Standardised Sampling Approach for Investigating Pathogens or Environmental Chemicals in Wild Game at Community Hunts
Simple Summary
Abstract
1. Introduction
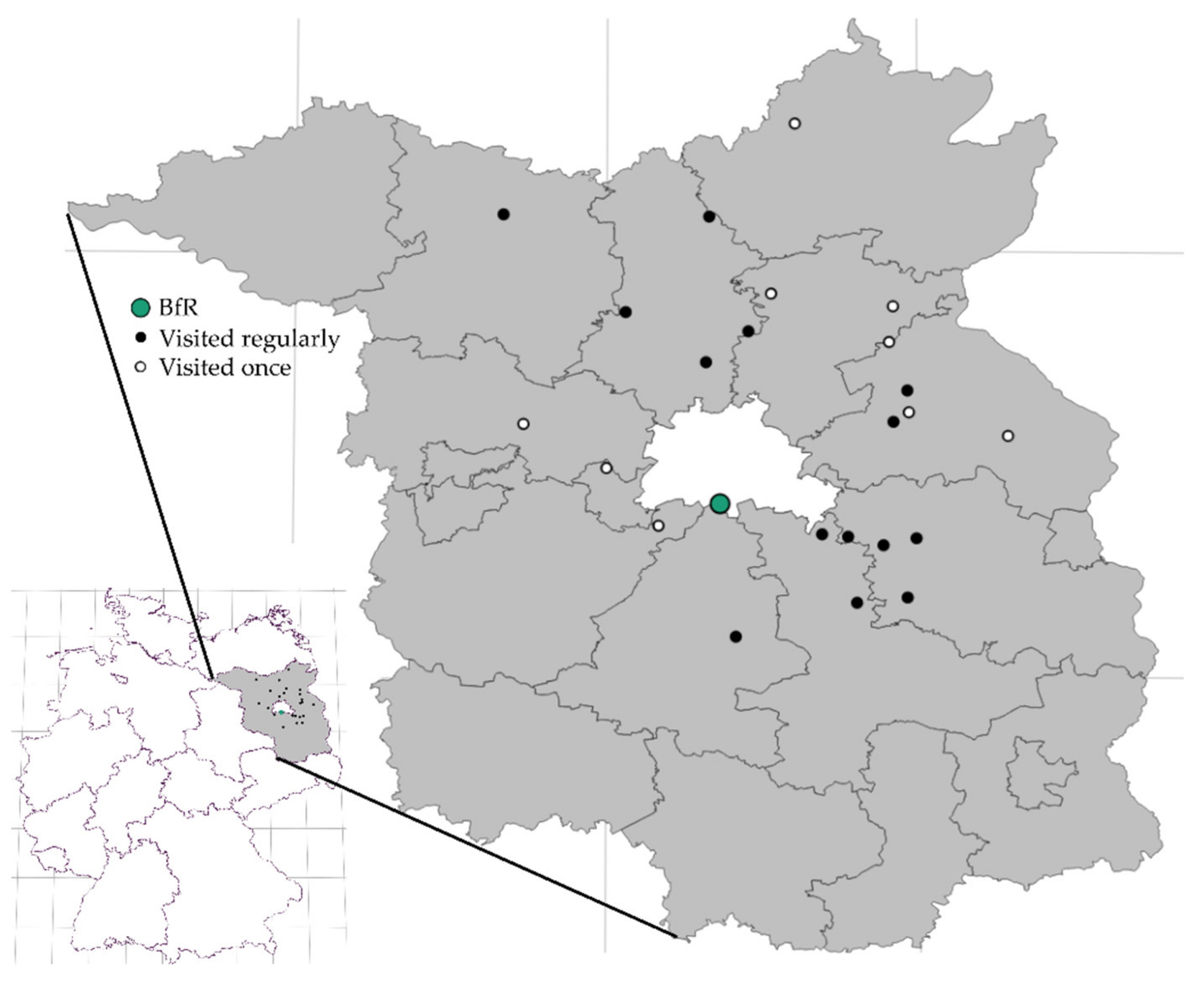
2. Materials and Methods
3. Results
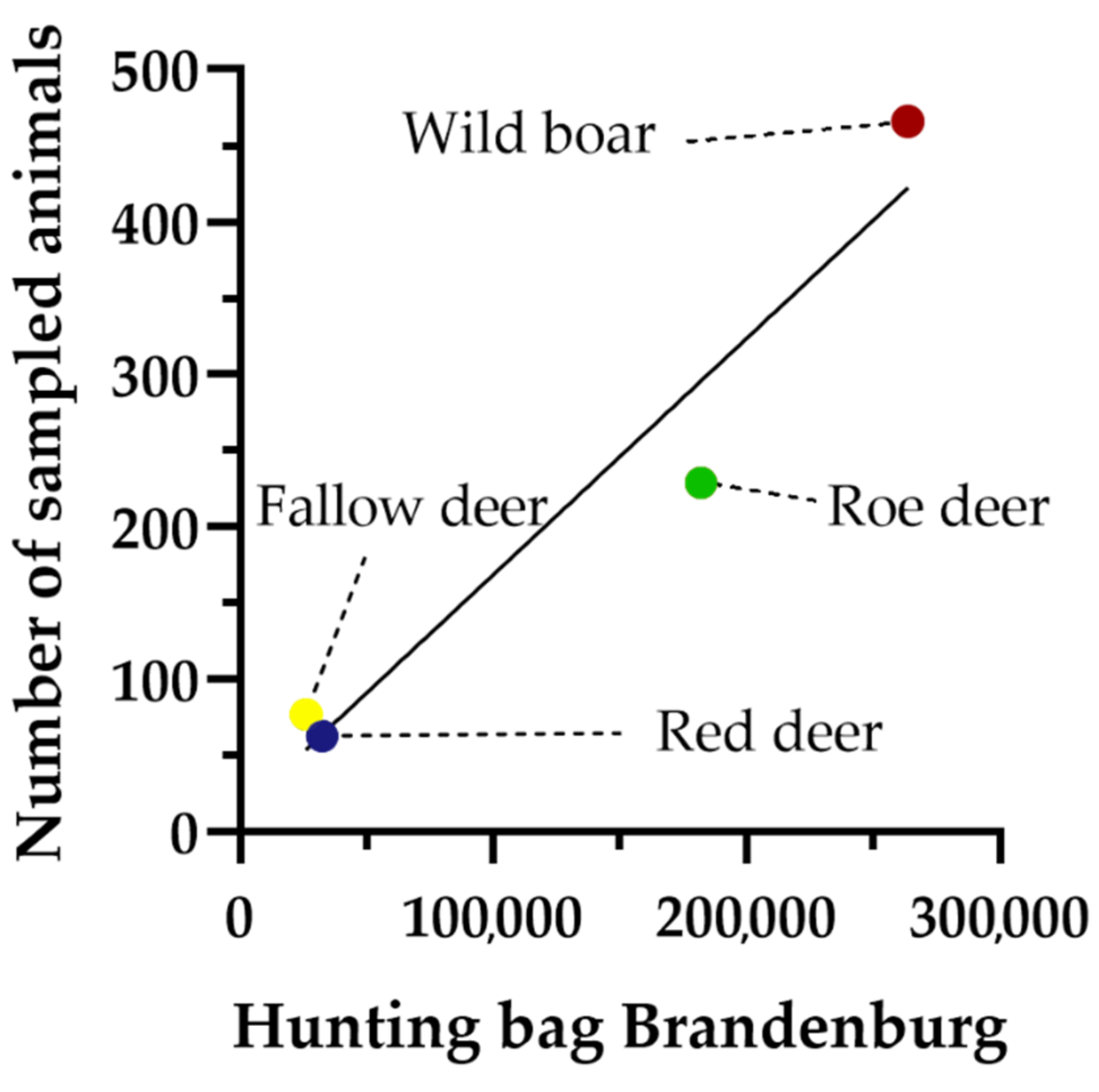
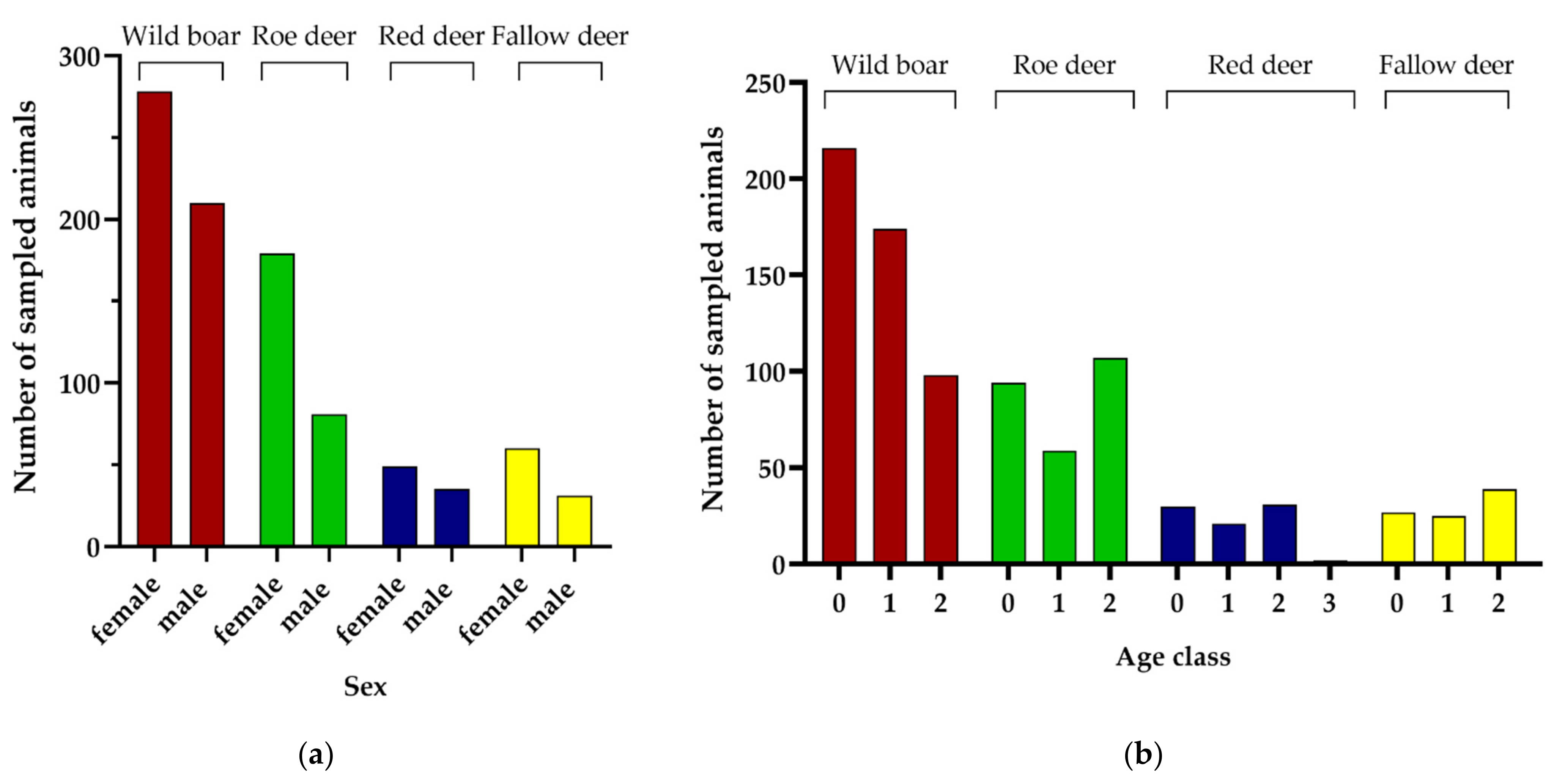
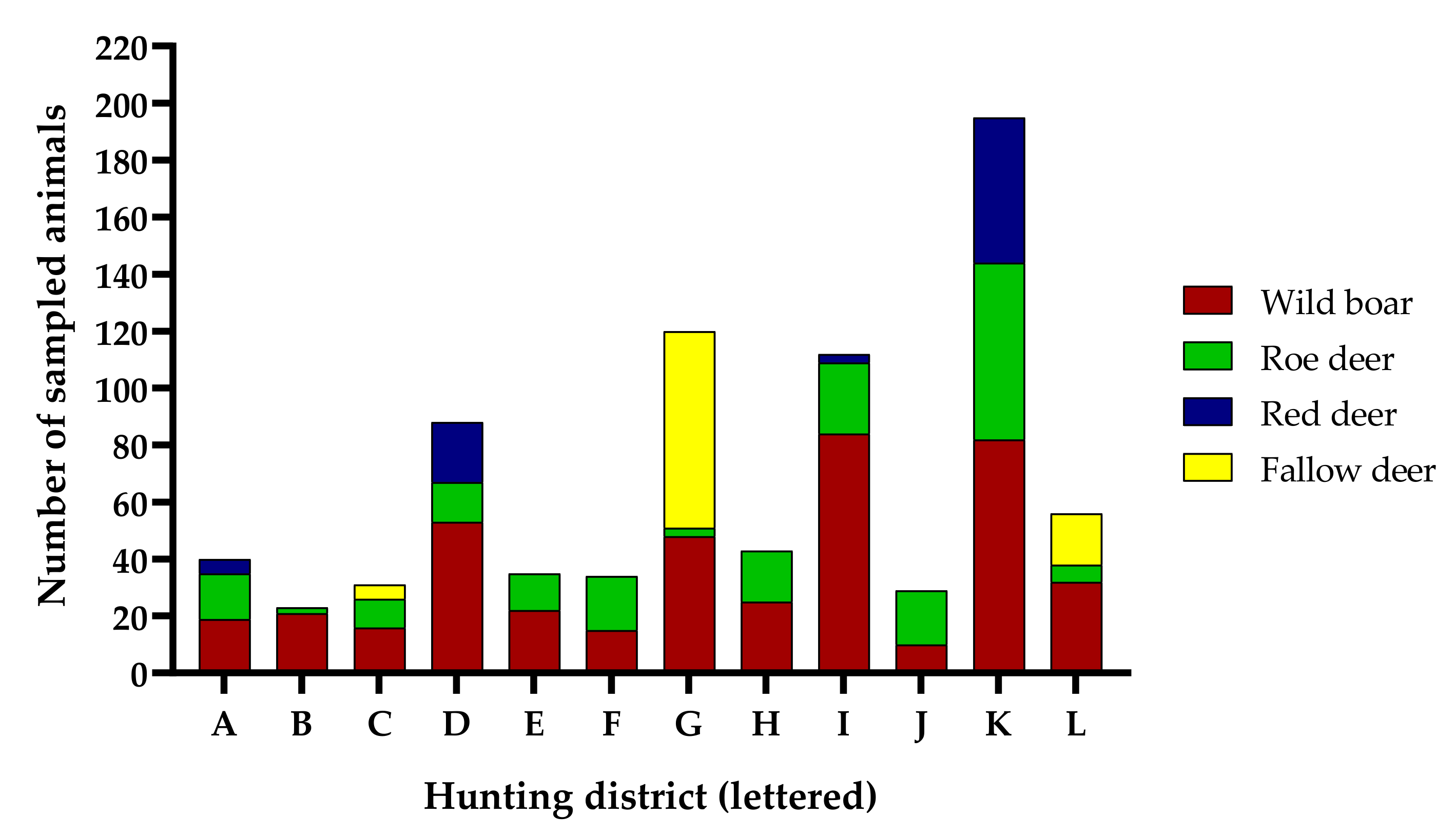
3.1. Performance of the Sampling Approach
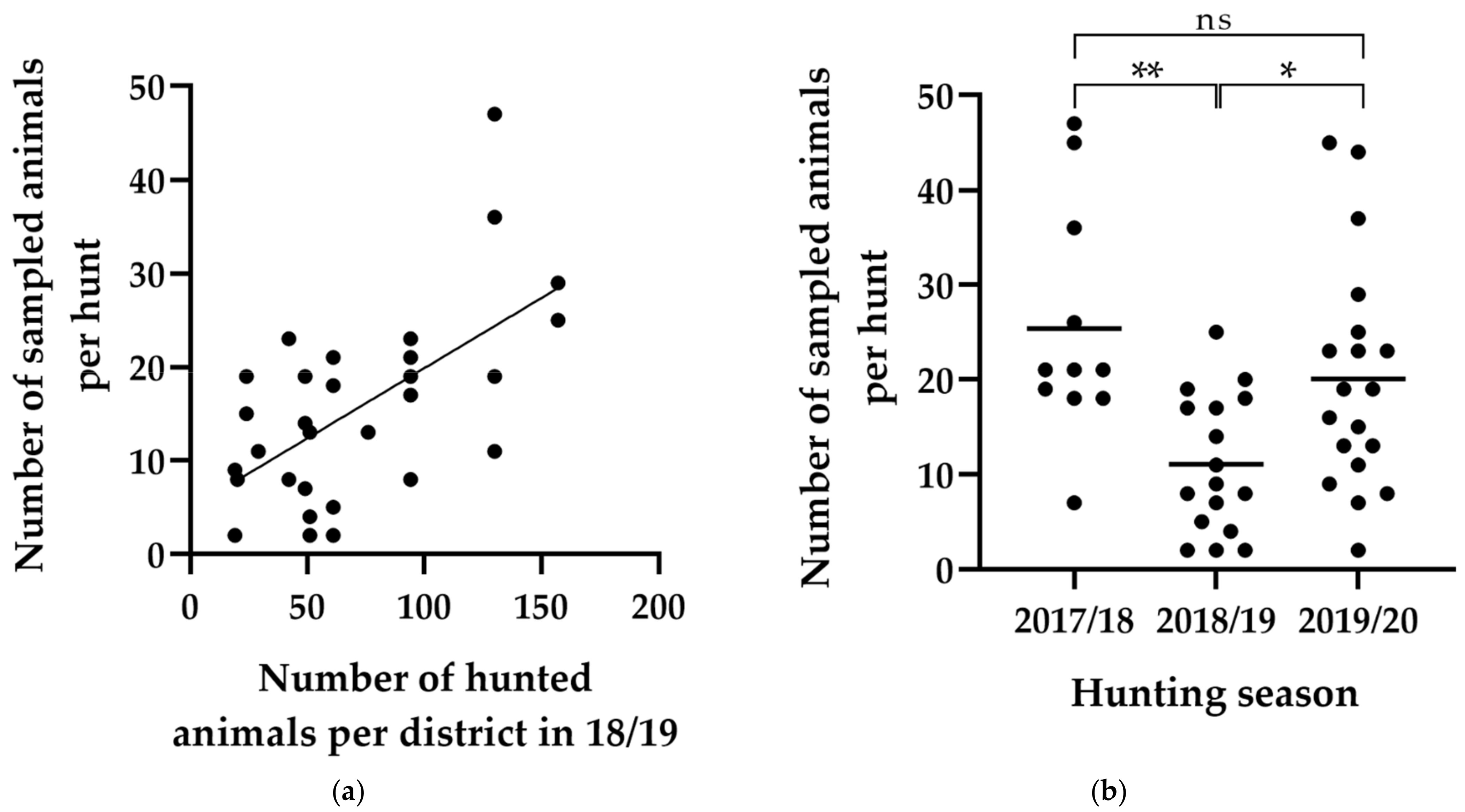
3.2. Parameters Affecting Sampling Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomassone, L.; Berriatua, E.; De Sousa, R.; Duscher, G.G.; Mihalca, A.D.; Silaghi, C.; Sprong, H.; Zintl, A. Neglected vector-borne zoonoses in Europe: Into the wild. Vet. Parasitol. 2018, 251, 17–26. [Google Scholar] [CrossRef] [PubMed]
- BVL. Monitoring 2020—BVL-Report 16.3; Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL): Berlin, Germany, 2020; p. 204. [Google Scholar]
- BVL. Monitoring 2012—BVL-Report 8.3; Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL): Berlin, Germany, 2012; p. 108. [Google Scholar]
- Chain, E.; Panel, O.C.I.T.F.; Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; et al. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. 2018, 16, e05333. [Google Scholar] [CrossRef]
- Anheyer-Behmenburg, H.E.; Szabo, K.; Schotte, U.; Binder, A.; Klein, G.; Johne, R. Hepatitis E Virus in Wild Boars and Spillover Infection in Red and Roe Deer, Germany, 2013–2015. Emerg. Infect. Dis. 2017, 23, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, J.; Numata, J.; Zimmermann, B.; Klinger, R.; Habedank, F.; Just, P.; Schafft, H.; Lahrssen-Wiederholt, M. Suitability of Wild Boar (Sus scrofa) as a Bioindicator for Environmental Pollution with Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS). Arch. Environ. Contam. Toxicol. 2018, 75, 594–606. [Google Scholar] [CrossRef]
- Němejc, K.; Sak, B.; Květoňová, D.; Hanzal, V.; Janiszewski, P.; Forejtek, P.; Rajský, D.; Ravaszová, P.; McEvoy, J.; Kváč, M. Cryptosporidium suis and Cryptosporidium scrofarum in Eurasian wild boars (Sus scrofa) in Central Europe. Vet. Parasitol. 2013, 197, 504–508. [Google Scholar] [CrossRef]
- Plaza-Rodríguez, C.; Alt, K.; Grobbel, M.; Hammerl, J.A.; Irrgang, A.; Szabo, I.; Stingl, K.; Schuh, E.; Wiehle, L.; Pfefferkorn, B.; et al. Wildlife as Sentinels of Antimicrobial Resistance in Germany? Front. Vet. Sci. 2020, 7, 627821. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; García-Presedo, I.; González-Warleta, M.; Mezo, M. Prevalence of Cryptosporidium and Giardia in roe deer (Capreolus capreolus) and wild boars (Sus scrofa) in Galicia (NW, Spain). Vet. Parasitol. 2011, 179, 216–219. [Google Scholar] [CrossRef]
- Bier, N.S.; Stollberg, K.; Mayer-Scholl, A.; Johne, A.; Nöckler, K.; Richter, M. Seroprevalence of Toxoplasma gondii in wild boar and deer in Brandenburg, Germany. Zoonoses Public Health 2020, 67, 601–606. [Google Scholar] [CrossRef]
- Takova, K.; Koynarski, T.; Minkov, I.; Ivanova, Z.; Toneva, V.; Zahmanova, G. Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria. Animals 2020, 10, 1521. [Google Scholar] [CrossRef]
- Montagnaro, S.; D’Ambrosi, F.; Petruccelli, A.; Ferrara, G.; D’Alessio, N.; Iovane, V.; Veneziano, V.; Fioretti, A.; Pagnini, U. A Serological Survey of Brucellosis in Eurasian Wild Boar (Sus scrofa) in Campania Region, Italy. J. Wildl. Dis. 2019, 56, 424. [Google Scholar] [CrossRef]
- Coelho, C.; Lopes, A.P.; Mesquita, J.R.; Cardoso, L.; Vieira-Pinto, M. Toxoplasma gondii Infection in Hunted Wild Boars (Sus scrofa): Heart Meat Juice as an Alternative Sample to Serum for the Detection of Antibodies. EcoHealth 2015, 12, 685–688. [Google Scholar] [CrossRef]
- Milner, J.M.; Bonenfant, C.; Mysterud, A.; Gaillard, J.-M.; Csányi, S.; Stenseth, N.C. Temporal and spatial development of red deer harvesting in Europe: Biological and cultural factors. J. Appl. Ecol. 2006, 43, 721–734. [Google Scholar] [CrossRef]
- Burbaitė, L.; Csányi, S. Roe deer population and harvest changes in Europe. Est. J. Ecol. 2009, 58, 169. [Google Scholar] [CrossRef]
- Burbaitė, L.; Csányi, S. Red deer population and harvest changes in Europe. Acta Zool. Litu. 2010, 20, 179–188. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Kotková, M.; Němejc, K.; Sak, B.; Hanzal, V.; Květoňová, D.; Hlásková, L.; Čondlová, Š.; McEvoy, J.; Kváč, M. Cryptosporidium ubiquitum, C. muris and Cryptosporidium deer genotype in wild cervids and caprines in the Czech Republic. Folia Parasitol. 2016, 63, 003. [Google Scholar] [CrossRef]
- Paulsen, P.; Ehebruster, J.; Irschik, I.; Lücker, E.; Riehn, K.; Winkelmayer, R.; Smulders, F.J.M. Findings of Alaria alata mesocercariae in wild boars (Sus scrofa) in eastern Austria. Eur. J. Wildl. Res. 2012, 58, 991–995. [Google Scholar] [CrossRef]
- Kaczyński, P.; Łozowicka, B.; Perkowski, M.; Zoń, W.; Hrynko, I.; Rutkowska, E.; Skibko, Z. Impact of broad-spectrum pesticides used in the agricultural and forestry sector on the pesticide profile in wild boar, roe deer and deer and risk assessment for venison consumers. Sci. Total Environ. 2021, 784, 147215. [Google Scholar] [CrossRef]
- Beňová, K.; Dvořák, P.; Tomko, M.; Falis, M. 137Cs monitoring in the meat of wild boar population in Slovakia. Potravinarstvo Slovak J. Food Sci. 2016, 10, 243–247. [Google Scholar] [CrossRef]
- Ghielmetti, G.; Hilbe, M.; Friedel, U.; Menegatti, C.; Bacciarini, L.; Stephan, R.; Bloemberg, G. Mycobacterial infections in wild boars (Sus scrofa) from Southern Switzerland: Diagnostic improvements, epidemiological situation and zoonotic potential. Transbound. Emerg. Dis. 2021, 68, 573–586. [Google Scholar] [CrossRef]
- Coelho, C.; Vieira-Pinto, M.; Faria, A.S.; Vale-Gonçalves, H.M.; Veloso, O.; Paiva-Cardoso, M.D.N.; Mesquita, J.R.; Lopes, A.P. Serological evidence of Toxoplasma gondii in hunted wild boar from Portugal. Vet. Parasitol. 2014, 202, 310–312. [Google Scholar] [CrossRef]
- Santoro, M.; Viscardi, M.; Sgroi, G.; Dʼalessio, N.; Veneziano, V.; Pellicano, R.; Brunetti, R.; Fusco, G. Real-time PCR detection of Toxoplasma gondii in tissue samples of wild boars (Sus scrofa) from southern Italy reveals high prevalence and parasite load. Parasites Vectors 2019, 12, 335. [Google Scholar] [CrossRef]
- Moutelíková, R.; Dufková, L.; Kamler, J.; Drimaj, J.; Plhal, R.; Prodělalová, J. Epidemiological survey of enteric viruses in wild boars in the Czech Republic: First evidence of close relationship between wild boar and human rotavirus A strains. Vet. Microbiol. 2016, 193, 28–35. [Google Scholar] [CrossRef]
- Hinić, V.; Brodard, I.; Thomann, A.; Holub, M.; Miserez, R.; Abril, C. IS711-based real-time PCR assay as a tool for detection of Brucellaspp. in wild boars and comparison with bacterial isolation and serology. BMC Vet. Res. 2009, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, M.; Hammerl, J.A.; Kunz, K.; Barac, A.; Nöckler, K.; Hertwig, S. Yersinia pseudotuberculosis Prevalence and Diversity in Wild Boars in Northeast Germany. Appl. Environ. Microbiol. 2018, 84, 84. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson-Ahomaa, M.; Wacheck, S.; Koenig, M.; Stolle, A.; Stephan, R. Prevalence of pathogenic Yersinia enterocolitica and Yersinia pseudotuberculosis in wild boars in Switzerland. Int. J. Food Microbiol. 2009, 135, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Alabau, E.; Mentaberre, G.; Camarero, P.R.; Castillo-Contreras, R.; Sánchez-Barbudo, I.S.; Conejero, C.; Fernández-Bocharán, M.S.; López-Olvera, J.R.; Mateo, R. Accumulation of diastereomers of anticoagulant rodenticides in wild boar from suburban areas: Implications for human consumers. Sci. Total Environ. 2020, 738, 139828. [Google Scholar] [CrossRef] [PubMed]
- Durkalec, M.; Szkoda, J.; Kolacz, R.; Opalinski, S.; Nawrocka, A.; Zmudzki, J. Bioaccumulation of Lead, Cadmium and Mercury in Roe Deer and Wild Boars from Areas with Different Levels of Toxic Metal Pollution. Int. J. Environ. Res. 2015, 9, 205–212. [Google Scholar]
- Vicente, J.; Domingo, M.; Segalés, J.; Höfle, U.; Balasch, M.; Plana-Durán, J.; Gortázar, C. Epidemiological study on porcine circovirus type 2 (PCV2) infection in the European wild boar (Sus scrofa). Vet. Res. 2004, 35, 243–253. [Google Scholar] [CrossRef]
- BImA. Jagd und Fischerei. Available online: https://www.bundesimmobilien.de/jagd-und-fischerei-b8f0c783cdcca84d (accessed on 1 March 2021).
- Tölle, C. Bundesanstalt für Immobilienaufgaben/Bundesforst. In Proceedings of the WALD.WILD.verBUND.FORSCHUNG, Multidisziplinäre Forschung und Feldstudien im verBUND über Wald und Wild, Berlin, Germany, 25–26 November 2019. [Google Scholar]
- Wildbewirtschaftungsrichtlinie. Gemeinsame Richtlinie für die Hege und Bejagung des Schalenwildes der Länder Brandenburg und Mecklenburg-Vorpommern (Wildbewirtschaftungsrichtlinie) vom 24 September 2001 (ABl./01, [Nr. 51], S.859). Available online: https://bravors.brandenburg.de/de/verwaltungsvorschriften-216896 (accessed on 16 March 2022).
- Deutscher_Jagdverband. Jagdstatistik für Einzelne Wildarten. Available online: https://web.archive.org/web/20210531130511/https://www.jagdverband.de/jagd-und-wildunfallstatistik (accessed on 31 May 2021).
- Kästner, C.; Bier, N.S.; Mayer-Scholl, A.; Nöckler, K.; Richter, M.H.; Johne, A. Prevalence of Alaria alata mesocercariae in wild boars from Brandenburg, Germany. Parasitol. Res. 2021, 120, 2103–2108. [Google Scholar] [CrossRef]
- Wotschikowsky, U. Ungulates and their management in Germany. In European Ungulates and their Management in the 21st Century; Appollonio, M., Andersen, R., Putman, R., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 201–222. [Google Scholar]
- BJagdG. Bundesjagdgesetz in der Fassung der Bekanntmachung vom 29 September 1976 (BGBl. I S. 2849), das zuletzt durch Artikel 291 der Verordnung vom 19 June 2020 (BGBl. I S. 1328) geändert worden ist. Available online: https://www.gesetze-im-internet.de/bjagdg/BJagdG.pdf (accessed on 30 January 2022).
- BbgJagdG. Jagdgesetz für das Land Brandenburg (BbgJagdG) vom 9 Oktober 2003 (GVBl.I/03, [Nr. 14], S.250) zuletzt geändert durch Artikel 3 des Gesetzes vom 10. July 2014 (GVBl.I/14, [Nr. 33]). Available online: https://bravors.brandenburg.de/de/gesetze-212920 (accessed on 30 January 2022).
- Stollberg, K.; Schares, G.; Mayer-Scholl, A.; Hrushetska, I.; Diescher, S.; Johne, A.; Richter, M.; Bier, N. Comparison of Direct and Indirect Toxoplasma gondii Detection and Genotyping in Game: Relationship and Challenges. Microorganisms 2021, 9, 1663. [Google Scholar] [CrossRef]
- Pareja-Carrera, J.; Rodríguez-Estival, J.; Martinez-Haro, M.; Ortiz, J.A.; Mateo, R. Age-dependent changes in essential elements and oxidative stress biomarkers in blood of red deer and vulnerability to nutritional deficiencies. Sci. Total Environ. 2018, 626, 340–348. [Google Scholar] [CrossRef]
- Smitka, P.; Tóthová, C.; Curlik, J.; Lazar, P.; Bíreš, J.; Pošiváková, T. Serum concentration of haptoglobin in European mouflon (Ovis musimon L.) from a game reserve. Acta Vet. Brno 2015, 84, 25–28. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Forth, J.H.; Probst, C.; Staubach, C.; Hlinak, A.; Rudovsky, A.; Holland, D.; Schlieben, P.; Göldner, M.; Schatz, J.; et al. Joining the club: First detection of African swine fever in wild boar in Germany. Transbound. Emerg. Dis. 2020, 68, 1744–1752. [Google Scholar] [CrossRef]
- Queirós, J.; Acevedo, P.; e Santos, J.P.V.; Barasona, J.; Beltran-Beck, B.; González-Barrio, D.; Armenteros, J.A.; Diez-Delgado, I.; Boadella, M.; De Mera, I.F.; et al. Red deer in Iberia: Molecular ecological studies in a southern refugium and inferences on European postglacial colonization history. PLoS ONE 2019, 14, e0210282. [Google Scholar] [CrossRef]
- Matuschka, F.-R.; Heiler, M.; Eiffert, H.; Fischer, P.; Lotter, H.; Spielman, A. Diversionary Role of Hoofed Game in the Transmission of Lyme Disease Spirochetes. Am. J. Trop. Med. Hyg. 1993, 48, 693–699. [Google Scholar] [CrossRef]
| Hunting Season | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2017/18 | 2018/19 | 2019/20 | 2020/21 | Total | ||||||
| number of hunting districts | 6 | 10 | 14 | 11 | 23 | |||||
| number of drive hunts | 11 | 17 | 19 | 15 | 62 | |||||
| game species | animals | samples | animals | samples | animals | samples | animals | samples | animals | samples |
| red deer | 41 | 114 | 23 | 51 | 13 | 45 | 8 | 31 | 85 | 241 |
| fallow deer | 0 | 0 | 1 | 1 | 62 | 275 | 29 | 142 | 92 | 418 |
| roe deer | 79 | 140 | 84 | 250 | 66 | 215 | 31 | 125 | 260 | 730 |
| wild boar | 146 | 456 | 80 | 379 | 240 | 818 | 22 | 109 | 488 | 1762 |
| unknown ungulate | 13 | 34 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 34 |
| total | 279 | 744 | 188 | 681 | 381 | 1353 | 90 | 407 | 938 | 3185 |
| Organ/Tissue | Pathogen Investigated | Detection Method | Disease in Humans | Cases in Germany 1 2001–2020 | Source |
|---|---|---|---|---|---|
| Liver | Hepatitis E virus * (Orthohepevirus A) | RNA | Hepatitis E | 32,144 | [5] |
| Heart | Toxoplasma gondii * | DNA, Antibodies (host response) | Toxoplasmosis (congenital/ postnatal) | 1438 (postnatal) | [13,24,40] |
| Blood/Serum | All pathogens | Antibodies (host response) | - | - | e.g., [10,11,12] |
| Faeces | Cryptosporidium spp. * | DNA, Oocysts | Cryptosporidiosis | 29,867 | [7,9,18] |
| Giardia sp. | Cysts | Giardiasis | 74,852 | [9] | |
| Campylobacter sp. * | Culture | Campylobacteriosis | 1,341,087 | [8] | |
| Shigatoxigenic Escherichia coli (STEC) * | Culture | Haemolytic uremic syndrome (HUS) and bloody diarrhoea | 2254 (HUS) 2 39,665 (EHEC) 3 | [8] | |
| Salmonella sp. | Culture | Salmonellosis | 687,133 | [8] | |
| Rotavirus sp. * | RNA | Rotaviral gastroenteritis | 962,075 | [25] | |
| Spleen | Brucella sp. * | DNA | Brucellosis | 745 | [26] |
| Tonsils | Yersinia sp. * | DNA, Culture | Yersiniosis | 87,337 | [27,28] |
| Nasal swabs | Methicillin-resistant Staphylococcus aureus (MRSA) * | Culture | Various manifestations | 35,936 | [8] |
| Lymph nodes | Mycobacterium spp. | DNA, Culture | Tuberculosis | 109,460 | [22] |
| Tongue, Abdominal fat | Alaria alata * | Mesocercariae, DNA | - | - | [19,36] |
| Foreleg musculature, tongue, diaphragm | Trichinella sp. | First larvae | Trichinellosis | 98 | Regulation (EC) 2015/1375 4 |
| Organ/Tissue | Chemical Investigated | Source |
|---|---|---|
| Liver | Per- and polyfluoroalkyl substances (PFAS) * | [6] |
| Pesticides (herbicides, insecticides, rodenticides) * | [20,29] | |
| Kidney | Heavy metals (cadmium, mercury, lead, …) | [30] |
| Fat tissue | Dioxins and dioxin-like PCB | [4] |
| Musculature | Cesium-137 | [21] |
| Organ/Tissue | Investigated for | Source |
|---|---|---|
| Blood | Nutrition (essential elements), oxidative stress | [41] |
| Haptoglobin (marker of inflammation and sub-clinical disease) | [42] | |
| African swine fever virus | [43] | |
| Spleen | Phylogeography | [44] |
| Attached ticks | Ticks and host reservoir function for Borrelia burgdorferi sensu lato | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maaz, D.; Gremse, C.; Stollberg, K.C.; Jäckel, C.; Sutrave, S.; Kästner, C.; Korkmaz, B.; Richter, M.H.; Bandick, N.; Steinhoff-Wagner, J.; et al. Standardised Sampling Approach for Investigating Pathogens or Environmental Chemicals in Wild Game at Community Hunts. Animals 2022, 12, 888. https://doi.org/10.3390/ani12070888
Maaz D, Gremse C, Stollberg KC, Jäckel C, Sutrave S, Kästner C, Korkmaz B, Richter MH, Bandick N, Steinhoff-Wagner J, et al. Standardised Sampling Approach for Investigating Pathogens or Environmental Chemicals in Wild Game at Community Hunts. Animals. 2022; 12(7):888. https://doi.org/10.3390/ani12070888
Chicago/Turabian StyleMaaz, Denny, Carl Gremse, Kaya C. Stollberg, Claudia Jäckel, Smita Sutrave, Carolyn Kästner, Birsen Korkmaz, Martin H. Richter, Niels Bandick, Julia Steinhoff-Wagner, and et al. 2022. "Standardised Sampling Approach for Investigating Pathogens or Environmental Chemicals in Wild Game at Community Hunts" Animals 12, no. 7: 888. https://doi.org/10.3390/ani12070888
APA StyleMaaz, D., Gremse, C., Stollberg, K. C., Jäckel, C., Sutrave, S., Kästner, C., Korkmaz, B., Richter, M. H., Bandick, N., Steinhoff-Wagner, J., Lahrssen-Wiederholt, M., & Mader, A. (2022). Standardised Sampling Approach for Investigating Pathogens or Environmental Chemicals in Wild Game at Community Hunts. Animals, 12(7), 888. https://doi.org/10.3390/ani12070888








