Impact of Maternal Feed Restriction at Different Stages of Gestation on the Proteomic Profile of the Newborn Skeletal Muscle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Protein Extraction
2.3. Protein Identification and Data Processing
2.4. Network Analyses
2.5. Statistical Analysis
3. Results
3.1. Differentially Abundant Proteins
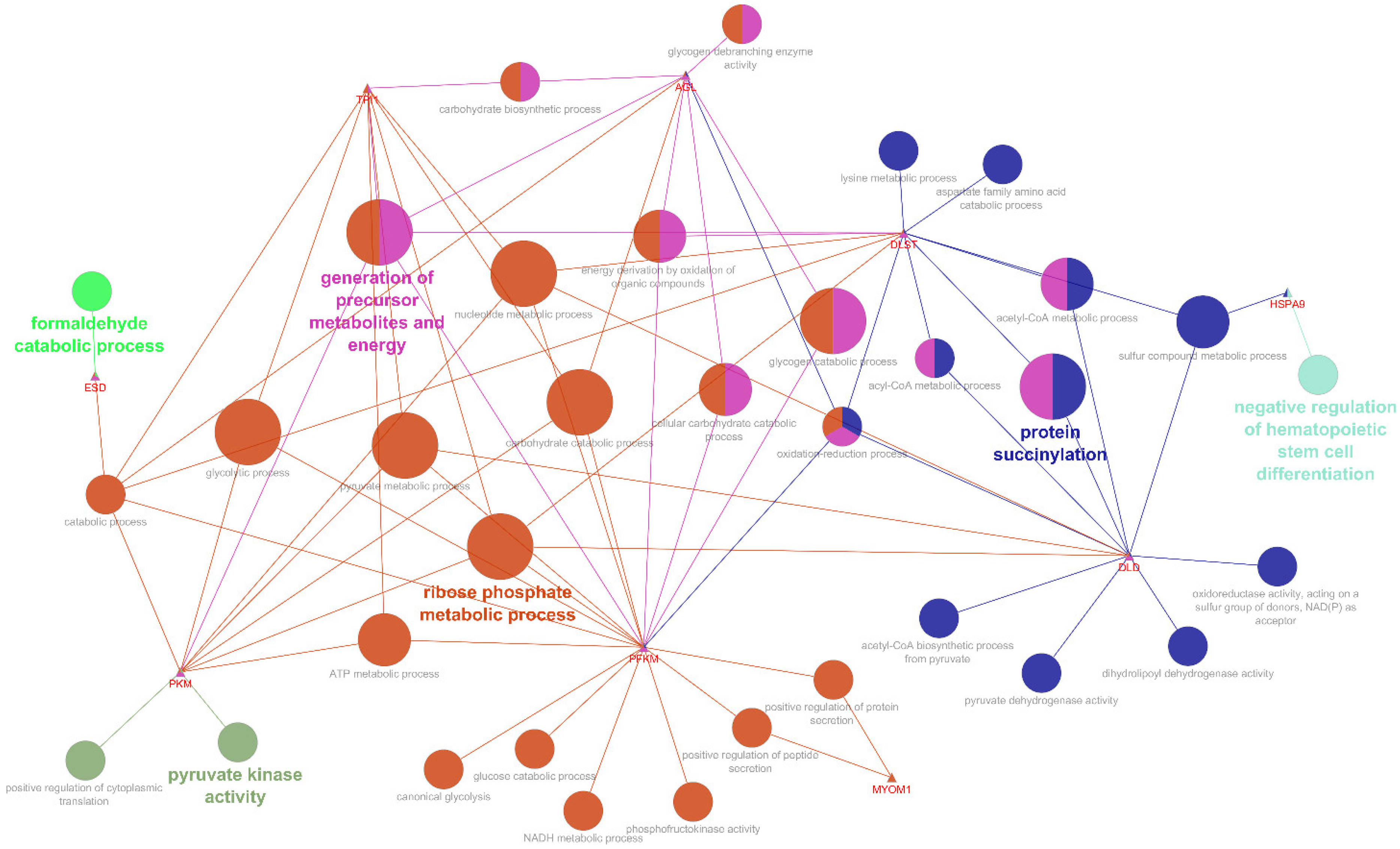
3.2. Protein-Protein Interaction Network and KEGG Pathways of the Exclusive Proteins
3.3. Interaction Network and Functional Enrichment of Differentially Abundant Proteins
4. Discussion
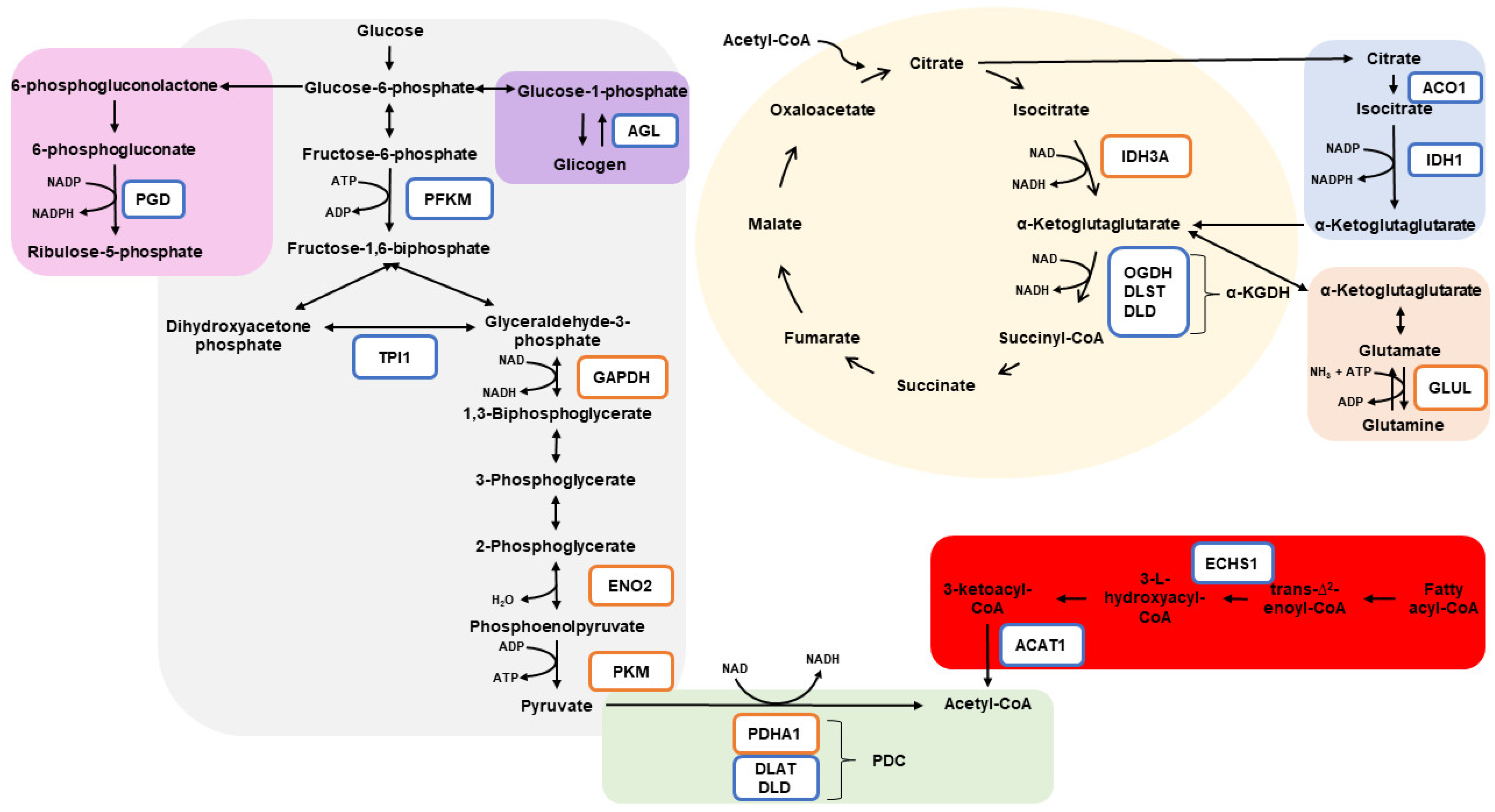
4.1. Glycolysis
4.2. Citrate Cycle (TCA)
4.3. Glutamine
4.4. Fatty Acid Degradation
4.5. Pentose Phosphate Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hornick, J.L.; Van Eenaeme, C.; Gérard, O.; Dufrasne, I.; Istasse, L. Mechanisms of Reduced and Compensatory Growth. Domest. Anim. Endocrinol. 2000, 19, 121–132. [Google Scholar] [CrossRef]
- Paradis, F.; Wood, K.M.; Swanson, K.C.; Miller, S.P.; McBride, B.W.; Fitzsimmons, C. Maternal Nutrient Restriction in Mid-to-Late Gestation Influences Fetal MRNA Expression in Muscle Tissues in Beef Cattle. BMC Genom. 2017, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, M.C.; Pillai, S.M.; Reed, S.A.; Stevens, J.R.; Hoffman, M.L.; Jones, A.K.; Zinn, S.A.; Govoni, K.E. Poor Maternal Nutrition during Gestation in Sheep Alters Prenatal Muscle Growth and Development in Offspring. J. Anim. Sci. 2020, 98, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal Programming of Skeletal Muscle Development in Ruminant Animals. J. Anim. Sci. 2010, 88, E51–E60. [Google Scholar] [CrossRef] [PubMed]
- Long, N.M.; Vonnahme, K.A.; Hess, B.W.; Nathanielsz, P.W.; Ford, S.P. Effects of Early Gestational Undernutrition on Fetal Growth, Organ Development, and Placentomal Composition in the Bovine. J. Anim. Sci. 2009, 87, 1950–1959. [Google Scholar] [CrossRef]
- Meyer, A.M.; Reed, J.J.; Vonnahme, K.A.; Soto-Navarro, S.A.; Reynolds, L.P.; Ford, S.P.; Hess, B.W.; Caton, J.S. Effects of Stage of Gestation and Nutrient Restriction during Early to Mid-Gestation on Maternal and Fetal Visceral Organ Mass and Indices of Jejunal Growth and Vascularity in Beef Cows. J. Anim. Sci. 2010, 88, 2410–2424. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Camacho, L.E.; Ebarb, S.M.; Swanson, K.C.; Vonnahme, K.A.; Stelzleni, A.M.; Johnson, S.E. Realimentation of Nutrient Restricted Pregnant Beef Cows Supports Compensatory Fetal Muscle Growth. J. Anim. Sci. 2013, 91, 4797–4806. [Google Scholar] [CrossRef]
- Sanglard, L.P.; Nascimento, M.; Moriel, P.; Sommer, J.; Ashwell, M.; Poore, M.H.; Duarte, M. de S.; Serão, N.V.L. Impact of Energy Restriction during Late Gestation on the Muscle and Blood Transcriptome of Beef Calves after Preconditioning. BMC Genom. 2018, 19, 1–18. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the Regulation of Protein Abundance from Proteomic and Transcriptomic Analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Abreu, R.S.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global Signatures of Protein and MRNA Expression Levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key Genes Regulating Skeletal Muscle Development and Growth in Farm Animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef]
- Du, M.; Ford, S.P.; Zhu, M.-J. Optimizing Livestock Production Efficiency through Maternal Nutritional Management and Fetal Developmental Programming. Anim. Front. 2017, 7, 5–11. [Google Scholar] [CrossRef]
- Feiner, G. Meat Products Handbook: Practical Science and Technology; CRC Press: Boca Raton, FL, USA, 2006; ISBN 1845691725. [Google Scholar]
- Scopes, R.K.; Stoter, A. [79] Purification of All Glycolytic Enzymes from One Muscle Extract. Methods Enzymol. 1982, 90, 479–490. [Google Scholar] [PubMed]
- Jia, X.; Hollung, K.; Therkildsen, M.; Hildrum, K.I.; Bendixen, E. Proteome Analysis of Early Post-Mortem Changes in Two Bovine Muscle Types:M. Longissimus Dorsi and M. Semitendinosis. Proteomics 2006, 6, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Sayd, T.; Morzel, M.; Chambon, C.; Franck, M.; Figwer, P.; Larzul, C.; Le Roy, P.; Monin, G.; Chérel, P.; Laville, E. Proteome Analysis of the Sarcoplasmic Fraction of Pig Semimembranosus Muscle: Implications on Meat Color Development. J. Agric. Food Chem. 2006, 54, 2732–2737. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.C.; Moura, F.H.; Souza, R.O.; Lopes, M.M.; Fontes, M.M.S.; Serão, N.V.L.; Sanglard, L.P.; Du, M.; Gionbelli, M.P.; Duarte, M.S. Effect of Maternal Feed Restriction in Dairy Goats at Different Stages of Gestation on Skeletal Muscle Development and Energy Metabolism of Kids at the Time of Births. Anim. Reprod. Sci. 2019, 206, 46–59. [Google Scholar] [CrossRef]
- Du, M.; Zhu, M.J.; Means, W.J.; Hess, B.W.; Ford, S.P. Nutrient Restriction Differentially Modulates the Mammalian Target of Rapamycin Signaling and the Ubiquitin-Proteasome System in Skeletal Muscle of Cows and Their Fetuses. J. Anim. Sci. 2005, 83, 117–123. [Google Scholar] [CrossRef]
- Aragão, R.S.; Guzmán, O.Q.; Pérez, G.G.; Manhães, R.C.; Bolaños, F.J. Maternal Protein Restriction Impairs the Transcriptional Metabolic Flexibility of Skeletal Muscle in Adult Rat Offspring. Br. J. Nutr. 2014, 112, 328–337. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Academy of Science: Washington, DC, USA, 2007; ISBN 978-0-309-10213-1. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Bei, Y.; Hong, P. A Novel Approach to Minimize False Discovery Rate in Genome-Wide Data Analysis. BMC Syst. Biol. 2013, 7, S1. [Google Scholar] [CrossRef][Green Version]
- Lewin, A.M.; Grieve, I.C. Grouping Gene Ontology Terms to Improve the Assessment of Gene Set Enrichment in Microarray Data. BMC Bioinform. 2006, 7, 1–9. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Goeminne, L.J.E.; Gevaert, K.; Clement, L. Experimental Design and Data-Analysis in Label-Free Quantitative LC/MS Proteomics: A Tutorial with MSqRob. J. Proteom. 2018, 171, 23–36. [Google Scholar] [CrossRef]
- Goeminne, L.J.E.; Gevaert, K.; Clement, L. Peptide-Level Robust Ridge Regression Improves Estimation, Sensitivity, and Specificity in Data-Dependent Quantitative Label-Free Shotgun Proteomics. Mol. Cell. Proteom. 2016, 15, 657–668. [Google Scholar] [CrossRef]
- Zhang, S.; Regnault, T.R.H.; Barker, P.L.; Botting, K.J.; McMillen, I.C.; McMillan, C.M.; Roberts, C.T.; Morrison, J.L. Placental Adaptations in Growth Restriction. Nutrients 2015, 7, 360–389. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Meyer, C.; Dostou, J.M.; Welle, S.L.; Gerich, J.E. Role of Human Liver, Kidney, and Skeletal Muscle in Postprandial Glucose Homeostasis. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E419–E427. [Google Scholar] [CrossRef]
- Akram, M. Mini-Review on Glycolysis and Cancer. J. Cancer Educ. 2013, 28, 454–457. [Google Scholar] [CrossRef]
- Ferguson, D.M.; Gerrard, D.E. Regulation of Post-Mortem Glycolysis in Ruminant Muscle. Anim. Prod. Sci. 2014, 54, 464–481. [Google Scholar] [CrossRef]
- Cox, M.M.; Nelson, D.L. Lehninger Principles of Biochemistry; Wh Freeman: New York, NY, USA, 2008; Volume 5. [Google Scholar]
- Roland, B.P.; Stuchul, K.A.; Larsen, S.B.; Amrich, C.G.; VanDemark, A.P.; Celotto, A.M.; Palladino, M.J. Evidence of a Triosephosphate Isomerase Non-Catalytic Function Crucial to Behavior and Longevity. J. Cell Sci. 2013, 126, 3151–3158. [Google Scholar] [PubMed]
- Orozco, J.M.; Krawczyk, P.A.; Scaria, S.M.; Cangelosi, A.L.; Chan, S.H.; Kunchok, T.; Lewis, C.A.; Sabatini, D.M. Dihydroxyacetone Phosphate Signals Glucose Availability to MTORC1. Nat. Metab. 2020, 2, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. MTOR Complex 1 Regulates Lipin 1 Localization to Control the Srebp Pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef]
- Grabež, V.; Kathri, M.; Phung, V.; Moe, K.M.; Slinde, E.; Skaugen, M.; Saarem, K.; Egelandsdal, B. Protein Expression and Oxygen Consumption Rate of Early Postmortem Mitochondria Relate to Meat Tenderness. J. Anim. Sci. 2015, 93, 1967–1979. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Meta-Proteomics for the Discovery of Protein Biomarkers of Beef Tenderness: An Overview of Integrated Studies. Food Res. Int. 2020, 127, 108739. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bonnet, M.; De Koning, L.; Picard, B. Reverse Phase Protein Array for the Quantification and Validation of Protein Biomarkers of Beef Qualities: The Case of Meat Color from Charolais Breed. Meat Sci. 2018, 145, 308–319. [Google Scholar] [CrossRef]
- Huang, H.; Larsen, M.R.; Karlsson, A.H.; Pomponio, L.; Costa, L.N.; Lametsch, R. Gel-Based Phosphoproteomics Analysis of Sarcoplasmic Proteins in Postmortem Porcine Muscle with PH Decline Rate and Time Differences. Proteomics 2011, 11, 4063–4076. [Google Scholar] [CrossRef]
- Nair, M.N.; Li, S.; Beach, C.; Rentfrow, G.; Suman, S.P. Intramuscular Variations in Color and Sarcoplasmic Proteome of Beef during Postmortem Aging. Meat Muscle Biol. 2018, 2, 92–101. [Google Scholar] [CrossRef]
- Wu, W.; Dai, R.T.; Bendixen, E. Comparing SRM and SWATH Methods for Quantitation of Bovine Muscle Proteomes. J. Agric. Food Chem. 2019, 67, 1608–1618. [Google Scholar] [CrossRef]
- Gagaoua, M.; Hughes, J.; Terlouw, E.M.C.; Warner, R.D.; Purslow, P.P.; Lorenzo, J.M.; Picard, B. Proteomic Biomarkers of Beef Colour. Trends Food Sci. Technol. 2020, 101, 234–252. [Google Scholar] [CrossRef]
- Merkulova, T.; Dehaupas, M.; Nevers, M.C.; Créminon, C.; Alameddine, H.; Keller, A. Differential Modulation of α, β and γ Enolase Isoforms in Regenerating Mouse Skeletal Muscle. Eur. J. Biochem. 2000, 267, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Doenst, T.; Schwarzer, M. Metabolic Pathways and Cycles. In The Scientist’s Guide to Cardiac Metabolism; Academic Press: San Diego, CA, USA, 2016; pp. 39–55. ISBN 9780128023945. [Google Scholar]
- Guevara, E.L.; Yang, L.; Birkaya, B.; Zhou, J.; Nemeria, N.S.; Patel, M.S.; Jordan, F. Global View of Cognate Kinase Activation by the Human Pyruvate Dehydrogenase Complex. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Biensø, R.S.; Knudsen, J.G.; Brandt, N.; Pedersen, P.A.; Pilegaard, H. Effects of IL-6 on Pyruvate Dehydrogenase Regulation in Mouse Skeletal Muscle. Pflugers Arch. Eur. J. Physiol. 2014, 466, 1647–1657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, M.; Umezawa, C.; Shin, T. Metabolic Pathways: Metabolism of Minerals and Vitamins. In Encyclopedia of Food Microbiology: Second Edition; Academic Press: Burlington, MA, USA, 2014; pp. 535–543. ISBN 9780123847331. [Google Scholar]
- Poljsak, B.; Milisav, I. Vitamin B3 Forms as Precursors to NAD+: Are They Safe? Trends Food Sci. Technol. 2018, 79, 198–203. [Google Scholar] [CrossRef]
- Volz, K. The Functional Duality of Iron Regulatory Protein 1. Curr. Opin. Struct. Biol. 2008, 18, 106–111. [Google Scholar] [CrossRef]
- McGahan, M.C.; Harned, J.; Mukunnemkeril, M.; Goralska, M.; Fleisher, L.; Ferrell, J.B. Iron Alters Glutamate Secretion by Regulating Cytosolic Aconitase Activity. Am. J. Physiol.-Cell Physiol. 2005, 288, C1117–C1124. [Google Scholar] [CrossRef]
- Koh, H.J.; Lee, S.M.; Son, B.G.; Lee, S.H.; Ryoo, Z.Y.; Chang, K.T.; Park, J.W.; Park, D.C.; Song, B.J.; Veech, R.L.; et al. Cytosolic NADP+-Dependent Isocitrate Dehydrogenase Plays a Key Role in Lipid Metabolism. J. Biol. Chem. 2004, 279, 39968–39974. [Google Scholar] [CrossRef]
- Tong, W.H.; Rouault, T.A. Metabolic Regulation of Citrate and Iron by Aconitases: Role of Iron-Sulfur Cluster Biogenesis. BioMetals 2007, 20, 549–564. [Google Scholar] [CrossRef]
- Moreno, M.; Ortega, F.; Xifra, G.; Ricart, W.; Fernández-Real, J.M.; Moreno-Navarrete, J.M. Cytosolic Aconitase Activity Sustains Adipogenic Capacity of Adipose Tissue Connecting Iron Metabolism and Adipogenesis. FASEB J. 2015, 29, 1529–1539. [Google Scholar] [CrossRef]
- Tretter, L.; Adam-Vizi, V. Alpha-Ketoglutarate Dehydrogenase: A Target and Generator of Oxidative Stress. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Zdzisińska, B.; Żurek, A.; Kandefer-Szerszeń, M. Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Arch. Immunol. Ther. Exp. 2017, 65, 21–36. [Google Scholar] [CrossRef]
- Krebs, H.A. Metabolism of Amino-Acids. Biochem. J. 1935, 29, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The Key Role of Anaplerosis and Cataplerosis for Citric Acid Cycle Function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B.; Volpi, E.; Wolfe, R.R. Whole Body and Skeletal Muscle Glutamine Metabolism in Healthy Subjects. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, E323–E333. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F. Glutamine and Skeletal Muscle. In Nutrition and Skeletal Muscle; Academic Press: San Diego, CA, USA, 2019; pp. 299–313. ISBN 9780128104224. [Google Scholar]
- Keogh, K.; Kenny, D.A.; Cormican, P.; McCabe, M.S.; Kelly, A.K.; Waters, S.M. Effect of Dietary Restriction and Subsequent Re-Alimentation on the Transcriptional Profile of Bovine Skeletal Muscle. PLoS ONE 2016, 11, e0149373. [Google Scholar]
- Goudarzi, A. The Recent Insights into the Function of ACAT1: A Possible Anti-Cancer Therapeutic Target. Life Sci. 2019, 232, 116592. [Google Scholar] [CrossRef]
- Li, H.; Ericsson, M.; Rabasha, B.; Budnik, B.; Chan, S.H.; Freinkman, E.; Lewis, C.A.; Doench, J.G.; Wagner, B.K.; Garraway, L.A.; et al. 6-Phosphogluconate Dehydrogenase Links Cytosolic Carbohydrate Metabolism to Protein Secretion via Modulation of Glutathione Levels. Cell Chem. Biol. 2019, 26, 1306–1314.e5. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef]


| Accession | Protein Name | Gene Name | FDR 1 | Fold Change 2 |
|---|---|---|---|---|
| (RM vs. MR) | ||||
| A0A452DPE6 | Calponin homology (CH) domain-containing protein | SMTNL1 | 0.0398564 | −0.90 |
| A0A452ET82 | Pyruvate kinase | PKM | 0.0398564 | −0.90 |
| A0A452FHP7 | Uncharacterized protein | FLNC | 0.0002162 | 0.25 |
| A0A452FBM2 | Uncharacterized protein | HSPA9 | 0.0138994 | 0.33 |
| A0A452G4K3 | ATP-dependent 6-phosphofructokinase | PFKM | 0.0398564 | 0.36 |
| A0A452FWD9; A0A452FWC6 | Dihydrolipoyl dehydrogenase | DLD | 0.0398564 | 0.42 |
| A0A452FX48I; A0A452FWP3 | Uncharacterized protein | AGL | 0.0002162 | 0.49 |
| A0A452ESM1; A0A452ERW8; A0A452ERN3 | Lipoyl-binding domain-containing protein | DLST | 0.0446152 | 0.53 |
| A0A452EYA9; A0A452EYB9; A0A452EY59 | Uncharacterized protein | SELENBP1 | 0.0006971 | 0.66 |
| A0A452FIG7 | S-formylglutathione hydrolase | ESD | 0.0398564 | 0.66 |
| A0A452EJS4 | Uncharacterized protein | MYOM1 | 0.0219753 | 0.69 |
| A0A452DWL1 | IF rod domain-containing protein | DES | 0.0000003 | 0.99 |
| A0A452ET55 | Triosephosphate isomerase | TPI1 | 0.0321358 | 1.18 |
| KEGG ID | Description | FDR 1 | Protein Names 2 |
|---|---|---|---|
| Treatment RM | |||
| oas04530 | Tight junction | 9.44 × 10−8 | ACTN1, ACTN4, EZR, LOC443340, MSN, MYH1, MYH13, MYH4, MYH8, MYL6, OMYHC2A, PPP2R1A, PPP2R1B, RDX, RHOA, YWHAQ |
| oas01200 | Carbon metabolism | 7.73 × 10−7 | ACAT1, ACO1, ADH5, DLAT, ECHS1, HADHA, IDH1, OGDH, OGDHL, PGD |
| oas00280 | Valine, leucine, and isoleucine degradation | 2.98 × 10−5 | ACAT1, ALDH2, ECHS1, HADHA, HIBADH, HSD17B10, OXCT1 |
| oas03015 | mRNA surveillance pathway | 5.52 × 10−5 | EIF4A3, PABPC1, PABPC4, PPP1CA, PPP1CB, PPP1CC, PPP2R1A, PPP2R1B |
| oas00071 | Fatty acid degradation | 6.03 × 10−5 | ACAT1, ADH5, ALDH2, ECHS1, ECI1, HADHA |
| oas03013 | RNA transport | 6.03 × 10−5 | EEF1A2, EIF4A1, EIF4A2, EIF4A3, EIF4EBP1, PABPC1, PABPC4, SEC13, SUMO2, SUMO3 |
| oas04720 | Long-term potentiation | 6.03 × 10−5 | PPP1CA, PPP1CB, PPP1CC, PPP1R1A, PPP3CA, PPP3CB, PPP3CC |
| oas00380 | Tryptophan metabolism | 9.75 × 10−5 | ACAT1, ALDH2, ECHS1, HADHA, OGDH, OGDHL |
| oas04810 | Regulation of actin cytoskeleton | 1.30 × 10−4 | ACTN1, ACTN4, EZR, LOC443340, MSN, PPP1CA, PPP1CB, PPP1CC, RDX, RHOA |
| oas00020 | Citrate cycle (TCA cycle) | 1.80 × 10−4 | ACO1, DLAT, IDH1, OGDH, OGDHL |
| Treatment MR | |||
| oas00010 | Glycolysis/Gluconeogenesis | 2.68 × 10−5 | AKR1A1, ALDH7A1, ENO2, PDHA1, PDHA2, GAPDH |
| oas00020 | Citrate cycle (TCA cycle) | 2.68 × 10−5 | IDH3A, IDH3G, PDHA1, PDHA2 |
| oas01230 | Biosynthesis of amino acids | 2.68 × 10−5 | ARG1, ENO2, GLUL, IDH3A, IDH3G, GAPDH |
| oas01200 | Carbon metabolism | 9.08 × 10−5 | ENO2, IDH3A, IDH3G, PDHA1, PDHA2, GAPDH |
| oas01100 | Metabolic pathways | 0.00064 | AKR1A1, ALDH7A1, ARG1, ENO2, GLUL, IDH3A, IDH3G, LAP3, MTHFD1, PDHA1, PDHA2, GAPDH |
| oas04066 | HIF-1 signaling pathway | 0.00074 | EIF4E, ENO2, PDHA1, PDHA2, GAPDH |
| oas00620 | Pyruvate metabolism | 0.0012 | ALDH7A1, PDHA1, PDHA2 |
| oas00330 | Arginine and proline metabolism | 0.0019 | ALDH7A1, ARG1, LAP3 |
| oas04145 | Phagosome | 0.0033 | RAC1, TUBB, TUBB1, TUBB2A |
| oas01210 | 2-Oxocarboxylic acid metabolism | 0.0055 | IDH3A, IDH3G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, T.C.; Dutra, L.L.; Mendes, T.A.d.O.; dos Santos, M.M.; Veroneze, R.; Gionbelli, M.P.; Duarte, M.d.S. Impact of Maternal Feed Restriction at Different Stages of Gestation on the Proteomic Profile of the Newborn Skeletal Muscle. Animals 2022, 12, 1011. https://doi.org/10.3390/ani12081011
Costa TC, Dutra LL, Mendes TAdO, dos Santos MM, Veroneze R, Gionbelli MP, Duarte MdS. Impact of Maternal Feed Restriction at Different Stages of Gestation on the Proteomic Profile of the Newborn Skeletal Muscle. Animals. 2022; 12(8):1011. https://doi.org/10.3390/ani12081011
Chicago/Turabian StyleCosta, Thaís Correia, Luana Lucas Dutra, Tiago Antônio de Oliveira Mendes, Marta Maria dos Santos, Renata Veroneze, Mateus Pies Gionbelli, and Marcio de Souza Duarte. 2022. "Impact of Maternal Feed Restriction at Different Stages of Gestation on the Proteomic Profile of the Newborn Skeletal Muscle" Animals 12, no. 8: 1011. https://doi.org/10.3390/ani12081011
APA StyleCosta, T. C., Dutra, L. L., Mendes, T. A. d. O., dos Santos, M. M., Veroneze, R., Gionbelli, M. P., & Duarte, M. d. S. (2022). Impact of Maternal Feed Restriction at Different Stages of Gestation on the Proteomic Profile of the Newborn Skeletal Muscle. Animals, 12(8), 1011. https://doi.org/10.3390/ani12081011








