1. Introduction
Ruminant animal production is dependent on the anaerobic microbial ecosystem (including bacteria, archaea, protozoa, and fungi) to ferment and transform human indigestible forages into high-grade dairy and meat products for human consumption. Ruminant animals, however, are major emitters of enteric methane (CH
4) due to the microbial breakdown of carbohydrates in the rumen [
1,
2], representing an unproductive loss of dietary energy [
3]. The rumen microbial fermentation process, also referred to as enteric fermentation, produces various gases, including carbon dioxide (CO
2) and CH
4, as by-products, exhaled or eructated by the ruminant (
Table 1). The eructation of gases via belching is important in bloat prevention and a primary route for CH
4 emission to the atmosphere [
4]. Estimates of the gas production rate in cattle range from less than 0.2 L/min in the fasted animal to 2.0 L/min following feeding [
5]. Generally, lower feed quality and higher feed intake lead to higher CH
4 emissions [
1]. Although feed intake is positively correlated with animal size, growth rate, level of activity, and production (e.g., milk production, wool growth, pregnancy, or work [
6]), it also varies among animal types and management practices for individual animal types (e.g., cattle in feedlots or grazing on grassland). From an energy perspective, enteric CH
4 emissions associated with rumen fermentation activities result in the loss of 6–12% of gross energy intake (GEI), or 8–14% of the digestible energy intake (DEI) of ruminants [
3,
7,
8], which could, in principle, otherwise be available for animal growth or milk production. Reducing enteric CH
4 emissions from cattle would benefit the environment and improve meat and milk production’s efficiency and economic profitability.
Livestock production systems face challenges posed by increasing food demand and environmental issues. When animal productivity is improved through nutrition, feeding management, reproduction, or genetics, CH
4 production per unit of meat or milk is reduced [
9]. Beauchemin and McGinn [
10] estimated that a 20% reduction in CH
4 production could allow growing cattle to gain an additional 75 g/d of body weight and 1 L/d more milk yield (MY) from dairy cows. Although total CH
4 emissions in cattle fed full mixed rations (TMR) increase with increasing concentrate feed levels [
11,
12,
13,
14], emissions per unit of milk produced [
15], or emissions per kg of average daily gain (ADG [
16]) generally decrease. However, much less evidence exists concerning the effect of dry matter intake (DMI), feed efficiency, rumen fermentation profiles, rumen microbiome changes, and enteric CH
4 emissions per unit of ADG or MY (CH
4 intensity; g CH
4/kg of MY) from dairy and beef cattle, respectively [
16,
17,
18].
Several reviews of enteric CH
4 production from cattle have been published [
1,
16,
19,
20,
21]. Unlike this review, they all focus more on mitigation options than understanding relationships among dietary and rumen properties that lead to CH
4 production associated with enteric CH
4 emissions factors (
Ym; % GEI) and CH
4 emissions intensity (product yield [
16,
20]). This review aims to explain how enteric CH
4 emissions are associated with DMI, GEI, ADG, MY, energy-corrected milk (ECM), rumen fermentation rate, and ruminal microbiota changes in dairy and beef cattle fed forage- and grain-based diets. The improved understanding of these relationships between enteric CH
4 emissions and animal productivities may provide insights into cost-effective means to reduce enteric CH
4 production.
2. Interrelationships between Methane (CH4) Production, Dry Matter Intake (DMI), and Gross Energy Intake (GEI)
In this analysis, a database of several studies examining the effects of mitigation strategies on enteric CH
4 emissions per unit of milk production, ADG, DMI, and GEI in dairy cows (
Table 2 and
Table 3) and beef cattle (
Table 4 and
Table 5) was created with enteric CH
4 emissions per unit of ECM (CH
4/kg of ECM) (
Table 2 and
Table 6) and rumen fermentation parameters (
Table 7) are also evaluated. Statistical analyses of the dataset [
16,
20] included calculations of slopes, correlation coefficients, and regression coefficients using the Proc Corr. procedure (SAS Institute Inc., Cary, NC, USA). A simple regression analysis using Proc Reg in SAS (SAS Institute Inc., Cary, NC, USA) was conducted to evaluate how DMI, GEI, milk production, ADG, and rumen fermentation profiles were related to CH
4 emissions from cattle (
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6). An ordinary least squares regression (OLS) was also used to estimate the impacts of animal performance on the enteric CH
4 emission in dairy and beef cattle, respectively (
Table 3,
Table 5,
Table 6,
Table 7 and
Table 8), used in Equation (1):
where
Yi denotes CH
4 production (enteric CH
4 emissions) per unit of output from dairy/beef cattle
i,
Xi is the animal performance of cattle
i (such as dry matter intake (DMI
i), gross energy intake (GEI
i), milk production
i, ADG
i, proipionate
i, A/P
i). The impact(s) of animal performance on enteric CH
4 emissions is/are denoted by
. In each analysis, a test the null hypothesis that
is zero was evaluated. When the regression analysis was conducted using
Table 3 and
Table 4, the null hypothesis that animal performance had no impact on enteric CH
4 emissions was rejected, as shown in
Table 3,
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8 and
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5. That is to say, CH
4 production (g/d) was significantly correlated with the animal performance- DMI
i, GEI
i, milk production
i, ADG
i, propionate
i, or A/P
i.
In temperate regions, our estimates of
have an impact on CH
4 emissions (18.53 and 18.93 g of CH
4/kg DMI for dairy and beef cattle, respectively;
Table 2) and were similar to the range of 19.6 to 21.5 g/kg DMI found in previously published studies [
73,
74,
75,
76]. This is consistent with both dairy cattle (fed temperate forages) and beef cattle (fed temperate and tropical forages) studies and reported that the relationships between CH
4 production and DMI were very similar (CH
4 production (g/day) = 20.7 ± 0.28 × DMI (kg/d);
R2 = 0.92,
p < 0.001) for all three production categories [
73]. However, individual determinations of enteric CH
4 carried out in respiration chambers found that the average CH
4 production for cattle (e.g., Brahman steers) fed tropical grasses ranged from 19.3 to 34.1 g CH
4/kg DMI [
77], indicating that tropical (C4) grasses contribute to enteric CH
4 emissions to a greater extent than temperate (C3) grasses [
78]. This is probably due to the difference in dietary composition between typical diets in temperate grasses (high-quality grasses) and tropical grasses (low-quality grasses), and the digestibility of these diets. Previously published studies showed variance in CH
4 production values from beef cattle, due to different CH
4-measurement methods, age, feed type, cattle breeds, day-to-day variations, individual physiological stage, and metabolic BW [
3,
6,
20,
36,
73,
79,
80,
81,
82]. The model of Chamley et al. [
73] also reported that these factors might mutually present an error of ~13.4% in predicting CH
4 emissions for individual animals. In the present study, measurements in the above dataset were from lactating Holstein–Friesian, Jersey, and cannulated dairy cows with a high DMI and high CH
4 production. The beef dataset consisted of growing/finishing steers or non-lactating heifers with lower BW and DMI and low CH
4 production. Data included CH
4 measurements from indoor respiration chambers (RC), using the sulfur-hexafluoride (SF
6) method, and the GreenFeed method (GF; C-Lock Inc., Rapid City, SD, USA), which may account for some of the variances in the dataset. It should be noted that Hammond et al. [
39,
83] used RC for the silage study, while the SF
6 technique was used for the grazing study. Recently, Min et al. [
82] indicated that the three different CH
4-measurement methods (RC, SF
6, and GF) might be highly variable in the relationship between daily CH
4 production and DMI (g/kg DMI). Based on Hammond et al. [
68,
84], the average estimate of CH
4 production (g/d) varied among the three measurement techniques (RC, SF
6, and GF).
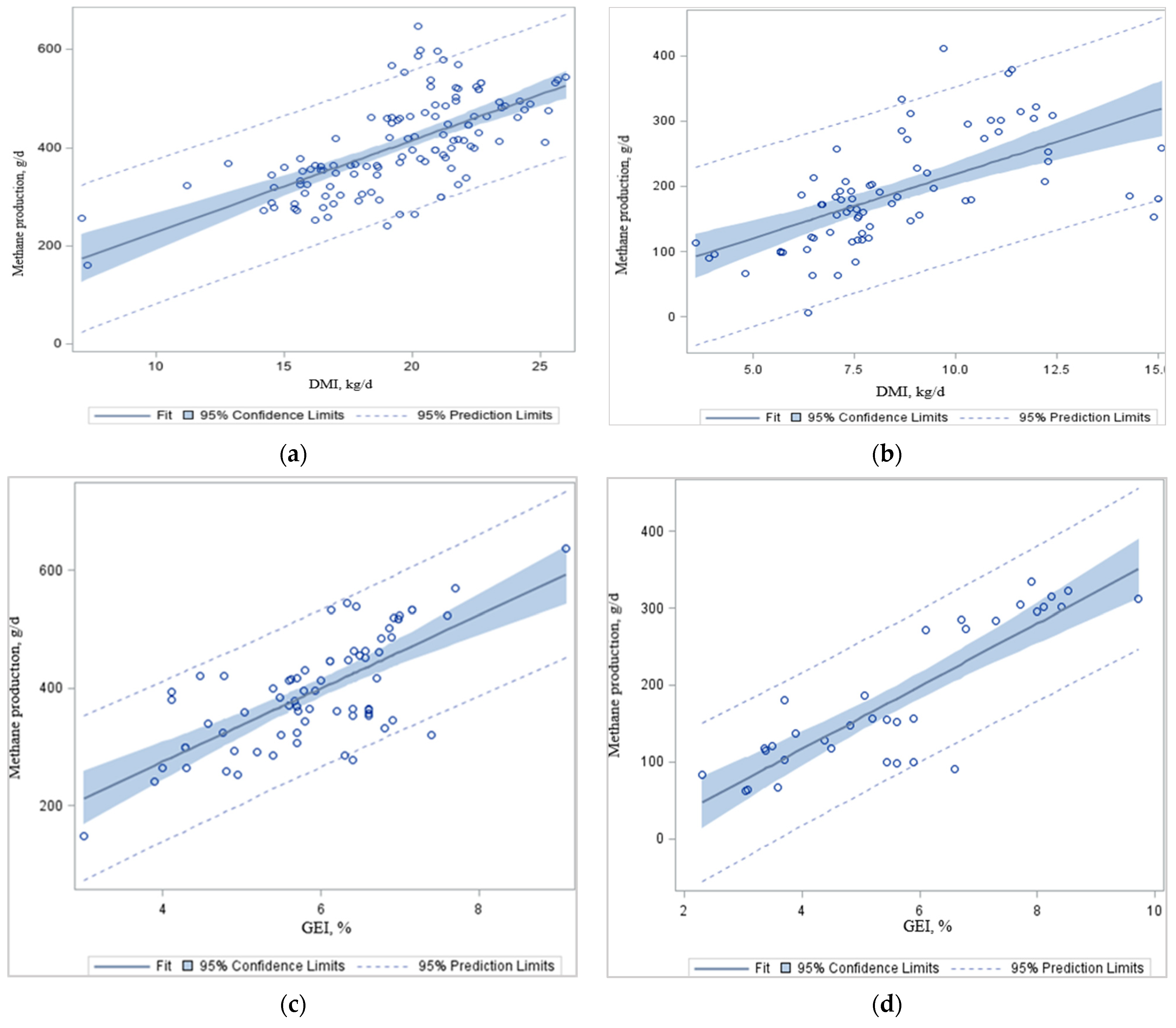
When the regression analysis was conducted using the data in
Table 2 and
Table 4, CH
4 productions (g/d) were significantly correlated with DMI
i, and GEI
i in dairy and beef cattle (
Table 2,
Table 3,
Table 4 and
Table 5 and
Figure 1a–d), respectively. In agreement with others, animal feed intake, either as GEI or DMI, had a strong linear relationship with CH
4 production: models based on these variables were of comparable accuracy with negligible bias [
80,
85,
86]. In the present analysis, total CH
4 production (g/d) increased with increasing DMI (
Figure 1a,b) and GEI (
Figure 1c,d) in dairy and beef cattle, simply because there was more feed available for rumen fermentation. Johnson and Johnson [
3] reported that, for each kg of increase in DMI, there was, on average, a 1.6% decrease of feed gross energy (GE) lost as CH
4. One study found a 2.1% reduction in the CH
4 conversion factor (
Ym; the proportion of the GEI converted to enteric CH
4 energy) per kg of DMI increase from dairy cows [
87]. Typical ruminant diets contain about 18.4 MJ of GE per kg of DM, and CH
4 has an energy content of 55.65 MJ/kg [
88]. The IPCC [
89] recommends
Ym ranges of 3.0 ± 1.0% GEI lost as CH
4 for feedlot cattle and 6.5 ± 1.0% GEI lost as CH
4 for dairy and other well-fed cattle consuming temperate-climate feed types [
89]. However, the
Ym does not consider other relevant animal or dietary characteristics that impact CH
4 emissions, such as digestibility, rumen fermentation characteristics, nutrient profiles, microbial community structure, diet composition, or cattle management.
The annual global CH
4 emission from dairy cows is approximately 18.9 Tg [
90], representing a loss of 5.5–6.5% of dietary GEI [
91]. However, CH
4, as a proportion of DMI or GEI (CH
4/kg of GEI), usually decreases as DMI increases above maintenance [
69,
92,
93], and is related to decreased DM digestibility at higher DMI [
1]. It has been reported that CH
4 production decreases with increasing levels of dietary concentrate fed [
94] and can be as low as 3% of GEI [
3] for diets with a high proportion (>60%) of concentrate. Metabolizable energy intake (MEI), neutral detergent fiber (NDF), acid detergent fiber (ADF), ether extract, lignin, and forage proportion need to be considered in the development of models to predict CH
4 emissions [
95]. Although the information on milk production would be relevant to assess the impact of animal performance on CH
4 estimates, data on milk production, ADG, rumen fermentation characteristics, and microbiome changes in CH
4 studies were insufficient.
3. Enteric Methane (CH4) Emissions, Milk Production, and Average Daily Gain (ADG) in Dairy and Beef Cattle
Numerous studies reported that a close relationship exists between DMI and milk production of dairy cows [
96,
97,
98,
99,
100], but limited information is available to calculate the relationships between milk production and CH
4 emissions in dairy cattle or ADG and CH
4 emissions in beef cattle. It has been reported that a linear relationship (
R2 = 0.47) exists between DMI and milk production [
101,
102]. The current analysis confirms a positive relationship (
p < 0.01;
Figure 2a) between DMI and milk production (
Table 5) in dairy cattle (y = 1.31x + 1.34 ± 2.70;
R2 = 0.34;
p < 0.001). We found that, as DMI increased by 1.0 kg/d, there was a 1.31 kg/d increase in milk production in dairy cattle (
Figure 2a). This agrees with Trupa et al. [
103], who proposed that, for every 2 kg of milk production, a cow consumes at least 1 kg of DMI (legume hay + concentrate). It has been documented that pasture DMI generally decreases when grazing cows are offered concentrate supplements, whereas total DMI and milk yield increase with concentrate feeding [
104]. This analysis confirmed this positive relationship (
Table 5;
Figure 2a). Min et al. [
105] reported that milk production increased by 1.7 and 0.9 kg for each additional kg of concentrate fed per day during the first and second years of lactation by dairy goats, respectively. The same authors reported that improved nutrition leads to an increase in daily milk yield (22%), peak yield (17%), time of peak yield (14 d), and persistency (8%; as the ability of a cow to continue milk production at a high level after the peak yield), compared with control treatment.
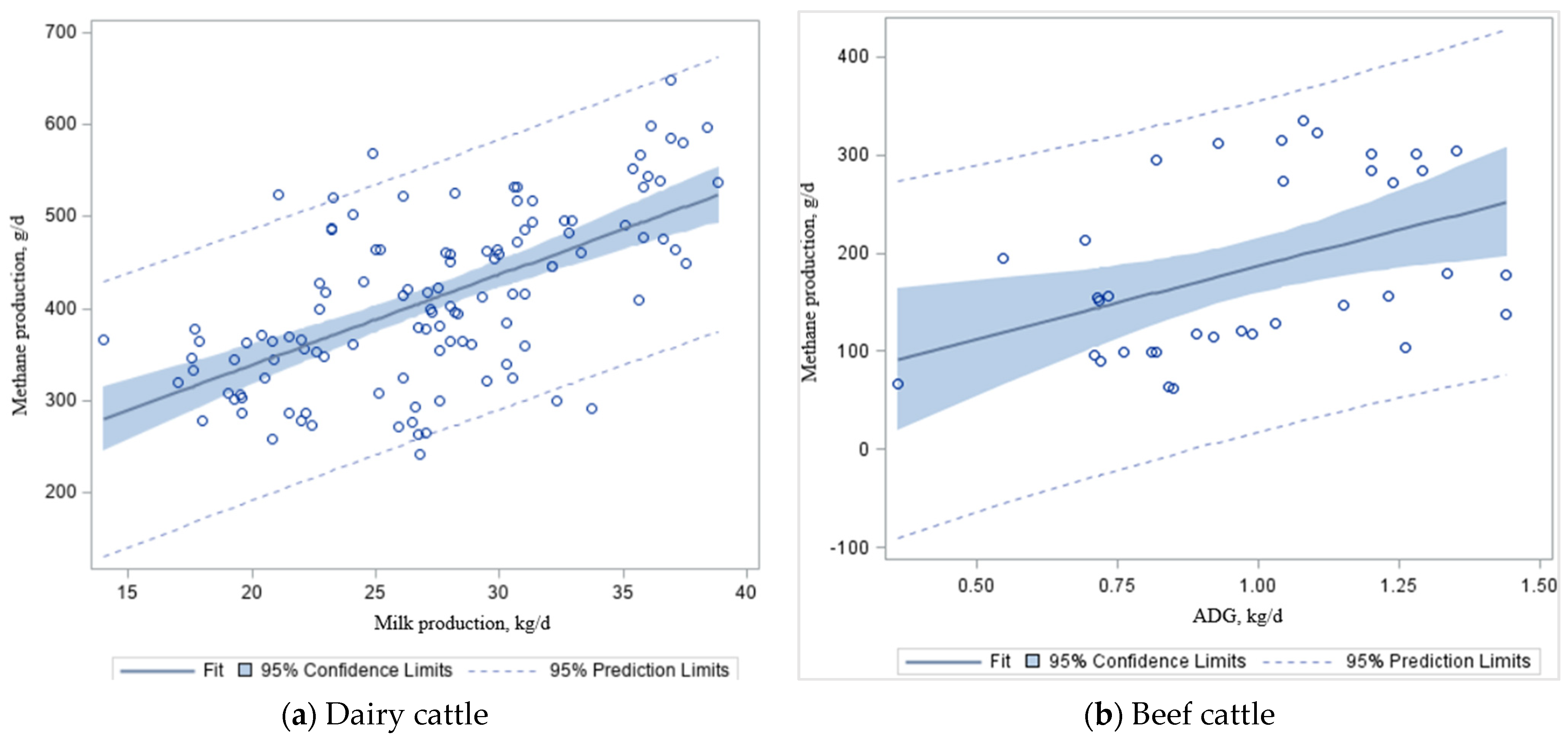
For our dataset, we found a positive relationship (
Table 6;
Figure 2b) between DMI and ADG (kg/d) in beef cattle (y = 0.09x + 2.44 ± 0.98;
R2 = 0.50;
p < 0.01), whereas DMI increased by 1.0 kg/d, and there were a 0.09 kg/d increase in ADG in beef cattle fed mixed (grazing + feedlot) diets (
Figure 2b). Other studies reported that each 1 kg increase in DMI increases ADG by 0.08–0.09 kg/d (silage-based diet) and 0.14–0.16 kg/d (grain-based diet) in finishing cattle [
59,
60,
106]. Along with DMI, intake of dietary energy and protein, or individual carbohydrate and protein contents, environmental stress, ration palatability, and feed processing may be important factors affecting milk and meat production, and require further analyses in the future [
103,
107]. The dietary energy associated with animal maintenance is about 70–75% in beef cattle and 50% in dairy cattle [
105]. The remaining nutritional energy is used to produce meat, milk, or gestation. Thus, as productivity increases, CH
4 emissions also increase (
Figure 3a,b), but CH
4 emissions per unit of product decrease [
106].
When the regression analysis was conducted on our dataset (
Table 3 and
Table 4), milk production was associated (
p < 0.001) with CH
4 production (
Figure 3a; y = 9.82x + 142. 69 ± 33.55);
R2 = 0.37) in dairy cattle (
Table 6). The ADG (kg/d) was also associated (
p < 0.01) with CH
4 emission (
Figure 3b; y = 117.33x + 38.34 ± 53.7);
R2 = 0.19) in beef steers (
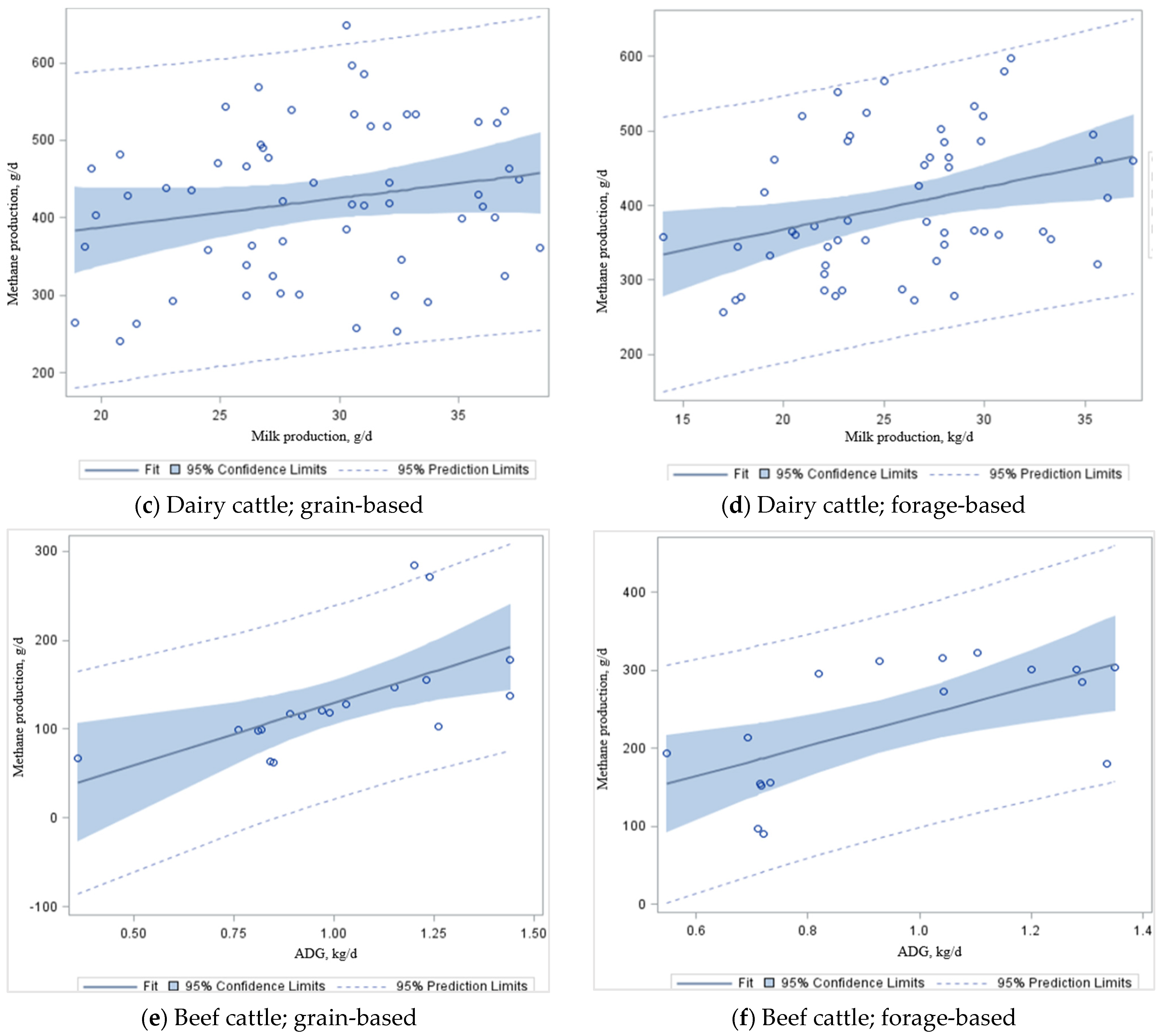
Table 6). Despite significance from the combined estimated slope (
Figure 3a), the relationship between milk production and CH
4 production in a grain-based diet (
Figure 3c) is not significant (
p = 0.12). However, there was a significant difference (
p < 0.01) in CH
4 emissions per kg ADG in beef cattle (
R2 = 0.38–0.40) fed grain-based (
Figure 3e) and forage-based (
Figure 3f) diets. This dataset took measurements on lactating Holstein–Friesian, Jersey, and cannulated dairy cows on high-quality dairy rations with some silage (e.g., corn, wheat, or grass silages) supplementation or high-quality grazing forage (e.g., alfalfa). These animals were found to have similar CH
4 production between high-forage and low-forage diets. In contrast, measurements in the beef dataset were from growing/finishing steers or non-lactating heifers with two different energy content diets (e.g., high forage- and high grain-based diets) that had significantly different CH
4 production between forage-based and grain-based diets. Adding grain to the feed ration increases the starch content. It reduces the amount of crude fiber, reducing rumen pH and promoting propionate production in the rumen while reducing the CH
4 yield [
103]. McGeough et al. [
60,
107] reported in their study that CH
4 emissions from beef cattle increased from 15.3 g/kg DMI for ad libitum concentrates to 25.9–30.1 g/kg DMI for whole crop wheat silage diets using the SF
6 technique. These data are comparable to those documented in the current study. Likewise, McGeough et al. [
60,
107] reported that CH
4 emissions increased from 22.1 g/kg DMI for the ad libitum grain-based diet to 26.2–29.4 g/kg DMI for diets based on corn silage from crops at various growth stages at harvest (supplemented with concentrates at 0.23 to 0.25 g/kg DM of the diet). Therefore, diet quality and ingredients have substantial effects on CH
4 production: if the feed quality is poor (e.g., high forage), the production of CH
4 is high (
Figure 3d,f). This is the primary cause of the loss of cow energy and, if it could be avoided, it would be critical to attaining increases in the ADG or milk production. However, improving productivity with the use of high-grain diets must be evaluated in terms of the cost of feed production and the use of fertilizers and machinery, which will increase fossil fuel use and increase N
2O emissions.
Research over the past century in dietary interventions, animal genetics, modified rumen microbial community structure, nutrition, and physiology has led to improvements in dairy production. Intensively managed dairy farms have GHG emissions as low as 1 kg of CO
2 equivalents (CO
2e)/kg of ECM, compared with >7 kg of CO
2eq/kg of ECM in less extensively managed farms [
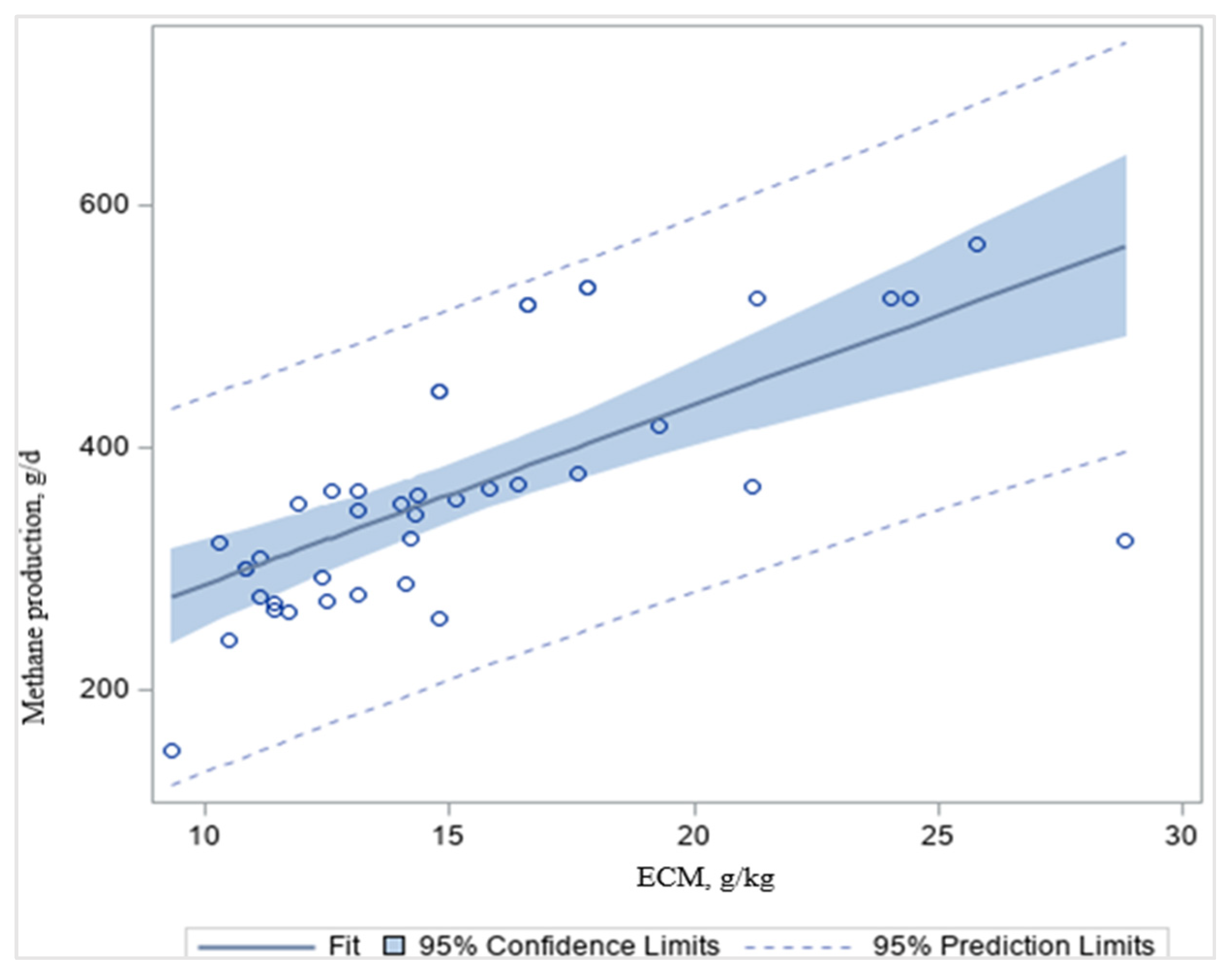
1]. High-quality grain-based diets deliver more energy for animal production as a proportion of the GEI or DMI (kg/d), and dilute the costs of maintenance more than low-quality forage-based diets or grazing, resulting in lower CH
4 g/kg ECM (
Table 8;
Figure 4), consistent with Knapp et al. [
1]. Accordingly, we found that CH
4 g/d decreased (
p < 0.001;
R2 = 0.46) with increasing ECM, g/kg in dairy cattle (
Figure 4). As a result, the enteric CH
4 emissions per unit of ECM (CH
4/ECM) are useful measurements in biology, nutrition, environmental quality, and economics [
1]. These data indicated that altering the forage quality and forage-to-concentrate ratio can affect enteric CH
4 emissions. Forage feeds are high in NDF, ADF, and lignin, which are more difficult to digest than concentrates [
60]. The slower digestion of a forage-based diet results in higher acetate formation in the rumen, and produces more CH
4 than the faster digestion of a grain-based diet (
Figure 4). Grain-based diets are high in starch and soluble carbohydrates and are more digestible than fibrous forage-based diets [
60]. It has been reported that a higher forage-to-concentrate ratio in the diets increases enteric CH
4 emissions and may decrease milk production depending upon the quality (digestibility) of the forage [
1]. Aguerre et al. [
14] found that enteric CH
4 emissions increased by 20% when increasing the forage-to-concentrate ratio from 47:53 to 68:32. However, grain-based diets can be more expensive, decrease milk fat content, and result in metabolic disorders [
107].
Alterations in milk pricing, from systems based on butterfat content to systems based on protein or other milk components, have been recommended to reduce CH
4 emissions [
106]. The fat content of milk accounts for about 9253 calories per gram of fat or 750 calories per 1 kg of 4% milk of the energy content of milk, and therefore reducing milk fat content will decrease the need for feed energy [
108], which, sequentially, will reduce enteric CH
4 emissions. A change in milk pricing based on solid-non-fat has been projected to reduce CH
4 emissions from U.S. milk cows by 15% [
106]. With the application of low-fat milk increasing, pricing based on milk protein will increase producers to adapt feeding systems to include highly digestible protein feeds, which will increase productivity and reduce CH
4 emissions. However, high protein ingredients are expensive in dairy rations, and excessive nitrogen (N) may be excreted in urine and feces. The impact on the environment as well as dietary feed accounts associated with such an approach must be assessed in terms of the overall profits that can be attained.
4. Enteric Methane Emissions and Rumen Fermentation Profiles
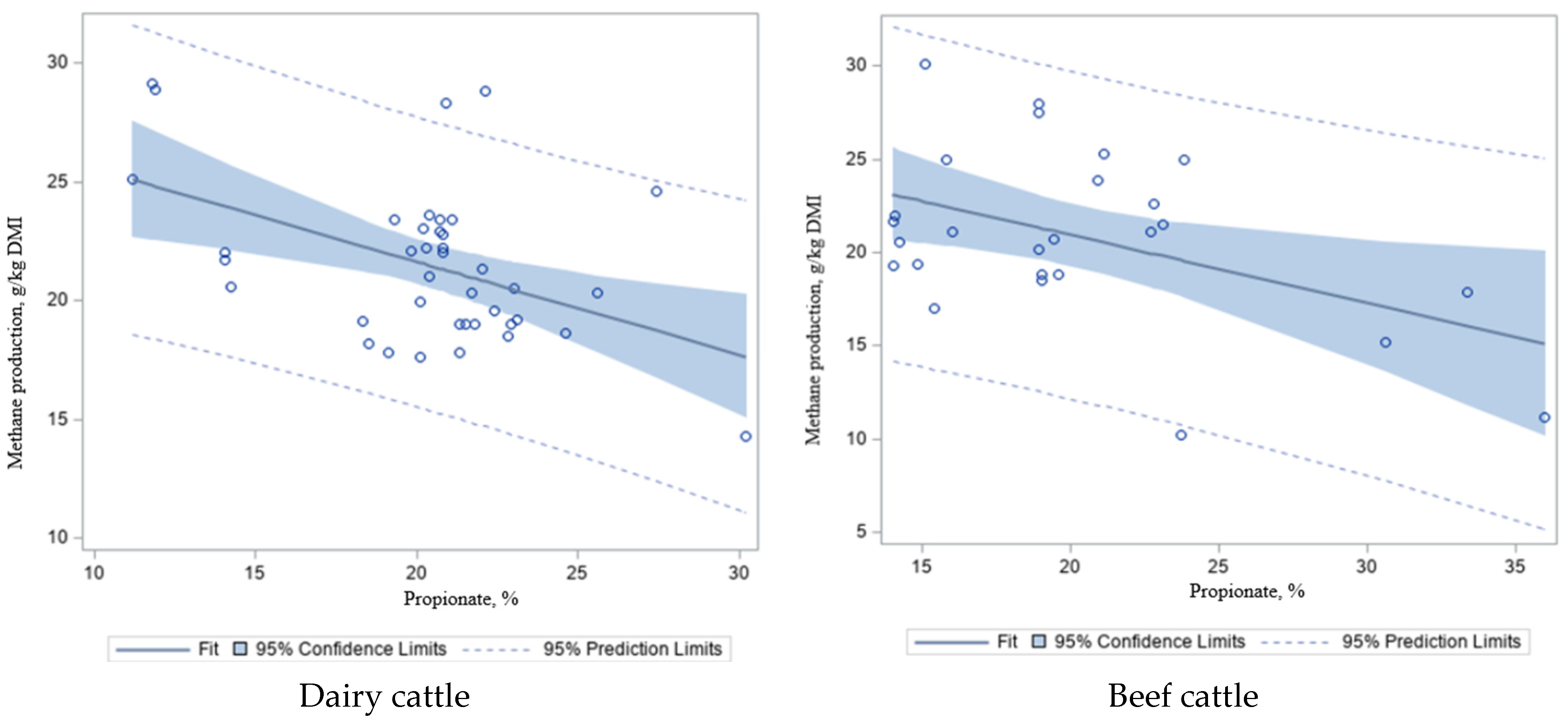
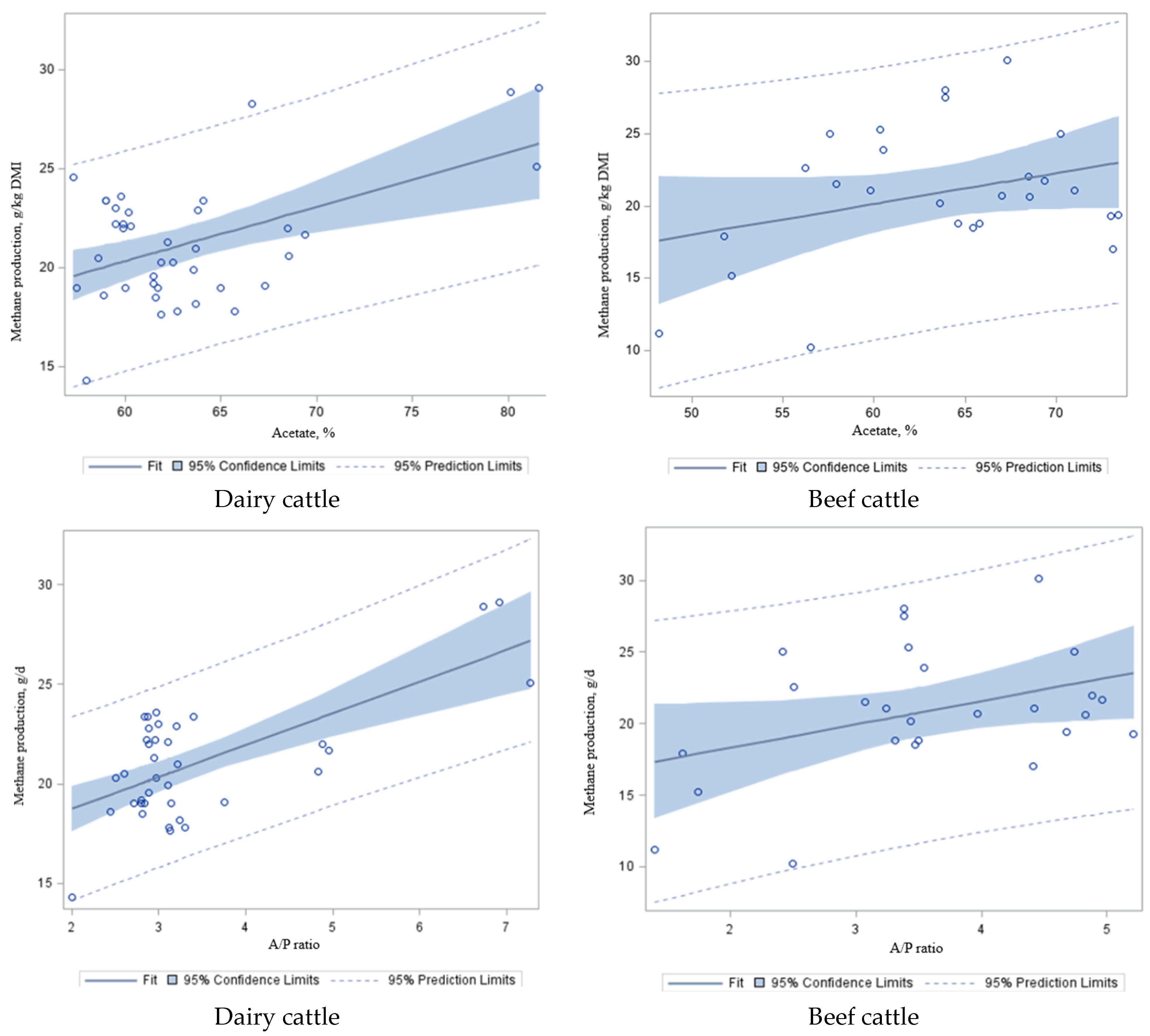
To further explore the effect of energy sources, as measured by volatile fatty acids (VFA;
Figure 5a–d) and acetate/propionate (A/P) ratio (
Figure 5e,f) on CH
4 emissions, these values were regressed against CH
4 in dairy and beef cattle in the study dataset (
Table 7). We found that there was a negative correlation between propionate concentration and CH
4 emissions in dairy (
R2 = 0.21;
p < 0.001;
Figure 5a) and beef cattle (
R2 = 0.21;
p < 0.02;
Figure 5b), and a positive correlation between acetate and CH
4 productions (more acetate, more CH
4 in the rumen) in dairy (
R2 = 0.28;
p < 0.001;
Figure 5c) and beef cattle (
R2 = 0.10;
p = 0.10;
Figure 5d), which is similar to the A/P ratio (
R2 = 0.45–0.15;
p < 0.001–0.05;
Figure 5e,f) and CH
4 emissions in dairy and beef cattle, respectively. Acetate is the most important intermediate substrate of CH
4 production (acetoclastic methanogenesis or syntrophic acetate oxidation coupled with hydrogenotrophic methanogenesis) during anaerobic digestion and the biogas process [
109]. Aceticlastic methanogenesis is carried out by
Methanosarcinaceae spp. and
Methanosaetaceae spp., while syntrophic acetate oxidation is performed by methanogens (mediated by
Methanobacteriales spp. and/or
Methanomicrobiales spp.) and acetate-oxidizing bacteria, including
Clostridium ultunense,
Syntrophaceticus schinkii,
Tepidanaerobacter acetatoxydan, and other thermophilic bacterial species [
110,
111,
112,
113,
114]. Likewise, Kittelmann et al. [
115] proposed that proportionally more propionate was present in one of the low CH
4 emitting cattle types in that study. Intrinsically, a dietary element or intervention that initiates a shift in support of propionate production will yield a reduction in CH
4 production per unit of feed fermented. In contrast, the opposite is true for acetate and butyrate [
115]. Danielsson et al. [
116] reported that the ruminal fermentation pattern of VFA showed that the proportion of propionate was higher in cluster L cows (low-CH
4 production), while the proportion of butyrate was higher in cluster H cows (high-CH
4 production). As a result, propionate fermentation is the most energy-efficient fermentation process due to energy assimilation from H
2 and propionate being the main precursor of gluconeogenesis in animals [
117,
118]. This phenomenon at least partially explains the relationship between propionate concentration, the A/P ratio, and CH
4 production observed in this study (
Figure 5e,f). Rumen fermentation that leads to propionate synthesis results in less H
2 being available for CH
4 production [
115,
119], which is primarily formed using H
2 by methanogenic archaea (CO
2 + 4H
2 – CH
4 +2H
2O [
120]).
Weimer et al. [
121] observed that the ruminal total VFA concentration and propionate proportion were higher in highly efficient cows than in low-efficiency cows. The primary energy sources for dairy and beef cattle are carbohydrates. Rumen microbes ferment these energy sources in the rumen to produce VFA (up to 200 mM) and various gases (
Table 1), which are used by ruminants as the energy source for milk and meat production, resulting in up to 75% of the cow’s metabolizable energy requirement [
117,
118]. It is reported that, as ruminal VFA production moves towards more propionate at the cost of acetate (e.g., a lower A/P), more ADG is achieved, and presumably more energy is utilized for animal growth [
115]. When glucose is metabolized into acetate, propionate, or butyrate, the animal’s energy efficiency relative to glucose is 62%, 109%, and 78%, respectively [
118,
122]. Accordingly, the production of acetate and butyrate results in the production of additional methanogenic substrates (formate and H
2), which may explain the increased amount of CH
4 emissions in high-CH
4 emitting animals.
5. Methanogenesis and Microbial Ecosystem
Several reports on the methanogenic potential of the rumen have garnered significant attention in the last decade due to the impact that methanogenesis has on ruminant animal performance and the environment [
21,
56,
74,
75,
82]. Methanogens exist within several locations within the rumen, including the association with the rumen epithelium, integration into biofilms, protozoa, and fungi [
21,
123,
124,
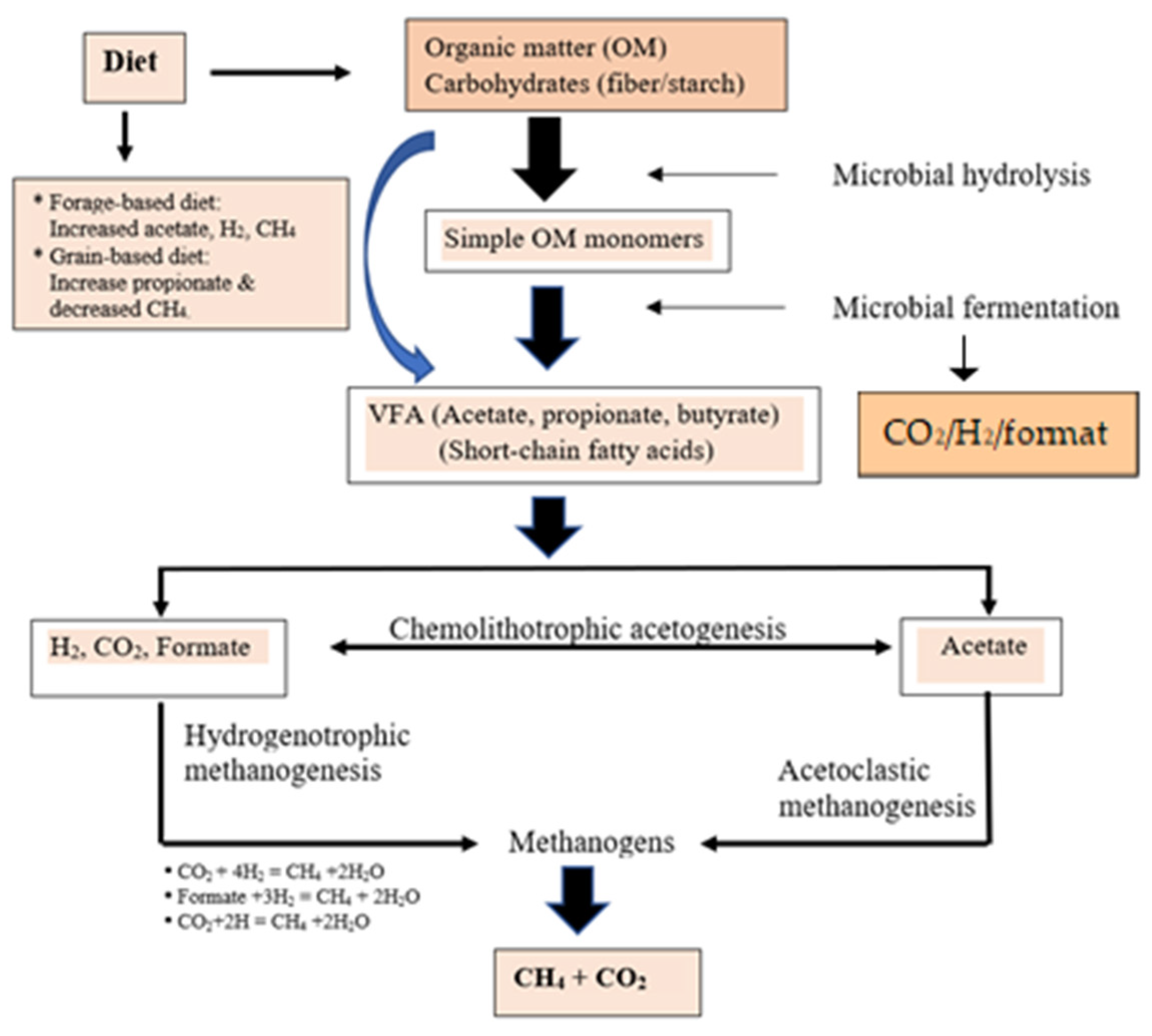
125]. A summary of the methanogenesis and microbial fermentation of dietary components in the rumen resulting in the production of VFA, CH
4, CO
2, and H
2 produced through belching is presented in
Figure 6. It has been noted that feeding concentrate diets that are high in energy substrates (non-structural carbohydrates) instantly lowered CH
4 emission (g/d and g/kg DMI); whereas high fiber diets (forages) resulted in increased CH
4 emissions. Ruminal methanogens utilize reducing equivalents produced by fermentative microflora (generally H
2-producing microorganisms) such as
Ruminococcus albus,
R. flavefaciens,
Neocalimastrix spp.,
Desulfovibrio, and ciliate protozoa [
126,
127,
128,
129]. According to Min et al. [
4],
R. albus and
R. flavefaciens (cellulolytic bacteria) produced the most H
2 among purified strains and sustained production of CH
4 when cocultured with the
Methanobrevibacte smithii that utilized the H
2 to reduce CO
2 to CH
4 [
130], which is also consistent with reports by Miller and Wolin [
131] and Wolin et al. [
132]. Syntrophic cooperation between H
2 consumers (e.g., methanogens) and H
2 producers alters the overall fermentation balance of the primary substrate toward the improved use of energy substances (Conrad et al. 1985). Subsequently, Kim et al. [
133] stated that the supplementation of acetogenic bacteria (
Proteiniphilum acetatigenes) isolated from Korean native goats (
Capra hircus coreanae) decreased methanogenic archaea. Hence, acetogens may function as a net H
2 sink that consequently reduces CH
4 emissions [
115].
Among the abundant bacterial phyla previously reported in numerous studies, Firmicutes and Bacteroidetes are the most abundant rumen microbiota in the guts of humans, mice, pigs, cattle, and meat goats [
134,
135,
136,
137,
138,
139]. Enteric CH
4 emissions from ruminants are mainly generated by hydrogenotrophic methanogenic archaea (i.e., methanogens) that support the normal function of the rumen ecosystem through the reduction (sink) of CO
2 by H
2 [
140,
141]. Fibrinolytic bacteria, especially cellulolytic
Ruminococcus and several
Eubacterium spp., are well documented H
2 producers. Conversely, the prominent cellulolytic flora,
Fibrobacter spp., does not produce H
2, while Bacteroidetes are net H
2 utilizers [
142]. Furthermore, the primary ciliate protozoa and fibrinolytic bacterial species in the rumen are H
2 producing microbes that counteract CH
4 reduction strategies that reduce available H
2 and may slow fiber digestion [
130,
143]. However, the constant removal of H
2 is vital to maintaining the biological fermentative function of the rumen because excessive H
2 accumulation constrains carbohydrate fermentation by preventing the regeneration of NAD
+ [
140,
144]. At an equivalent level of DMI, cattle diets with a higher amount of concentrate are more rapidly fermented, which results in a higher ruminal digesta passage rate, a shorter digestion time between feed particles and methanogens, and subsequently, reduced CH
4 production and numbers of archaeal methanogens [
145,
146,
147]. Moreover, feeding efficiently fermentable carbohydrates lowers ruminal pH and the number of cellulolytic bacteria and protozoa, resulting in reduced fiber degradation, proportionally less acetate and more propionate (thus also less free hydrogen), and, finally, less CH
4 production, because propionate serves as an H
2 sink [
86]. A potential explanation for this could be competition for the same substrate, as
Methanobrevibacter species are hydrogenotrophic [
148] and use H
2 and formate as substrates for CH
4 production (
Figure 6). These findings imply that the prevailing microbes in the rumen (Firmicutes and Bacteroidetes; F/B), ciliates protozoa, and methanogen archaea populations might have a role in adapting host biological parameters to reduce CH
4 production, and can potentially be utilized to estimate CH
4 emissions [
149,
150]. It has been reported that the richness of Firmicutes and the F/B ratio was positively associated with ADG due to lower A/P ratios [
138,
139] and positively correlated with enhanced CH
4 emissions (
Figure 5e,f [
149]). These same authors confirmed that Firmicutes populations were linked to lower VFA levels when CH
4 production was high, demonstrating that the F/B ratio could be used as an indicator to analyze rumen microbiome and GHG emissions. In addition, a significant positive relationship between fecal methanogen archaea concentration (µg/g fecal DM) and CH
4 emissions, expressed on a DMI basis (g/kg DMI), was found (R
2 = 0.53; n = 20) [
86]. A reduction of methanogenesis or methanogens in the rumen should be associated with a decrease in methanogen archaea.
As the single producers of CH
4, a reasonable assumption would consider an increased abundance of methanogens within the rumen environment, producing a greater CH
4 emission. However, the composition, rather than the abundance, of the rumen methanogen is more closely related to CH
4 production [
144]. An earlier study with 21 dairy cows fed mixed diets containing concentrate and silage showed no differences in the abundance of methanogens between high and low CH
4-emitter dairy cows [
116]. However, the same authors reported an increased relative abundance of
Methanobrevibacter gottschalkii (1.5-fold more abundant) and
Methanobrevibacter ruminantium (1.3-fold more abundant) that was linked with high and low CH
4-emitting dairy cows, respectively. In addition, Lettat et al. [
151] reported that CH
4 reduction was related to the decrease in protozoa populations in multiparous dairy cattle fed different types of silage diets (corn silage vs. alfalfa silages). Correspondingly, particular species of the methanogen archaea community, rather than the overall abundance of Archaea, were found to be related to enteric CH
4 emissions in New Zealand sheep [
70,
114]. However, the precise mechanism causing the high and low CH
4 emissions phenotypes detected in sheep and cattle remains unclear [
19,
82,
152]. Concerning the microbial community structure, previous studies reported a decrease in CH
4 production when the archaeal richness and diversity were reduced [
82,
153,
154]. In addition to the alterations observed within the microbiome community structure, an adaptation in the methanogenic archaeal community structure toward less efficient CH
4-producing species is still poorly defined, and deserves further investigation.
Ciliate protozoa are important H
2 producers that play an essential role in the interspecies H
2 transfer and CH
4 emissions within the rumen microbial ecosystem [
155,
156]. A relatively strong interaction between protozoal numbers and CH
4 emissions has been reported and suggests that protozoa might be a good target for CH
4 mitigation [
82,
156,
157]. Rumen methanogen archaea can represent as much as 1–2% of the host ciliate volume [
158]. Up to 20% of rumen methanogens can be found attached to protozoa [
159]. In addition, dietary strategies to reduce CH
4 by eliminating or inhibiting ciliate protozoa were reviewed by Hegarty [
160] and Boadi et al. [
107]. These nutritional strategies to mitigate the protozoa population included an increase in the proportion of the grain-based diet, the use of selected fatty acids (lauric- [C12:0], myristic- [C14:0] or linolenic acid [C18:3]), trace minerals (Cu and Zn), and various feed additives, such as saponins, ionophore, and monensin. Rumen ciliate protozoa are prodigious H
2 producers, the main substrate for methanogenesis in the rumen, and their removal (defaunation; protozoa-free) yielded an average 13–45% lower CH
4 emissions in vivo [
107,
155,
160,
161], but the results are not always consistent [
141,
150,
162,
163]. Most studies have used sheep, goat, or beef cattle as experimental models, and the effects of defaunation on the productivity of highly productive dairy cows fed intensive diets are not well known [
164]. As stated in previous data [
165,
166,
167,
168], the proportion of methanogens relative to total bacteria was more evenly distributed between the liquid and solid rumen content phases in wether sheep with unaltered protozoa populations, while defaunated sheep had a lower proportion of methanogens associated with the liquid phase. These results indicate that methanogenesis is regulated not only by methanogen activity, but also impacted by various factors such as diets and varying biological ecosystems with protozoa, bacteria (Firmicutes/Bacteroidetes), and fungi community diversity affected by VFA (acetate, butyrate, and propionate), H
2, and other substrate availability [
120,
149,
164,
165]. Therefore, future work relating to microbial diversity and the function of this community associated with animal products, especially methanogens, could be helpful to improve our understanding of the mechanisms involved in methanogenesis pathways in the rumen. In addition, cost-effective ways to change the microbial ecology to reduce H
2 production, to re-partition H
2 into products other than CH
4, or to promote methanotrophic microbes with the ability to oxidize CH
4 still need to be found and developed.
6. Conclusions
New technologies offer the potential to manipulate the rumen microbiome through genetic selection and varying degrees by various dietary intervention strategies to reduce CH4 emissions. Strategies to reduce GHG emissions, however, still need to be developed, which increase ruminant production efficiency, whereas reducing the production of CH4 from cattle, sheep, and goats. Many of the approaches discussed are only partial strategies; all approaches to reducing enteric CH4 emissions should consider the economic impacts on farm profitability and the relationships between enteric CH4 and other GHG. Numerous dietary mitigation interventions have been identified, which could help reduce CH4 emissions, and other strategies currently being explored and identified. The greatest declines in CH4 emissions are likely to be achieved through a combination of approaches, including dietary modification and improved rumen fermentation for improving feed conversion efficiency.
Dietary manipulation influences CH4 production by directly influencing the rumen microbiome. There is the potential to affect the rumen fermentation profiles and microbiota community structure positively and meet sustainability goals by reducing CH4 emissions from cattle production systems. Increased animal productivity resulted in reduced enteric CH4 production per animal production (milk and ADG) and improved feed efficiency. Animal DMI, GEI, ECM, ADG, and A/P ratio are the most important predictors of CH4 production; however, diet quality and type, rumen fermentation profiles (acetate, propionate), and microbial community structure (methanogens, bacteria, protozoa) can significantly affect this relationship. Approaches to mitigating enteric CH4 emissions from beef and dairy cattle production can improve animal performance and feed efficiency, while helping to reduce atmospheric GHG emissions that contribute to global warming. One possible strategy to reduce GHG emissions is a beneficial modification of the rumen microbiome to maintain a low A/P ratio and limit H2 production via feed management. The populations of prevailing microbial types in the rumen (Firmicutes: Bacteroidetes ratio), ciliate protozoa, and methanogen archaea might have a role in adapting host biological parameters to reduce CH4 production, and can potentially be utilized to estimate CH4 emissions. Properly designed dietary interventions can reduce enteric CH4 production without detrimental impacts on animal production. Therefore, GHG reduction strategies should be established to increase ruminant production efficiency, while minimizing losses of CH4 energy from cattle production systems.