Genome-Wide Selection Sweep between Wild and Local Pigs from Europe for the Investigation of the Hereditary Characteristics of Domestication in Sus Scrofa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
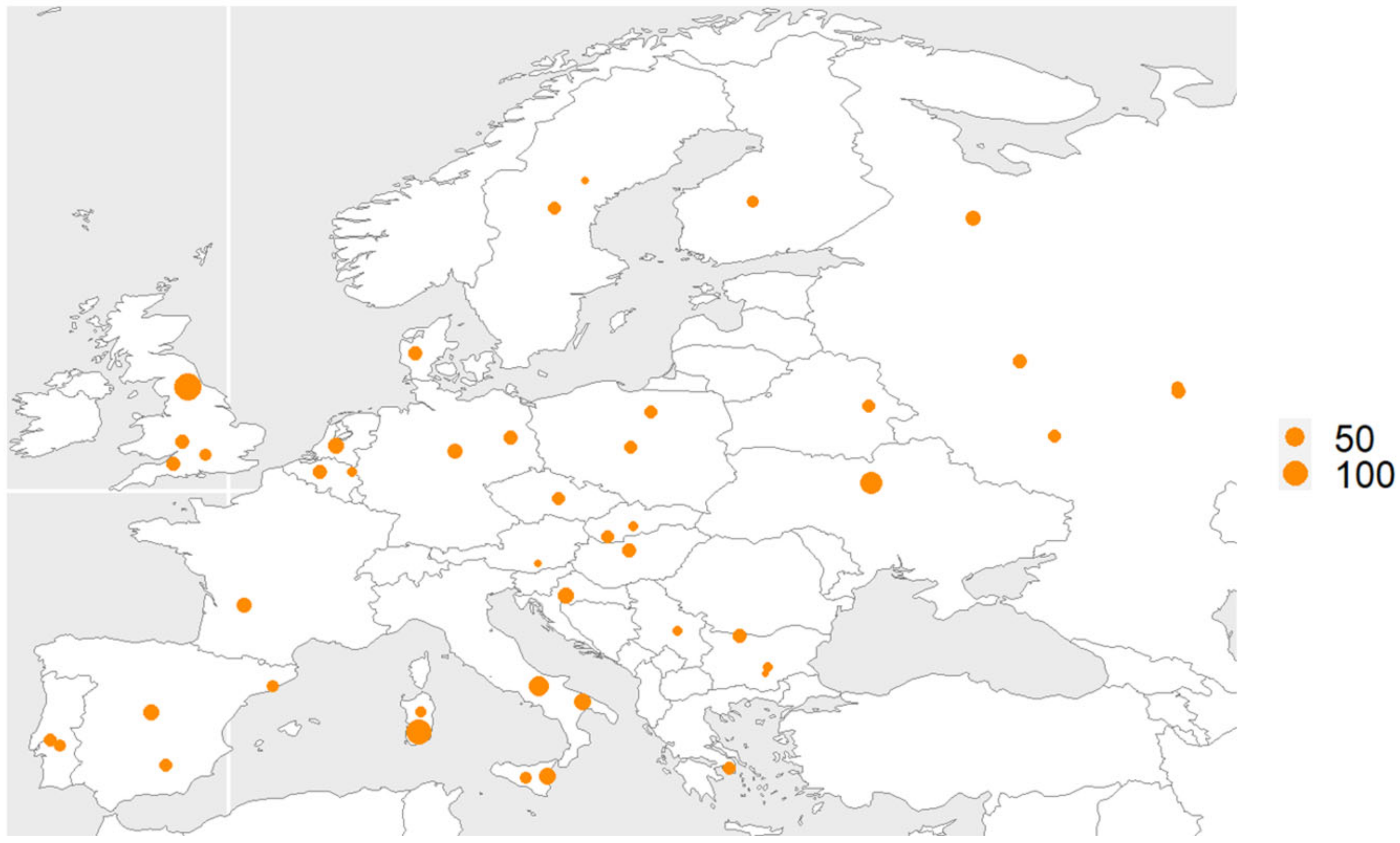
2.1. Ethics Statement and Sample Collection
2.2. Data Analysis
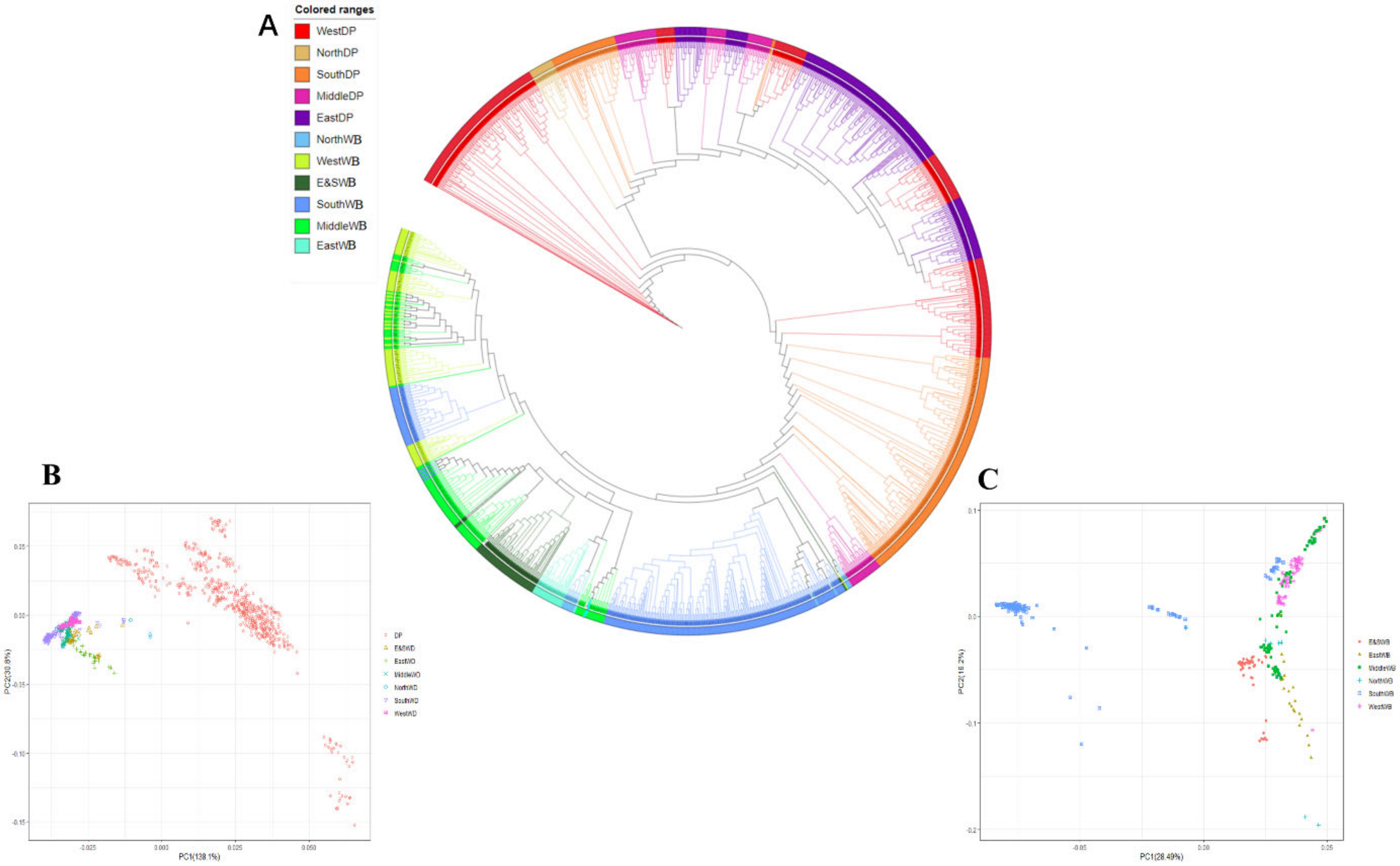
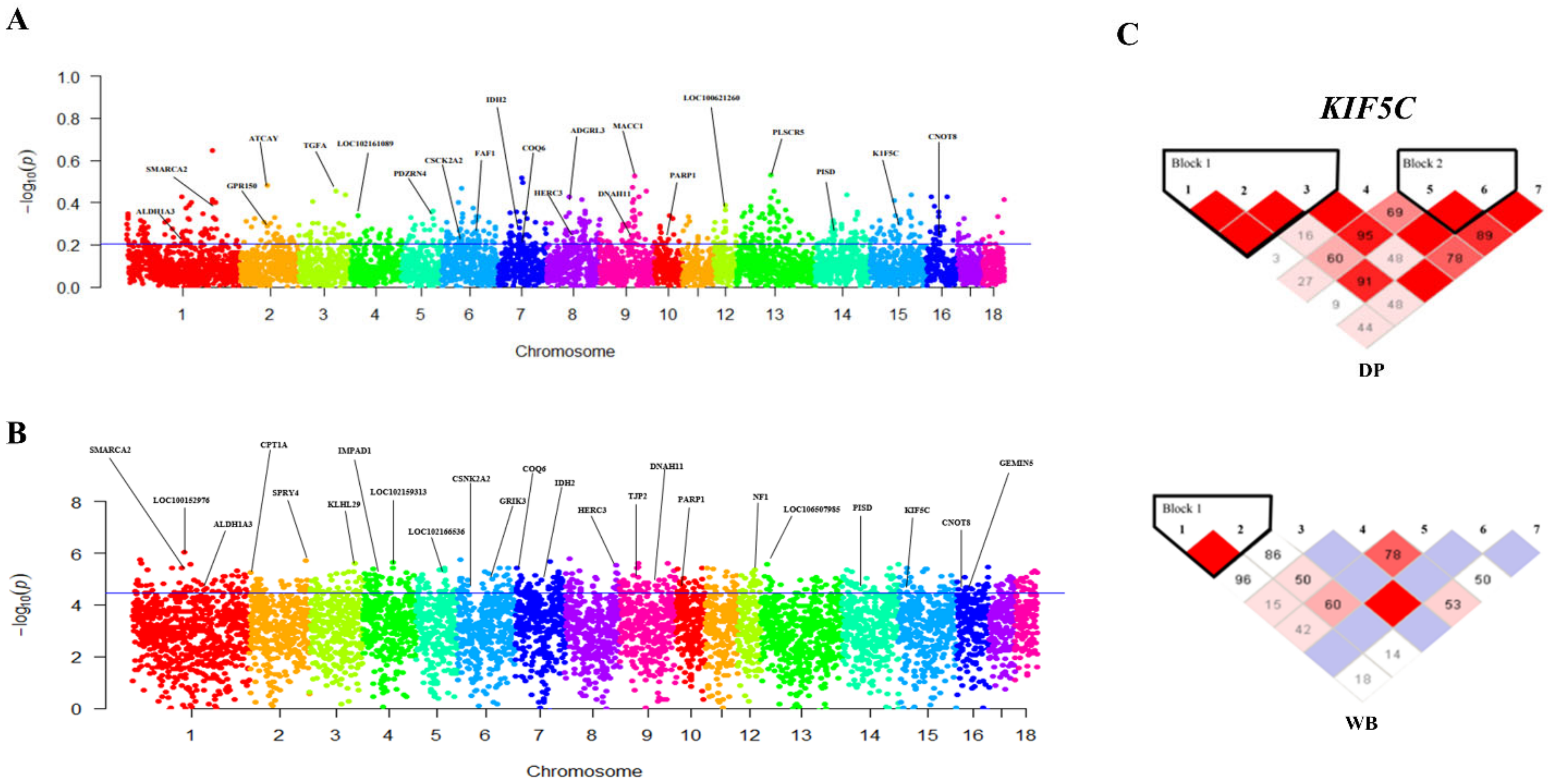
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Valiño, L.; Sarasa, C.; Duarte, R. Economy-wide effects of a sustainable pathway in the pig sector: A case study in Aragon (Spain). J. Environ. Manag. 2019, 239, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Sans, P.; Combris, P. World meat consumption patterns: An overview of the last fifty years (1961–2011). Meat Sci. 2015, 109, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, M.; Yang, B.; Wu, Z.; Deng, Z.; Hou, Y.; Ren, J.; Huang, L. Introgression of Eastern Chinese and Southern Chinese haplotypes contributes to the improvement of fertility and immunity in European modern pigs. Gigascience 2020, 9, giaa014. [Google Scholar] [CrossRef]
- Muñoz, M.; Bozzi, R.; García, F.; Núñez, Y.; Geraci, C.; Crovetti, A.; García-Casco, J.; Alves, E.; Škrlep, M.; Charneca, R.; et al. Diversity across major and candidate genes in European local pig breeds. PLoS ONE 2018, 13, e0207475. [Google Scholar] [CrossRef]
- Humphray, S.J.; Scott, C.E.; Clark, R.; Marron, B.; Bender, C.; Camm, N.; Davis, J.; Jenks, A.; Noon, A.; Patel, M.; et al. A high utility integrated map of the pig genome. Genome Biol. 2007, 8, R139. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Han, X.M.; Huang, C.P.; Zhong, L.; Adeola, A.C.; Irwin, D.M.; Xie, H.B.; Zhang, Y.P. Population Genomics Analysis Revealed Origin and High-altitude Adaptation of Tibetan Pigs. Sci Rep. 2019, 9, 11463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Gong, H.; Cui, L.; Zhang, W.; Ma, J.; Chen, C.; Ai, H.; Xiao, S.; Huang, L.; et al. Genetic correlation of fatty acid composition with growth, carcass, fat deposition and meat quality traits based on GWAS data in six pig populations. Meat Sci. 2019, 150, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, P.H.; Zhu, M.X.; He, L.C.; Sui, S.P.; Gao, S.; Su, G.S.; Ding, N.S.; Huang, Y.; Lu, Z.Q.; et al. Genome-wide association analysis reveals genomic regions on Chromosome 13 affecting litter size and candidate genes for uterine horn length in Erhualian pigs. Animal 2018, 12, 2453–2461. [Google Scholar] [CrossRef]
- Xu, P.; Ni, L.; Tao, Y.; Ma, Z.; Hu, T.; Zhao, X.; Yu, Z.; Lu, C.; Zhao, X.; Ren, J. Genome-wide association study for growth and fatness traits in Chinese Sujiang pigs. Anim. Genet. 2020, 51, 314–318. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Huang, M.; Tang, J.; Yang, L.; Yu, Z.; Li, D.; Li, G.; Jiang, Y.; Sun, Y.; et al. Whole-genome SNP markers reveal conservation status, signatures of selection, and introgression in Chinese Laiwu pigs. Evol. Appl. 2020, 14, 383–398. [Google Scholar] [CrossRef]
- Druml, T.; Salajpal, K.; Dikic, M.; Urosevic, M.; Grilz-Seger, G.; Baumung, R. Genetic diversity, population structure and subdivision of local Balkan pig breeds in Austria, Croatia, Serbia and Bosnia-Herzegovina and its practical value in conservation programs. Genet. Sel. Evol. 2012, 44, 5. [Google Scholar] [CrossRef] [PubMed]
- Vigne, J.-D.; Zazzo, A.; Saliège, J.-F.; Poplin, F.; Guilaine, J.; Simmons, A. Pre-Neolithic wild boar management and introduction to Cyprus more than 11,400 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 16135–16138. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Hongo, H. The Archaeology of Pig Domestication in Eurasia. J. Archaeol. Res. 2020, 8, 557–615. [Google Scholar] [CrossRef]
- Khederzadeh, S.; Kusza, S.; Huang, C.P.; Markov, N.; Scandura, M.; Babaev, E.; Šprem, N.; Seryodkin, I.V.; Paule, L.; Esmailizadeh, A.; et al. Maternal genomic variability of the wild boar (Sus scrofa) reveals the uniqueness of East-Caucasian and Central Italian populations. Ecol. Evol. 2019, 9, 9467–9478. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Albarella, U.; Dobney, K.; Rowley-Conwy, P.; Schibler, J.; Tresset, A.; Vigne, J.D.; Edwards, C.J.; Schlumbaum, A.; Dinu, A.; et al. Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. Proc. Natl. Acad. Sci. USA 2007, 104, 15276–15281. [Google Scholar] [CrossRef] [PubMed]
- Krause-Kyora, B.; Makarewicz, C.; Evin, A.; Flink, L.G.; Dobney, K.; Larson, G.; Hartz, S.; Schreiber, S.; von Carnap-Bornheim, C.; von Wurmb-Schwark, N.; et al. Use of domesticated pigs by Mesolithic hunter-gatherers in northwestern Europe. Nat. Commun. 2013, 4, 2348. [Google Scholar] [CrossRef] [PubMed]
- Gethöffer, F.; Pfarrer, C.; Siebert, U. Histology confirms that macroscopic evaluation of ovaries is a valid method for the assessment of the reproductive status in wild boar. Theriogenology 2018, 113, 192–196. [Google Scholar] [CrossRef]
- Sales, J.; Kotrba, R. Meat from wild boar (Sus scrofa L.): A review. Meat Sci. 2013, 94, 187–201. [Google Scholar] [CrossRef]
- Torres, R.T.; Fernandes, J.; Carvalho, J.; Cunha, M.V.; Caetano, T.; Mendo, S.; Serrano, E.; Fonseca, C. Wild boar as a reservoir of antimicrobial resistance. Sci. Total Env. 2020, 717, 135001. [Google Scholar] [CrossRef]
- Johann, F.; Handschuh, M.; Linderoth, P.; Dormann, C.F.; Arnold, J. Adaptation of wild boar (Sus scrofa) activity in a human-dominated landscape. BMC Ecol. 2020, 20, 4. [Google Scholar] [CrossRef]
- Li, M.; Tian, S.; Jin, L.; Zhou, G.; Li, Y.; Zhang, Y.; Wang, T.; Yeung, C.K.; Chen, L.; Ma, J.; et al. Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars. Nat. Genet. 2013, 45, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Asano, M.; Kuninaga, N.; Suzuki, M. Monitoring relative abundance index and age ratios of wild boar (Sus scrofa) in small scale population in Gifu prefecture, Japan during classical swine fever outbreak. J. Vet. Med. Sci. 2020, 82, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, B.; Frank, K.; Astuti, P.K.; Szemethy, D.; Szendrei, L.; Szemethy, L.; Kusza, S.; Stéger, V. Population Genetic Structure of the Wild Boar (Sus scrofa) in the Carpathian Basin. Genes 2020, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Pittiglio, C.; Khomenko, S.; Beltran-Alcrudo, D. Wild boar mapping using population-density statistics: From polygons to high resolution raster maps. PLoS ONE 2018, 13, e0193295. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cui, L.; Perez-Enciso, M.; Traspov, A.; Crooijmans, R.P.M.A.; Zinovieva, N.; Schook, L.B.; Archibald, A.; Gatphayak, K.; Knorr, C.; et al. Genome-wide SNP data unveils the globalization of domesticated pigs. Genet. Sel. Evol. 2017, 49, 71, Erratum in Genet. Sel. Evol. 2020, 52, 30. [Google Scholar] [CrossRef] [PubMed]
- Iacolina, L.; Scandura, M.; Goedbloed, D.J.; Alexandri, P.; Crooijmans, R.P.; Larson, G.; Archibald, A.; Apollonio, M.; Schook, L.B.; Groenen, M.A.; et al. Genomic diversity and differentiation of a managed island wild boar population. Heredity 2016, 116, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Co mprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef]
- Weir, B.S.; Hill, W.G. Estimating F-statistics. Annu. Rev. Genet. 2002, 36, 721–750. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Caliebe, A.; Nebel, A.; Makarewicz, C.; Krawczak, M.; Krause-Kyora, B. Insights into early pig domestication provided by ancient DNA analysis. Sci. Rep. 2017, 7, 44550. [Google Scholar] [CrossRef]
- Larson, G.; Dobney, K.; Albarella, U.; Fang, M.; Matisoo-Smith, E.; Robins, J.; Lowden, S.; Finlayson, H.; Brand, T.; Willerslev, E.; et al. Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science 2005, 307, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Scandura, M.; Iacolina, L.; Apollonio, M. Genetic diversity in the European wild boar Sus scrofa: Phylogeography, population structure and wild x domestic hybridization. Mammal. Rev. 2011, 41, 125–137. [Google Scholar] [CrossRef]
- Vernesi, C.; Crestanello, B.; Pecchioli, E.; Tartari, D.; Caramelli, D.; Hauffe, H.; Bertorelle, G. The genetic impact of demographic decline and reintroduction in the wild boar (Sus scrofa): A microsatellite analysis. Mol. Ecol. 2003, 12, 585–595. [Google Scholar] [CrossRef]
- Saez-Royuela, C.; Telleria, J.L. The increased population of the wild boar (Sus scrofa L.) in Europe. ‘Fonseca, C. Population dynamics and management of wild boar (Sus scrofa L.) in Central Portugal and Southeastern Poland. Mammal Rev. 1986, 16, 97–101. [Google Scholar]
- Frank, B.F.; Monaco, A.; Bath, A.J. Beyond standard wildlife management: A pathway to encompass human dimension findings in wild boar management. Eur. J. Wildl. Res. 2015, 61, 723–730. [Google Scholar] [CrossRef]
- White, S. From globalized pig breeds to capitalist pigs: A study in animal cultures and evolutionary history. Env. Hist. 2011, 16, 94–120. [Google Scholar] [CrossRef]
- Scandura, M.; Iacolina, L.; Cossu, A.; Apollonio, M. Effects of human perturbation on the genetic make-up of an island population: The case of the Sardinian wild boar. Heredity 2011, 106, 1012–1020. [Google Scholar] [CrossRef][Green Version]
- Sommer Wilkens, B. La fauna sarda durante l’Olocene: Le conoscenze attuali. Sard. Cors. Balear. Antiq. 2003, 1, 181–197. [Google Scholar]
- Danilkin, A.A. The wild boar: An unprecedented spread or restoration of the species range? Dokl. Biol. Sci. 2001, 380, 457–460. [Google Scholar] [CrossRef]
- Nie, S.; Qian, X.; Shi, M.; Li, H.; Peng, C.; Ding, X.; Zhang, S.; Zhang, B.; Xu, G.; Lv, Y.; et al. ALDH1A3 Accelerates Pancreatic Cancer Metastasis by Promoting Glucose Metabolism. Front. Oncol. 2020, 10, 915. [Google Scholar] [CrossRef]
- Huitema, L.F.; Apschner, A.; Logister, I.; Spoorendonk, K.M.; Bussmann, J.; Hammond, C.L.; Schulte-Merker, S. Entpd5 is essential for skeletal mineralization and regulates phosphate homeostasis in zebrafish. Proc. Natl. Acad. Sci. USA 2012, 109, 21372–21377. [Google Scholar] [CrossRef] [PubMed]
- Bergaggio, E.; Riganti, C.; Garaffo, G.; Vitale, N.; Mereu, E.; Bandini, C.; Pellegrino, E.; Pullano, V.; Omedè, P.; Todoerti, K.; et al. IDH2 inhibition enhances proteasome inhibitor responsiveness in hematological malignancies. Blood 2019, 133, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Mondesir, J.; Willekens, C.; Touat, M.; de Botton, S. IDH1 and IDH2 mutations as novel therapeutic targets: Current perspectives. J. Blood Med. 2016, 7, 171–180. [Google Scholar] [PubMed]
- Chen, J.S.; Wu, F.; Yang, H.S.; Li, F.N.; Jiang, Q.; Liu, S.J.; Kang, B.J.; Li, S.; Adebowale, T.O.; Huang, N.; et al. Growth performance, nitrogen balance, and metabolism of calcium and phosphorus in growing pigs fed diets supplemented with alpha- ketoglutarate. Anim. Feed Sci. Technol. 2017, 226, 21–28. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Liang, C.; Bao, M.; Tian, Y.; Zhu, J.; Zhang, T.; Yang, J.; Wang, Z. ALDH1A3 serves as a predictor for castration resistance in prostate cancer patients. BMC Cancer 2020, 20, 387. [Google Scholar] [CrossRef]
- Duan, J.J.; Cai, J.; Guo, Y.F.; Bian, X.W.; Yu, S.C. ALDH1A3, a metabolic target for cancer diagnosis and therapy. Int. J. Cancer 2016, 139, 965–975. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, Y.; Zhu, Z.; Wang, T.Y.; Morris, D.L.; Shen, J.J.; Zhang, H.; Pan, H.F.; Yang, J.; Yang, S.; et al. Identification of ST3AGL4, MFHAS1, CSNK2A2 and CD226 as loci associated with systemic lupus erythematosus (SLE) and evaluation of SLE genetics in drug repositioning. Ann. Rheum. Dis. 2018, 77, 1078–1084. [Google Scholar] [CrossRef]
- Kosowska, A.; Cadenas-Fernández, E.; Barroso, S.; Sánchez-Vizcaíno, J.M.; Barasona, J.A. Distinct African Swine Fever Virus Shedding in Wild Boar Infected with Virulent and Attenuated Isolates. Vaccines 2020, 8, 767. [Google Scholar] [CrossRef]
- Kovalenko, G.; Molozhanova, A.; Halka, I.; Nychyk, S. Antibody Prevalence to Influenza Type A in Wild Boar of Northern Ukraine. Vector Borne Zoonotic Dis. 2017, 17, 836–839. [Google Scholar] [CrossRef]
- Magnani, L.; Cabot, R.A. Developmental arrest induced in cleavage stage porcine embryos following microinjection of mRNA encoding Brahma (Smarca 2), a chromatin remodeling protein. Mol. Reprod. Dev. 2007, 74, 1262–1267. [Google Scholar] [CrossRef]
- Orłowska, L.; Rembacz, W.; Florek, C. Carcass weight, condition and reproduction of wild boar harvested in north-western Poland. Pest Manag. Sci. 2013, 69, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Martinat-Botté, F.; Bariteau, F.; Badouard, B.; Terqui, M. Control of pig reproduction in a breeding programme. J. Reprod. Fertil. Suppl. 1985, 33, 211–228. [Google Scholar] [PubMed]
- Frantz, L.A.; Schraiber, J.G.; Madsen, O.; Megens, H.J.; Cagan, A.; Bosse, M.; Paudel, Y.; Crooijmans, R.P.; Larson, G.; Groenen, M.A. Evidence of long-term gene flow and selection during domestication from analyses of Eurasian wild and domestic pig genomes. Nat. Genet. 2015, 47, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Knap, P.W.; Merks, J.W.M. A note on the genetics of aggressiveness of primiparous sows towards their piglets. Livest. Prod. 1987, 17, 161–167. [Google Scholar] [CrossRef]
- van der Steen, H.A.M.; Schaeffer, L.R.; de Jong, H.; de Groot, P.N. Aggressive behavior of sows at parturition. J. Anim. 1988, 66, 271–279. [Google Scholar] [CrossRef]
- Quilter, C.R.; Blott, S.C.; Wilson, A.E.; Bagga, M.R.; Sargent, C.A.; Oliver, G.L.; Southwood, O.I.; Gilbert, C.L.; Mileham, A.; Affara, N.A. Porcine maternal infanticide as a model for puerperal psychosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144, 862–868. [Google Scholar] [CrossRef]
- Chen, C.; Gilbert, C.L.; Yang, G.; Guo, Y.; Segonds-Pichon, A.; Ma, J.; Evans, G.; Brenig, B.; Sargent, C.; Affara, N.; et al. Maternal infanticide in sows: Incidence and behavioural comparisons between savaging and non-savaging sows at parturition. Appl. Anim. Behav. 2008, 109, 238–248. [Google Scholar] [CrossRef]
- Baxter, E.M.; Jarvis, S.; Sherwood, L.; Farish, M.; Roehe, R.; Lawrence, A.B.; Edwards, S.A. Genetic and environmental effects on piglet survival and maternal behaviour of the farrowing sow. Appl. Anim. Behav. Sci. 2011, 130, 28–41. [Google Scholar] [CrossRef]
- Fraser, D. The role of behaviour in swine production: A review of research. Appl. Anim. Ethol. 1984, 11, 317–339. [Google Scholar] [CrossRef]
- Løvendahl, P.; Damgaard, L.H.; Nielsen, B.L.; Thodberg, K.; Su, G.; Rydhmer, L. Aggressive behaviour of sows at mixing and maternal behaviour are heritable and genetically correlated traits. Livest. Prod. 2005, 93, 73–85. [Google Scholar] [CrossRef]
- Turner, S.P.; White, I.M.S.; Brotherstone, S.; Farnworth, M.J.; Knap, P.W.; Penny, P.; Mendl, M.; Lawrence, A.B. Heritability of post-mixing aggressiveness in grower-stage pigs and its relationship with production traits. Anim. Sci. 2006, 82, 615–620. [Google Scholar] [CrossRef]
- Stukenborg, A.; Traulsen, I.; Stamer, E.; Puppe, B.; Presuhn, U.; Krieter, J. Heritabilities of agonistic behavioural traits in pigs and their relationships within and between different age groups. Livest. Sci. 2012, 149, 25–32. [Google Scholar] [CrossRef]
- Appel, A.K.; Voß, B.; Tönepöhl, B.; König von Borstel, U.; Gauly, M. Genetic associations between maternal traits and aggressive behaviour in Large White sows. Animal 2016, 10, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, K.; Stamer, E.; Traulsen, I.; Krieter, J. Estimation of genetic parameters for agonistic behaviour of pigs at different ages. J. Agric. 2016, 154, 732–741. [Google Scholar] [CrossRef]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef]
- Goldstein, L.S.; Lawrence, S.B.; Yang, Z. Microtubule-based transport systems inneurons: The roles of kinesins and dyneins. Annu. Rev. Neurol. 2000, 23, 39–71. [Google Scholar] [CrossRef]
- Hollenbeck, P.J. Phosphorylation of neuronal kinesin heavy and light chains in vivo. J. Neurochem. 1993, 60, 2265–2275. [Google Scholar] [CrossRef]
- Justine Perrin, R.; Rousset-Rouvière, C.; Garaix, F.; Cano, A.; Conrath, J.; Boyer, O.; Tsimaratos, M. COQ6 mutation in patients with nephrotic syndrome, sensorineural deafness, and optic atrophy. JIMD Rep. 2020, 54, 37–44. [Google Scholar] [CrossRef]
- Kaiser, S.; Hennessy, M.B.; Sachser, N. Domestication affects the structure, development and stability of biobehavioural profiles. Front. Zool. 2015, 12 (Suppl. 1), S19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Zhang, H.-Y.; Yuan, Y.; He, Y.; Zhang, W.; Han, Y.; Na, R.; Zeng, Y.; Luo, J.; Yang, H.; et al. Genome-Wide Selection Sweep between Wild and Local Pigs from Europe for the Investigation of the Hereditary Characteristics of Domestication in Sus Scrofa. Animals 2022, 12, 1037. https://doi.org/10.3390/ani12081037
Gong Y, Zhang H-Y, Yuan Y, He Y, Zhang W, Han Y, Na R, Zeng Y, Luo J, Yang H, et al. Genome-Wide Selection Sweep between Wild and Local Pigs from Europe for the Investigation of the Hereditary Characteristics of Domestication in Sus Scrofa. Animals. 2022; 12(8):1037. https://doi.org/10.3390/ani12081037
Chicago/Turabian StyleGong, Yiming, Hao-Yuan Zhang, Ying Yuan, Yongmeng He, Weiyi Zhang, Yanguo Han, Risu Na, Yan Zeng, Jia Luo, Haili Yang, and et al. 2022. "Genome-Wide Selection Sweep between Wild and Local Pigs from Europe for the Investigation of the Hereditary Characteristics of Domestication in Sus Scrofa" Animals 12, no. 8: 1037. https://doi.org/10.3390/ani12081037
APA StyleGong, Y., Zhang, H.-Y., Yuan, Y., He, Y., Zhang, W., Han, Y., Na, R., Zeng, Y., Luo, J., Yang, H., Huang, Y., Zhao, Y., Zhao, Z., & E, G.-X. (2022). Genome-Wide Selection Sweep between Wild and Local Pigs from Europe for the Investigation of the Hereditary Characteristics of Domestication in Sus Scrofa. Animals, 12(8), 1037. https://doi.org/10.3390/ani12081037






