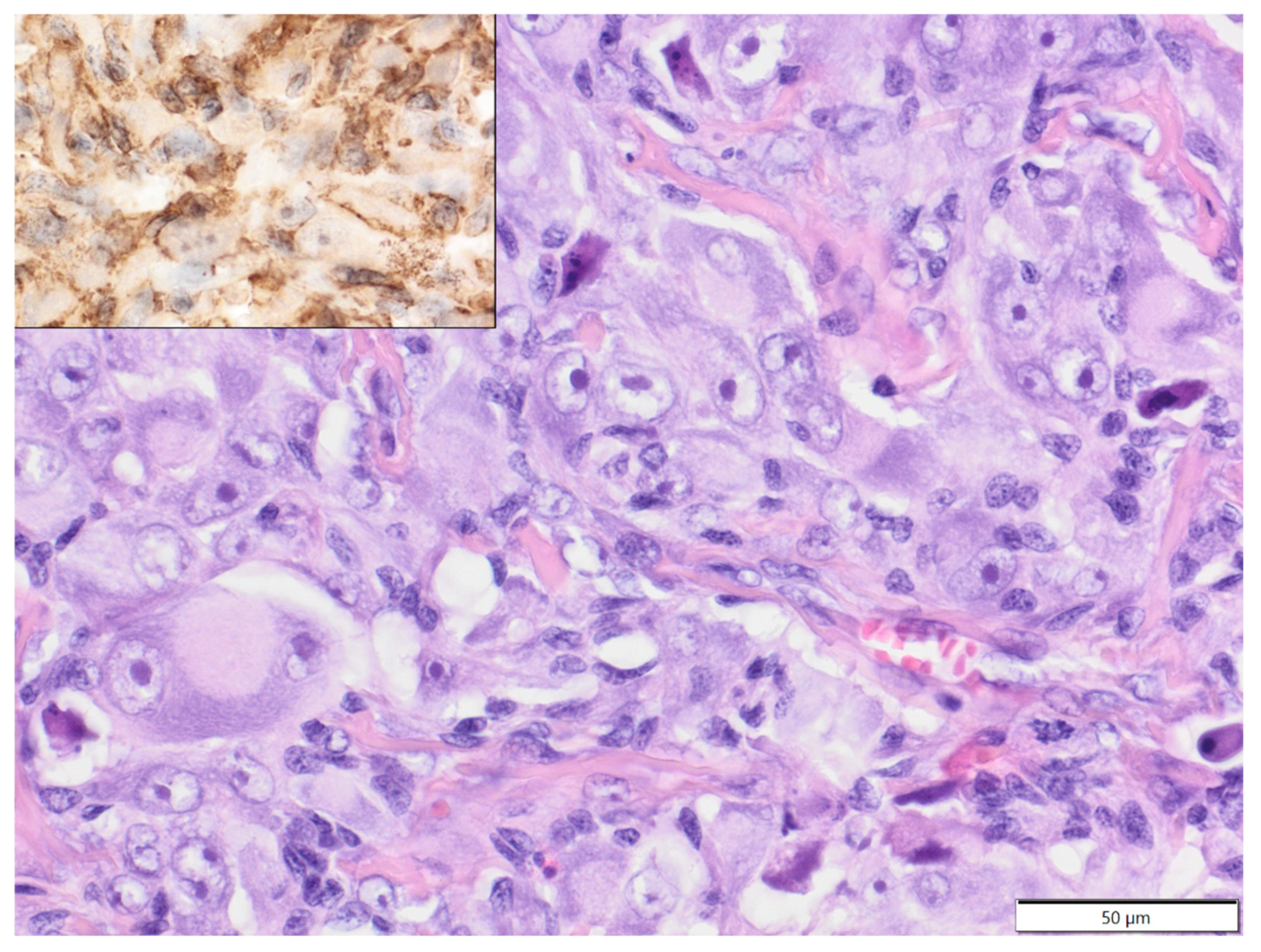
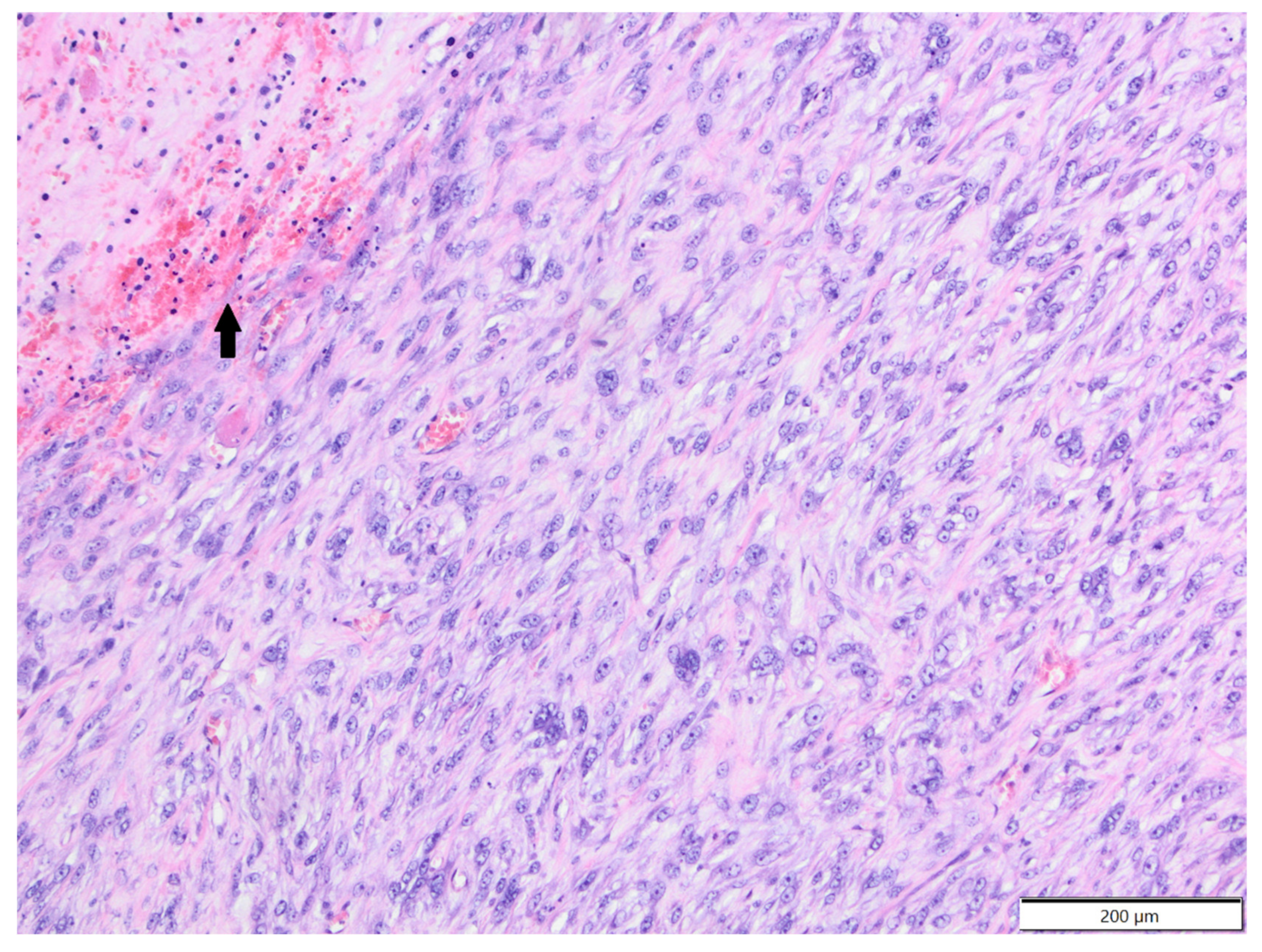
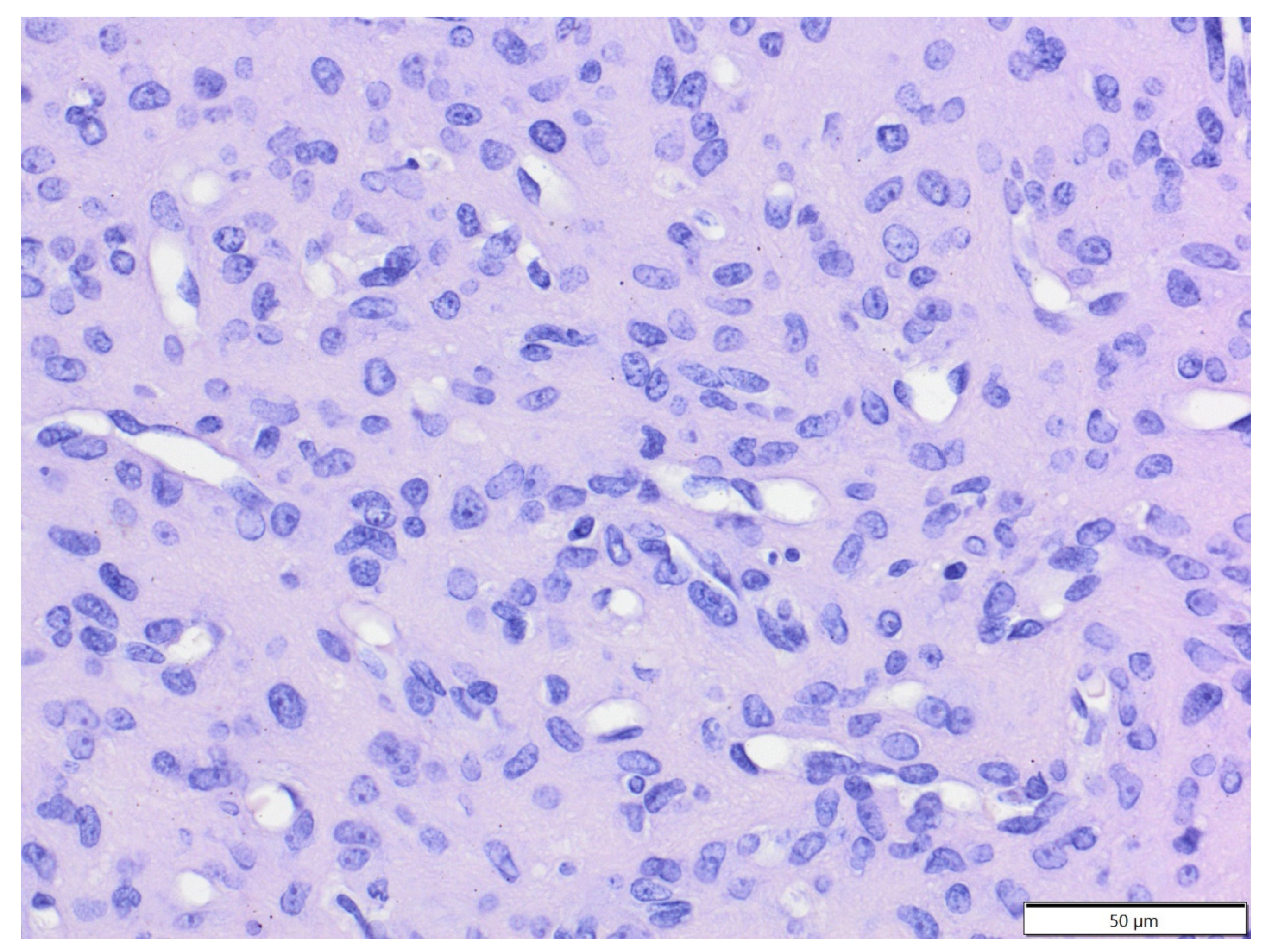
| Primary Antibody | Clone | Dilution | Source | Visualisation System |
|---|---|---|---|---|
| Iba-1 | Polyclonal rabbit | 1:200 | Wako Pure Chemical Industries, Ltd., Osaka, Japan | Impress HRP Universal Antibody (Anti-Mouse/Rabbit IgG) a |
| CD3 | Polyclonal rabbit anti-human | 1:50 | Dako, Glostrup, Denmark | Impress HRP Universal Antibody (Anti-Mouse/Rabbit IgG) a |
| CD79a | Monoclonal mouse anti-human HM57 | 1:100 | Bio-Rad Laboratories Inc., Hercules, CA, USA | EnVision+ System-HRP, Mouse (DAB) b |
| CD20 | Monoclonal rabbit anti-human SP32 | 1:100 | Abcam, Cambridge, UK | Impress HRP Universal Antibody (Anti-Mouse/Rabbit IgG) a |
| Vimentin | Monoclonal mouse anti-bovine VIM 3B4 | 1:100 | Dako, Glostrup, Denmark | EnVision+ System-HRP, Mouse (DAB) b |
| Monoclonal mouse anti-porcine V9 | 1:100 | |||
| Desmin | Monoclonal mouse anti-human D33 | 1:50 | Dako, Glostrup, Denmark | EnVision+ System-HRP, Mouse (DAB) b |
| α-SMA | Monoclonal mouse anti-human 1A4 | 1:50 | Dako, Glostrup, Denmark | EnVision+ System-HRP, Mouse (DAB) b |
| Species | Vimentin (V9) | Vimentin (3B4) | α-SMA | Desmin | Iba-1 | CD3 | CD79a | CD20 |
|---|---|---|---|---|---|---|---|---|
| guinea pig | + | + | + | + | + | + | + | - |
| rat | + | - | + | + | + | + | + | + |
| pet rabbit | + | + | + | + | nd | nd | + | nd |
| ferret | + | - | + | + | + | + | + | + |
| golden hamster | + | - | + | + | + | + | + | + |
| Djungarian hamster | + | - | + | + | + | + | + | + |
| degu | + | + | + | + | + | + | - | + |
| African pygmy hedgehog | - | + | + | + | + | + | + | - |
| Mongolian gerbil | + | - | + | + | nd | nd | nd | nd |
| chinchilla | + | - | + | + | + | + | + | - |
| Nr. of Cases | Tumour Type | Frequency | Females | Males | Mean Age |
|---|---|---|---|---|---|
| Guinea pigs | |||||
| 46 | Lipomas | 44.7% | 22 | 23 | 2.9 years |
| 15 | Trichofolliculoma | 14.6% | 11 | 4 | 3.9 years |
| 13 | Fibrosarcoma | 12.6% | 6 | 7 | 3.7 years |
| 9 | Liposarcoma | 8.7% | 4 | 4 | 3.6 years |
| 7 | Trichoepithelioma | 6.8% | 5 | 2 | 3.4 years |
| 3 | Fibroma | 2.9% | 2 | 1 | 3.8 years |
| 2 | Haemangioma | 1.9% | 0 | 2 | 2.5 years |
| 1 | Haemangiosarcoma | 1% | 0 | 1 | 9 years |
| 1 | Perivascular wall tumour | 1% | 0 | 1 | 2.5 years |
| 1 | Poorly differentiated sarcoma | 1% | 0 | 1 | 5.4 years |
| 1 | Sebaceous adenoma | 1% | 1 | 0 | 3 years |
| 1 | Epitheliomatous sebaceous carcinoma | 1% | 0 | 1 | 5 years |
| 1 | Apocrine adenocarcinoma | 1% | 0 | 1 | 5 years |
| 1 | Squamous cell carcinoma | 1% | 1 | 0 | 4 years |
| 1 | Compound melanocytoma | 1% | 0 | 1 | 5 years |
| Rats | |||||
| 15 | Fibrosarcoma | 28.3% | 4 | 11 | 17.9 months |
| 9 | Fibroma | 17% | 2 | 7 | 23.4 months |
| 8 | Squamous cell carcinoma | 15.1% | 1 | 7 | 24.3 months |
| 4 | Perivascular wall tumour | 7.5% | 1 | 3 | 21.3 months |
| 3 | Pleomorphic fibrosarcoma | 5.7% | 1 | 1 | 24 months |
| 2 | Histiocytic sarcoma | 3.8% | 1 | 1 | 20 months |
| 2 | Myxosarcoma | 3.8% | 0 | 2 | 31 months |
| 2 | Malignant peripheral nerve sheath tumour | 3.8% | 1 | 1 | 26 months |
| 2 | Simple lipoma | 3.8% | 1 | 1 | 27 months |
| 1 | Benign peripheral nerve sheath tumour | 1.9% | 0 | 1 | 24 months |
| 1 | Neurofibroma | 1.9% | 0 | 1 | 6 months |
| 1 | Sebaceous adenoma | 1.9% | 0 | 1 | 24 months |
| 1 | Apocrine ductal adenoma | 1.9% | unspecified | 18 months | |
| 1 | Pilomatricoma | 1.9% | 1 | 0 | 24 months |
| 1 | Melanocytoma | 1.9% | 1 | 0 | 33 months |
| Pet rabbits | |||||
| 18 | Trichoblastoma | 41.9% | 5 | 11 | 6 years |
| 7 | Fibrosarcoma | 16.3% | 3 | 4 | 7.4 years |
| 3 | Lipomas | 7% | 3 | 0 | 2.3 years |
| 3 | Fibroma | 7% | 0 | 3 | 10.5 years |
| 2 | Melanoma | 4.7% | 2 | 0 | 7 years |
| 2 | Squamous cell carcinoma | 4.7% | 0 | 2 | 5.8 years |
| 1 | Apocrine adenocarcinoma | 2.3% | 0 | 1 | 6 years |
| 1 | Basal cell carcinoma | 2.3% | 1 | 0 | 5 years |
| 1 | Trichoepithelioma | 2.3% | 0 | 1 | 8 years |
| 1 | Papilloma | 2.3% | 0 | 1 | 3 years |
| 1 | Benign peripheral nerve sheath tumour | 2.3% | 0 | 1 | 9 years |
| 1 | Malignant peripheral nerve sheath tumour | 2.3% | 0 | 1 | 6 years |
| 1 | Haemangioma | 2.3% | 0 | 1 | 1.5 years |
| 1 | Compound melanocytoma | 2.3% | 0 | 1 | 4.5 years |
| Ferrets | |||||
| 7 | Mast cell tumour | 33.3% | 2 | 4 | 3 years |
| 4 | Squamous cell carcinoma | 19% | 0 | 4 | 3.8 years |
| 4 | Sebaceous epithelioma | 19% | 2 | 2 | 6.8 years |
| 2 | Poorly differentiated sarcoma | 14.3% | 1 | 1 | 6 years |
| 1 | Sebaceous carcinoma | 4.8% | 0 | 1 | 5 years |
| 1 | Secretory apocrine adenoma | 4.8% | 0 | 1 | 5 years |
| 1 | Apocrine adenocarcinoma | 4.8% | 0 | 1 | 4 years |
| 1 | Malignant peripheral nerve sheath tumour | 4.8% | 0 | 1 | unspecified |
| Hamsters | |||||
| 5 | Trichoepithelioma | 29.4% | 2 | 3 | 15.2 months |
| 3 | Fibrosarcoma | 17.6% | 1 | 2 | 18.7 months |
| 2 | Melanoma | 11.8% | 2 | 0 | 9.5 months |
| 1 | Poorly differentiated sarcoma | 5.9% | 1 | 0 | 21 months |
| 1 | Trichofolliculoma | 5.9% | 0 | 1 | 23 months |
| 1 | Sebaceous epithelioma | 5.9% | 0 | 1 | 13 months |
| 1 | Apocrine adenocarcinoma | 5.9% | 0 | 1 | 12 months |
| 1 | Apocrine ductal adenocarcinoma | 5.9% | 0 | 1 | 15 months |
| 1 | Simple lipoma | 5.9% | 1 | 0 | 12 months |
| 1 | Epitheliotropic T-cell lymphoma | 5.9% | 0 | 1 | 18 months |
| Degus | |||||
| 2 | Fibrosarcoma | 25% | 1 | 0 | 6 years |
| 2 | Poorly differentiated sarcoma | 25% | 0 | 2 | 4.3 years |
| 1 | Myxosarcoma | 12.5% | 0 | 1 | 5 years |
| 1 | Anaplastic sarcoma with giant cells | 12.5% | 1 | 0 | 1.5 years |
| 1 | Malignant peripheral nerve sheath tumour | 12.5% | 1 | 0 | 3.5 years |
| 1 | Epitheliotropic T-cell lymphoma | 12.5% | 1 | 0 | 5 years |
| African pygmy hedgehogs | |||||
| 1 | Fibrosarcoma | 20% | 1 | 0 | 4 years |
| 1 | Liposarcoma | 20% | 0 | 1 | 4 years |
| 1 | Malignant peripheral nerve sheath tumour | 20% | 0 | 1 | 2 years |
| 1 | Histiocytic sarcoma | 20% | 0 | 1 | 7 years |
| 1 | Poorly differentiated sarcoma | 20% | 1 | 0 | 5 years |
| Mongolian gerbils | |||||
| 2 | Scent gland epithelioma | 66.7% | 0 | 2 | 2 years |
| 1 | Histiocytic sarcoma | 33.3% | 1 | 0 | unspecified |
| Chinchillas | |||||
| 2 | Fibrosarcoma | 66.7% | 1 | 1 | 15.5 years |
| 1 | Haemangioma | 33.3% | 0 | 1 | unspecified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otrocka-Domagała, I.; Paździor-Czapula, K.; Fiedorowicz, J.; Mikiewicz, M.; Piotrowska, A.; Gesek, M. Cutaneous and Subcutaneous Tumours of Small Pet Mammals—Retrospective Study of 256 Cases (2014–2021). Animals 2022, 12, 965. https://doi.org/10.3390/ani12080965
Otrocka-Domagała I, Paździor-Czapula K, Fiedorowicz J, Mikiewicz M, Piotrowska A, Gesek M. Cutaneous and Subcutaneous Tumours of Small Pet Mammals—Retrospective Study of 256 Cases (2014–2021). Animals. 2022; 12(8):965. https://doi.org/10.3390/ani12080965
Chicago/Turabian StyleOtrocka-Domagała, Iwona, Katarzyna Paździor-Czapula, Joanna Fiedorowicz, Mateusz Mikiewicz, Agnieszka Piotrowska, and Michał Gesek. 2022. "Cutaneous and Subcutaneous Tumours of Small Pet Mammals—Retrospective Study of 256 Cases (2014–2021)" Animals 12, no. 8: 965. https://doi.org/10.3390/ani12080965
APA StyleOtrocka-Domagała, I., Paździor-Czapula, K., Fiedorowicz, J., Mikiewicz, M., Piotrowska, A., & Gesek, M. (2022). Cutaneous and Subcutaneous Tumours of Small Pet Mammals—Retrospective Study of 256 Cases (2014–2021). Animals, 12(8), 965. https://doi.org/10.3390/ani12080965







