Epidemiology and Survival of Dogs Diagnosed with Splenic Lymphoid Hyperplasia, Complex Hyperplasia, Stromal Sarcoma and Histiocytic Sarcoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
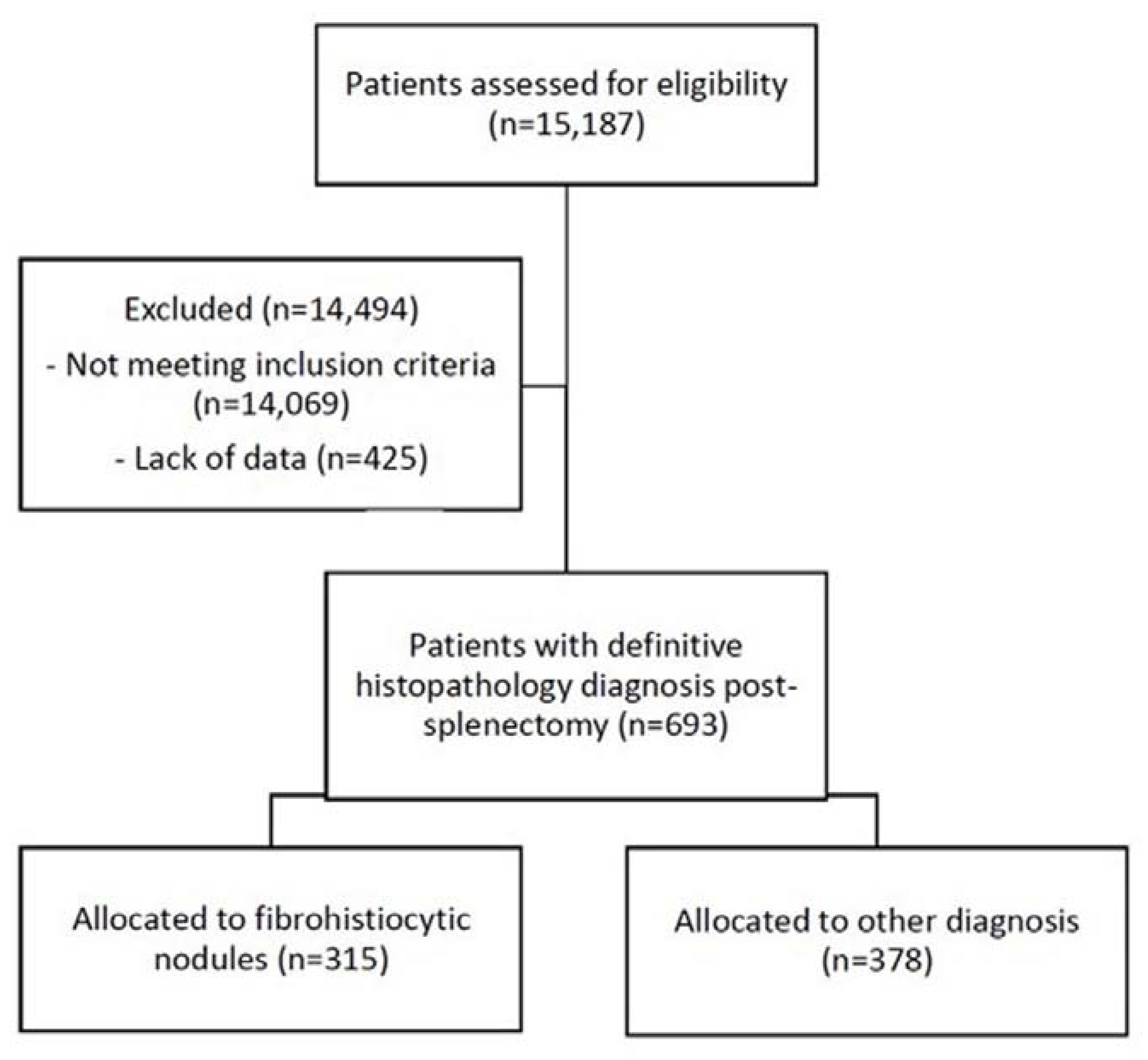
2.1. Preliminary Screening
2.2. Patient Identification
2.3. Data Management
2.4. Statistical Analysis
3. Results
3.1. Descriptive Results
3.2. Risk Factors for Type of Splenic Fibrohistiocytic Nodule Diagnosis
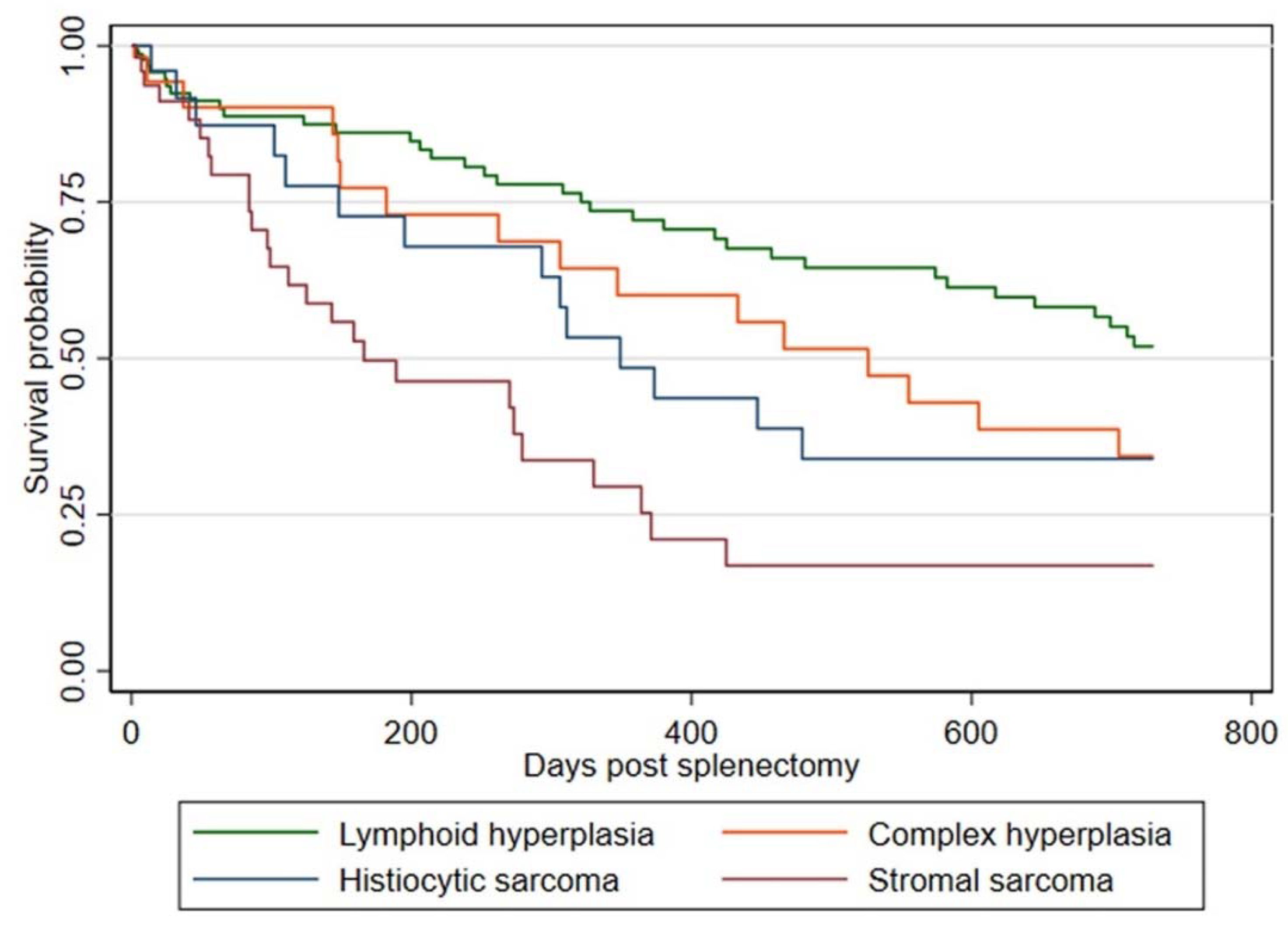
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleming, J.; Creevy, K.; Promislow, D. Mortality in North American dogs from 1984 to 2004: An investigation into age, size, and breed-related causes of death. J. Vet. Intern. Med. 2011, 25, 187–198. [Google Scholar] [CrossRef]
- Spangler, W.L.; Culbertson, M.R. Prevalence, type, and importance of splenic diseases in dogs: 1480 cases (1985–1989). J. Am. Vet. Med. Assoc. 1992, 200, 829–834. [Google Scholar] [PubMed]
- Spangler, W.L.; Kass, P.H. Pathologic factors affecting postsplenectomy survival in dogs. J. Vet. Intern. Med. 1997, 11, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Ishmael, J.; Howell, J.M. Neoplasia of the spleen of the dog with a note on nodular hyperplasia. J. Comp. Pathol. 1968, 78, 59–67. [Google Scholar] [CrossRef]
- Pintar, J.; Breitschwerdt, E.B.; Hardie, E.M.; Spaulding, K.A. Acute nontraumatic hemoabdomen in the dog: A retrospective analysis of 39 cases (1987–2001). J. Am. Anim. Hosp. Assoc. 2003, 39, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Backschat, P.S.; Nishiya, A.T.; Toyota, F.T.; Guerra, J.L. Estudo casuístico retrospectivo de neoformações primárias esplênicas [Retrospective case study of primary splenic neoformations]. Rev. Cient. Med. Vet. 2012, 10, 1–5. [Google Scholar]
- Van den Broek Campanelli, T.; Alves, D.L.; Lima, S.R.; Braga, G.; Dall’Olio, A.J. Estudo Retrospectivo de exames histopatologicos esplenic na rotina laboratorial do Hospital Escola Veterinario UNIFAJ de 2015 A 2020 [Retrospective study of histopathologic splenic exams in the routine laboratory of the Veterinary School Hospital UNIFAJ from 2015 to 2020]. Vet. Zootec. 2021, 28, 1–7. [Google Scholar]
- Bettini, G.; Mandrioli, L.; Brunetti, B.; Marcato, P. Canine splenic pathology: A retrospective study of 109 surgical samples, with special emphasis on fibrohistiocytic nodules. Eur. J. Vet. Pathol. 2001, 7, 101–110. [Google Scholar]
- Spangler, W.L.; Kass, P.H. Pathologic and prognostic characteristics of splenomegaly in dogs due to fibrohistiocytic nodules: 98 cases. Vet. Pathol. 1998, 35, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.S.; Frimberger, A.E.; Sullivan, N.; Moore, P.F. Histologic and immunohistochemical review of splenic fibrohistiocytic nodules in dogs. J. Vet. Intern. Med. 2012, 26, 1164–1168. [Google Scholar] [CrossRef]
- Linden, D.; Liptak, J.M.; Vinayak, A.; Grimes, J.A.; Sandey, M.; Smiley, W.; Matz, B.M. Outcomes and prognostic variables associated with primary abdominal visceral soft tissue sarcomas in dogs: A Veterinary Society of Surgical Oncology retrospective study. Vet. Comp. Oncol. 2019, 17, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; McInnes, E.F.; Bostock, D.E.; Hoather, T.M.; Dobson, J.M. Immunohistochemical and histopathologic features of 14 malignant fibrous histiocytomas from Flat-Coated Retrievers. Vet. Pathol. 2002, 39, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erich, S.A.; Constantino-Casas, F.; Dobson, J.M.; Teske, E. Morphological distinction of histiocytic sarcoma from other tumor types in Bernese Mountain dogs and Flatcoated Retrievers. In Vivo 2018, 32, 7–17. [Google Scholar] [PubMed] [Green Version]
- Ballegeer, E.A.; Forrest, L.J.; Dickinson, R.M.; Schutten, M.M.; Delaney, F.A.; Young, K.M. Correlation of ultrasonographic appearance of lesions and cytologic and histologic diagnoses in splenic aspirates from dogs and cats: 32 cases (2002–2005). J. Am. Vet. Med. Assoc. 2007, 230, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.S.; Muramoto, C.; Fontes, T.N.; Meneses, I.D.; Cardoso, P.G.; Vieira Filho, C.H.; Estrela-Lima, A.; Peixoto, T.C. Lesions in 224 spleens of splenectomized dogs and evalution of alternative techniques for previous microscopic diagnosis. Pesqui. Vet. Bras. 2019, 39, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Biriş, A.; Marian, B.; Toma, C.; Negru, M.; Cătoi, C.J. LUCRĂRI ŞTIINŢIFICE. In Presented at the International Symposium “Young People and Veterinary Medicine Research”; VETERINARĂ, MEDICINĂ: Timișoara, Romania, 2019. [Google Scholar]
- Fernandez, S.; Lang, J.M.; Maritato, K.C. Evaluation of Nodular Splenic Lesions in 370 Small-Breed Dogs (<15 kg). J. Am. Anim. Hosp. Assoc. 2019, 55, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Leyva, F.J.; Loughin, C.A.; Dewey, C.W.; Marino, D.J.; Akerman, M.; Lesser, M.L. Histopathologic characteristics of biopsies from dogs undergoing surgery with concurrent gross splenic and hepatic masses: 125 cases (2012–2016). BMC Res. Notes 2018, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, M.J.; Casale, S. Incidence of malignancy and outcomes for dogs undergoing splenectomy for incidentally detected nonruptured splenic nodules or masses: 105 cases (2009–2013). J. Am. Vet. Med. Assoc. 2016, 248, 1267–1273. [Google Scholar] [CrossRef]
- Latifi, M.; Tuohy, J.L.; Coutermarsh-Ott, S.L.; Klahn, S.L.; Leeper, H.; Dervisis, N. Clinical outcomes in dogs with localized splenic histiocytic sarcoma treated with splenectomy with or without adjuvant chemotherapy. J. Vet. Intern. Med. 2020, 34, 2645–2650. [Google Scholar] [CrossRef]
- VCA VetCompass Australia. Available online: https://www.vetcompass.com.au/ (accessed on 21 November 2020).
- ANKC Breed Groups. Available online: https://ankc.org.au/Breed/UmbracoDataDetail/2370 (accessed on 20 August 2019).
- Sherwood, J.M.; Haynes, A.M.; Klocke, E.; Higginbotham, M.L.; Thomson, E.M.; Weng, H.Y.; Towle Millard, H.A. Occurrence and Clinicopathologic Features of Splenic Neoplasia Based on Body Weight: 325 Dogs (2003–2013). J. Am. Anim. Hosp. Assoc. 2016, 52, 220–226. [Google Scholar] [CrossRef]
- O’Byrne, K.; Hosgood, G. Splenic mass diagnosis in dogs undergoing splenectomy according to breed size. J. Vet. Rec. 2019, 184, 620–625. [Google Scholar]
- Wendelburg, K.M.; O’Toole, T.E.; McCobb, E.; Price, L.L.; Lyons, J.A.; Berg, J. Risk factors for perioperative death in dogs undergoing splenectomy for splenic masses: 539 cases (2001–2012). J. Am. Vet. Med. Assoc. 2014, 245, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Sabattini, S.; Lopparelli, R.M.; Rigillo, A.; Giantin, M.; Renzi, A.; Matteo, C.; Capitani, O.; Dacasto, M.; Mengoli, M.; Bettini, G. Canine Splenic Nodular Lymphoid Lesions: Immunophenotyping, Proliferative Activity, and Clonality Assessment. Vet. Pathol. 2018, 55, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meireles, C.B. Diagnóstico Esplênico Pós-Esplenectomia em Cães–Estudo Retrospectivo (2004–2014) [Post-Splenectomy Splenic Diagnosis in Dogs—Retrospective Study (2004–2014)]; Universidade de Brasilia: Brasilia, Brazil, 2015. [Google Scholar]
- Mallinckrodt, M.J.; Gottfried, S.D. Mass-to-splenic volume ratio and splenic weight as a percentage of body weight in dogs with malignant and benign splenic masses: 65 cases (2007–2008). J. Am. Vet. Med. Assoc. 2011, 239, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.M.d.F. Estudo Retrospetivo de 107 Casos de Esplenectomia em Cães e Gatos [Retrospective Study of 107 Cases of Splenectomy in Dogs and Cats]; Universidade de Lisboa, Faculdade de Medicina Veterinária: Lisboa, Portugal, 2017. [Google Scholar]
- Bandinelli, M.B.; Pavarini, S.P.; Oliveira, E.C.; Gomes, D.C.; Cruz, C.E.; Driemeier, D. Retrospective study of disorders in spleens from splenectomized dogs: 179 cases. Pesqui. Vet. Bras. 2011, 31, 697–701. [Google Scholar] [CrossRef] [Green Version]
- Abadie, J.; Hédan, B.; Cadieu, E.; De Brito, C.; Devauchelle, P.; Bourgain, C.; Parker, H.G.; Vaysse, A.; Margaritte-Jeannin, P.; Galibert, F. Epidemiology, pathology, and genetics of histiocytic sarcoma in the Bernese mountain dog breed. J. Hered. 2009, 100 (Suppl. 1), S19–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dervisis, N.G.; Kiupel, M.; Qin, Q.; Cesario, L. Clinical prognostic factors in canine histiocytic sarcoma. Vet. Comp. Oncol. 2017, 15, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
| Histopathological Diagnosis | Histopathological Diagnosis Extended Terminology |
|---|---|
| Lymphoid hyperplasia | Benign hyperplastic nodules/Fibrohistiocytic grade 1/Nodular hyperplasia/Lymphoid follicular hyperplasia |
| Complex hyperplasia | Complex nodular hyperplasia/Fibrohistiocytic grade 2/Nodular hyperplasia complex |
| Histiocytic sarcoma | Histiocytic/ Histiocytic sarcoma/Fibrohistiocytic grade 3/Malignant histiocytosis (splenic) |
| Stromal sarcoma | Stromal/Splenic Sarcoma/Sarcoma grade 3 |
| Variable | Category | Freq. | Percent (%) |
|---|---|---|---|
| Testing | Biopsy | 685 | 98.9 |
| Necropsy | 8 | 1.2 | |
| Diagnosis | Lymphoid hyperplasia | 169 | 24.4 |
| Complex hyperplasia | 55 | 7.9 | |
| Histiocytic sarcoma | 32 | 4.6 | |
| Stromal sarcoma | 59 | 8.5 | |
| Hemangiosarcoma | 258 | 37.2 | |
| Haemangioma | 4 | 0.6 | |
| Haematoma | 42 | 6.1 | |
| Other sarcoma | 3 | 0.4 | |
| Lymphoma | 15 | 2.2 | |
| Carcinoma | 3 | 0.4 | |
| Lipoma | 3 | 0.4 | |
| Leiomyoma | 1 | 0.1 | |
| Myeloproliferative | 13 | 1.9 | |
| Normal | 9 | 1.3 | |
| Torsion | 3 | 0.4 | |
| Splenomegaly | 24 | 3.5 | |
| Treatment | Yes | 32 | 4.6 |
| No | 0 | 0.0 | |
| Unknown | 661 | 95.4 | |
| Total | 693 | 100 |
| Lymphoid Hyperplasia (%) | Complex Hyperplasia (%) | Histiocytic Sarcoma (%) | Stromal Sarcoma (%) | Total (%) | |
|---|---|---|---|---|---|
| Age category (years) | |||||
| <8 | 33 (63.5) | 11 (21.2) | 2 (3.8) | 6 (11.5) | 52 (100) |
| 8–<10 | 30 (40.5) | 12 (16.2) | 16 (21.6) | 16 (21.6) | 74 (100) |
| ≥10 | 106 (56.1) | 32 (16.9) | 14 (7.4) | 37 (19.6) | 189 (100) |
| Sex | |||||
| Female entire | 17 (56.7) | 3 (10) | 5 (16.7) | 5 (16.7) | 30 (100) |
| Female neutered | 59 (53.2) | 19 (17.1) | 11 (9.9) | 22 (19.8) | 111 (100) |
| Male entire | 40 (65.6) | 7 (11.5) | 6 (9.8) | 8 (13.1) | 61 (100) |
| Male neutered | 53 (46.9) | 26 (23) | 10 (8.8) | 24 (21.2) | 113 (100) |
| Breed * | |||||
| Toys | 24 (47.1) | 8 (15.7) | 8 (15.7) | 11 (21.6) | 51 (100) |
| Terriers | 35 (57.4) | 12 (19.7) | 6 (9.8) | 8 (13.1) | 61 (100) |
| Gundogs | 34 (55.7) | 9 (14.8) | 7 (11.5) | 11 (18) | 61 (100) |
| Hounds | 19 (61.3) | 4 (13) | 1 (3.2) | 7 (22.6) | 31 (100) |
| Working | 29 (56.8) | 8 (15.7) | 3 (5.9) | 11 (21.6) | 51 (100) |
| Utility | 14 (41.2) | 10 (29.4) | 4 (11.7) | 6 (17.7) | 34 (100) |
| Non-sporting | 10 (50) | 3 (15) | 3 (15) | 4 (20) | 20 (100) |
| Total | 169 (53.6) | 55 (17.5) | 32 (10.2) | 59 (18.7) | 315 (100) |
| Diagnosis | Variable/Category | RRR (95% CI) | p-Value |
|---|---|---|---|
| Lymphoid hyperplasia | Reference | ||
| Complex hyperplasia | Age (years) | ||
| <8 | Reference | ||
| 8–<10 | 0.9 (0.3–2.5) | 0.84 | |
| ≥10 | 2.5 (1.3–5.1) | 0.49 | |
| Sex | |||
| Female entire | 0.3 (0.1–1.3) | 0.12 | |
| Female neutered | 0.6 (0.3–1.3) | 0.19 | |
| Male entire | 0.3 (0.1–1) | 0.04 | |
| Male neutered | Reference | ||
| Breed | |||
| Toys | Reference | ||
| Terriers | 1.3 (0.4–3.7) | 0.66 | |
| Gundogs | 0.8 (0.3–2.5) | 0.72 | |
| Hounds | 0.7 (0.2–2.8) | 0.61 | |
| Working dogs | 0.8 (0.2–2.4) | 0.62 | |
| Utility | 2.1 (0.7–6.8) | 0.19 | |
| Non-sporting | 1.0 (0.2–4.7) | 0.99 | |
| Histiocytic sarcoma | Age (Years) | ||
| <8 | Reference | ||
| 8–<10 | 8.4 (1.7–41) | 0.01 | |
| ≥10 | 1.9 (0.4–9.6) | 0.39 | |
| Sex | |||
| Female entire | 1.7 (0.4–6.0) | 0.44 | |
| Female neutered | 1.2 (0.4–3.3) | 0.70 | |
| Male entire | 0.8 (0.3–2.7) | 0.77 | |
| Male neutered | Reference | ||
| Breed | |||
| Toys | Reference | ||
| Terriers | 0.6 (0.2–2.2) | 0.47 | |
| Gundogs | 0.6 (0.2–1.9) | 0.38 | |
| Hounds | 0.2 (0.01–1.5) | 0.10 | |
| Working dogs | 0.4 (0.08–1.6) | 0.16 | |
| Utility | 0.7 (0.2–2.9) | 0.61 | |
| Non-sporting | 0.9 (0.2–4.5) | 0.91 | |
| Stromal sarcoma | Age (Years) | ||
| <8 | Reference | ||
| 8–<10 | 2.8 (0.9–8.5) | 0.05 | |
| ≥10 | 1.8 (0.7–4.8) | 0.22 | |
| Sex | |||
| Female entire | 0.5 (0.2–1.8) | 0.31 | |
| Female neutered | 0.8 (0.4–1.7) | 0.70 | |
| Male entire | 0.5 (0.2–1.2) | 0.14 | |
| Male neutered | Reference | ||
| Breed | |||
| Toys | Reference | ||
| Terriers | 0.5 (0.2–1.6) | 0.28 | |
| Gundogs | 0.7 (0.3–1.9) | 0.50 | |
| Hounds | 0.8 (0.3–2.7) | 0.79 | |
| Working dogs | 0.8 (0.3–2.4) | 0.80 | |
| Utility | 0.8 (0.2–2.8) | 0.80 | |
| Non-sporting | 0.9 (0.2–3.7) | 0.90 |
| Variable/Category | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Diagnosis | ˂0.001 | |
| Lymphoid hyperplasia | Reference | |
| Complex hyperplasia | 2.0 (1.1–3.8) | 0.03 |
| Histiocytic sarcoma | 2.5 (1.3–5.1) | 0.01 |
| Stromal sarcoma | 4.0 (2.3–7) | 0.00 |
| Age (years) | 0.02 | |
| <8 | 0.9 (0.3–2.7) | 0.90 |
| 8–<10 | Reference | |
| ≥10 | 2.0 (1.1–3.6) | 0.02 |
| Sex | 0.08 | |
| Female entire | 1.0 (0.3–3.0) | 0.97 |
| Female neutered | 1.9 (1.1–3.1) | 0.01 |
| Male entire | 1.3 (0.6–2.9) | 0.45 |
| Male neutered | Reference | |
| Breed | 0.47 | |
| Toys | Reference | |
| Terriers | 1.2 (0.6–2.4) | 0.54 |
| Gundogs | 1.6 (0.8–3.2) | 0.14 |
| Hounds | 1.3 (0.6–3) | 0.56 |
| Working dogs | 0.9 (0.4–1.8) | 0.71 |
| Utility | 1.9 (0.9–4.2) | 0.10 |
| Non-sporting | 1.0 (0.3–3.2) | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spröhnle-Barrera, C.H.; McGhie, J.; Allavena, R.E.; Owen, H.C.; Palmieri, C.; Barnes, T.S. Epidemiology and Survival of Dogs Diagnosed with Splenic Lymphoid Hyperplasia, Complex Hyperplasia, Stromal Sarcoma and Histiocytic Sarcoma. Animals 2022, 12, 960. https://doi.org/10.3390/ani12080960
Spröhnle-Barrera CH, McGhie J, Allavena RE, Owen HC, Palmieri C, Barnes TS. Epidemiology and Survival of Dogs Diagnosed with Splenic Lymphoid Hyperplasia, Complex Hyperplasia, Stromal Sarcoma and Histiocytic Sarcoma. Animals. 2022; 12(8):960. https://doi.org/10.3390/ani12080960
Chicago/Turabian StyleSpröhnle-Barrera, Cleide H., Jayne McGhie, Rachel E. Allavena, Helen C. Owen, Chiara Palmieri, and Tamsin S. Barnes. 2022. "Epidemiology and Survival of Dogs Diagnosed with Splenic Lymphoid Hyperplasia, Complex Hyperplasia, Stromal Sarcoma and Histiocytic Sarcoma" Animals 12, no. 8: 960. https://doi.org/10.3390/ani12080960
APA StyleSpröhnle-Barrera, C. H., McGhie, J., Allavena, R. E., Owen, H. C., Palmieri, C., & Barnes, T. S. (2022). Epidemiology and Survival of Dogs Diagnosed with Splenic Lymphoid Hyperplasia, Complex Hyperplasia, Stromal Sarcoma and Histiocytic Sarcoma. Animals, 12(8), 960. https://doi.org/10.3390/ani12080960








